Nimesulide-Induced Fixed Drug Eruption Followed by Etoricoxib-Induced Fixed Drug Eruption: An Unusual Case Report and Review of the Literature
Abstract
1. Introduction
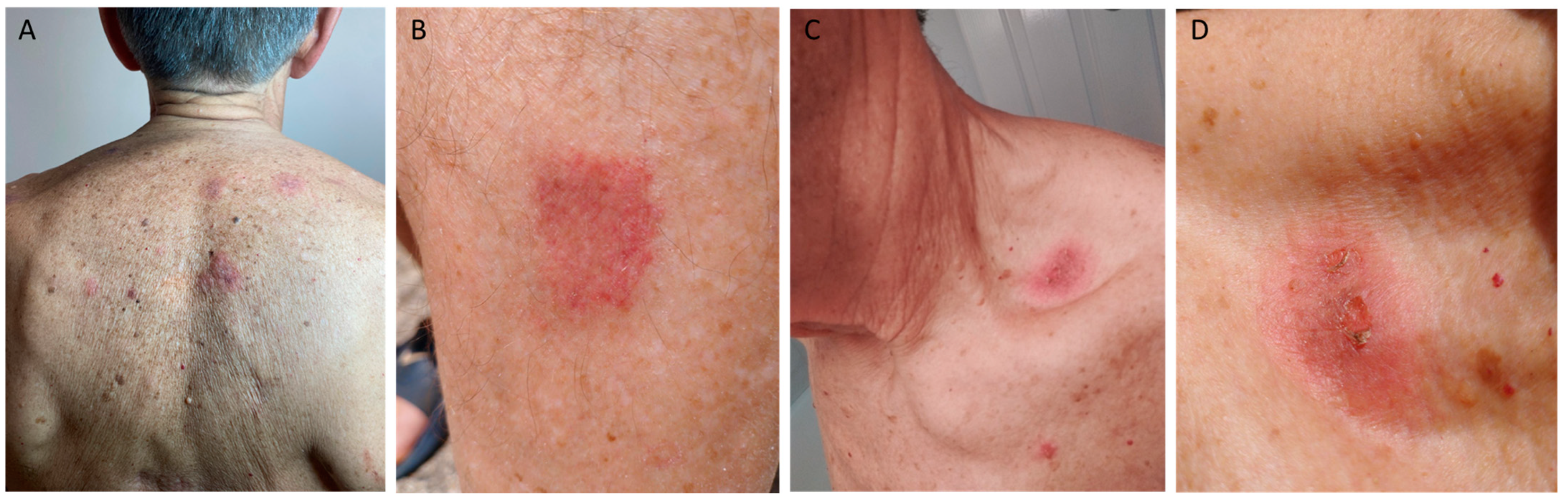
2. Case Description
3. Review of the Literature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sehgal, V.N.; Srivastava, G. Fixed drug eruption (FDE): Changing scenario of incriminating drugs. Int. J. Dermatol. 2006, 45, 897–908. [Google Scholar] [CrossRef]
- Savin, J.A. Current causes of fixed drug eruption in the U.K. Br. J. Dermatol. 2001, 145, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.J.; Lee, J.B. A Review of Fixed Drug Eruption with a Special Focus on Generalized Bullous Fixed Drug Eruption. Medicina 2021, 57, 925. [Google Scholar] [CrossRef]
- Patel, S.; John, A.M.; Handler, M.Z.; Schwartz, R.A. Fixed Drug Eruptions: An Update, Emphasizing the Potentially Lethal Generalized Bullous Fixed Drug Eruption. Am. J. Clin. Dermatol. 2020, 21, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Ardern-Jones, M.R.; Mockenhaupt, M.; Aberer, W.; Barbaud, A.; Caubet, J.C.; Spiewak, R.; Torres, M.J.; Mortz, C.G. EAACI position paper on how to classify cutaneous manifestations of drug hypersensitivity. Allergy 2019, 74, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, T. Fixed drug eruption: Pathogenesis and diagnostic tests. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 316–321. [Google Scholar] [CrossRef]
- Flowers, H.; Brodell, R.; Brents, M.; Wyatt, J.P. Fixed drug eruptions: Presentation, diagnosis, and management. South. Med. J. 2014, 107, 724–727. [Google Scholar] [CrossRef]
- Mizukawa, Y.; Shiohara, T. Fixed drug eruption: A prototypic disorder mediated by effector memory T cells. Curr. Allergy Asthma Rep. 2009, 9, 71–77. [Google Scholar] [CrossRef]
- Barbaud, A.; Gonçalo, M.; Bruynzeel, D.; Bircher, A. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermat. 2001, 45, 321–328. [Google Scholar] [CrossRef]
- Ammar, H.; Ben Fredj, N.; Ben Romdhane, H.; Chaabane, A.; Chadli, Z.; Ben Fadhel, N.; Aouam, K. Cross-reactivity between nonsteroidal anti-inflammatory drugs in fixed drug eruption: Two unusual cases and a literature review. Br. J. Clin. Pharmacol. 2023, 89, 561–573. [Google Scholar] [CrossRef]
- Ouni, B.; Fathallah, N.; Ben-Sayed, N.; Abdessayed, N.; Slim, R.; Ben Salem, C. Fixed drug eruption (FDE) induced by mefenamic acid with unusually cross-reactivity to diclofenac: A case report. Br. J. Clin. Pharmacol. 2021, 87, 1582–1583. [Google Scholar] [CrossRef]
- Movsisyan, M.; Fiandor, A.; González-Muñoz, M.; Quirce, S.; Bellón, T.; Hakobyan, A.; Marques-Mejias, M.A.; Domínguez-Ortega, J.; Cabañas, R. The Lymphocyte Transformation Test Is Useful in the Diagnosis of Fixed Drug Eruption Induced by Etoricoxib. J. Investig. Allergol. Clin. Immunol. 2019, 29, 307–309. [Google Scholar] [CrossRef]
- Carneiro-Leão, L.; Rodrigues Cernadas, J. Bullous Fixed Drug Eruption Caused by Etoricoxib Confirmed by Patch Testing. J. Allergy Clin. Immunol. Pract. 2019, 7, 1629–1630. [Google Scholar] [CrossRef] [PubMed]
- Ben Romdhane, H.; Ammar, H.; Ben Fadhel, N.; Chadli, Z.; Ben Fredj, N.; Boughattas, N.A.; Chaabane, A.; Aouam, K. Piroxicam-induced fixed drug eruption: Cross-reactivity with meloxicam. Contact Dermat. 2019, 81, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; Brinca, A.; Gonçalo, M. Patch testing in fixed drug eruptions--a 20-year review. Contact Dermat. 2011, 65, 195–201. [Google Scholar] [CrossRef]
- Ozdemir, S.K.; Erkekol, F.O.; Aydin, O.; Celik, G.E.; Misirligil, Z. Fixed drug eruption due to meloxicam. J. Investig. Allergol. Clin. Immunol. 2011, 21, 419–420. [Google Scholar] [PubMed]
- Pérez-Calderón, R.; Gonzalo-Garijo, M.A.; Pérez-Rangel, I.; Sánchez-Vega, S.; Zambonino, M.A. Fixed drug eruption due to nabumetone in a patient with previous fixed drug eruptions due to naproxen. J. Investig. Allergol. Clin. Immunol. 2011, 21, 153–154. [Google Scholar]
- Ozkaya, E. Fixed drug eruption at sites of ear piercing. Clin. Exp. Dermatol. 2008, 33, 345–347. [Google Scholar] [CrossRef]
- Linares, T.; Marcos, C.; Gavilan, M.J.; Arenas, L. Fixed drug eruption due to aceclofenac. Contact Dermat. 2007, 56, 291–292. [Google Scholar] [CrossRef]
- Ozkaya-Bayazit, E. Specific site involvement in fixed drug eruption. J. Am. Acad. Dermatol. 2003, 49, 1003–1007. [Google Scholar] [CrossRef]
- Oliveira, H.; Gonvçalo, M.; Reis, J.; Figueiredo, A.A. Fixed drug eruption to piroxicam. Positive patch tests with cross-sensitivity to tenoxicam. J. Dermatol. Treat. 1999, 10, 209–212. [Google Scholar] [CrossRef]
- Ordoqui, E.; De Barrio, M.; Rodríguez, V.M.; Herrero, T.; Gil, P.J.; Baeza, M.L. Cross-sensitivity among oxicams in piroxicam-caused fixed drug eruption: Two case reports. Allergy 1995, 50, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Alanko, K. Topical provocation of fixed drug eruption. A study of 30 patients. Contact Dermat. 1994, 31, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Gastaminza, G.; Echechipía, S.; Navarro, J.A.; Fernández de Corrés, L. Fixed drug eruption from piroxicam. Contact Dermat. 1993, 28, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, A.J.; Bharija, S.C.; Singh, M.; Belhaj, M.S. Ninety-eight fixed drug eruptions with provocation tests. Dermatologica 1988, 177, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Alanko, K.; Stubb, S.; Reitamo, S. Topical provocation of fixed drug eruption. Br. J. Dermatol. 1987, 116, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, A.J.; Belhaj, M.S.; Bharija, S.C.; Mohammed, M. Drugs causing fixed eruptions. J. Dermatol. 1984, 11, 383–385. [Google Scholar] [CrossRef]
- Pasricha, J.S. Drugs causing fixed eruptions. Br. J. Dermatol. 1979, 100, 183–185. [Google Scholar] [CrossRef]
- Pandhi, R.K.; Dedi, T.R. Letter: Fixed skin eruption caused by oxyphenbutazone with cross-reactivity to phenylbutazone. Arch. Dermatol. 1975, 111, 131. [Google Scholar] [CrossRef]
- Nayyar, K.C.; Pasricha, J.S. Fixed drug eruption to oxyphenbutazone and phenylbutazone. Dermatologica 1972, 144, 214–216. [Google Scholar] [CrossRef]
- Worboys, M.; Toon, E. Phenylbutazone (Bute, PBZ, EPZ): One drug across two species. Hist. Philos. Life Sci. 2018, 40, 27. [Google Scholar] [CrossRef]
- Cho, Y.T.; Lin, J.W.; Chen, Y.C.; Chang, C.Y.; Hsiao, C.H.; Chung, W.H.; Chu, C.-Y. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J. Am. Acad. Dermatol. 2014, 70, 539–548. [Google Scholar] [CrossRef]
- Mizukawa, Y.; Yamazaki, Y.; Shiohara, T. In vivo dynamics of intraepidermal CD8+ T cells and CD4+ T cells during the evolution of fixed drug eruption. Br. J. Dermatol. 2008, 158, 1230–1238. [Google Scholar] [CrossRef]
- Phillips, E.J.; Bigliardi, P.; Bircher, A.J.; Broyles, A.; Chang, Y.S.; Chung, W.H.; Lehloenya, R.; Mockenhaupt, M.; Mmed, J.P.; Pirmohamed, M.; et al. Controversies in drug allergy: Testing for delayed reactions. J. Allergy Clin. Immunol. 2019, 143, 66–73. [Google Scholar] [CrossRef]
- Barbaud, A. Skin testing in delayed reactions to drugs. Immunol. Allergy Clin. N. Am. 2009, 29, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, A.M.; Demoly, P. Drug provocation tests: Up-date and novel approaches. Allergy Asthma Clin. Immunol. 2013, 9, 12. [Google Scholar] [CrossRef]
- Kowalski, M.L.; Asero, R.; Bavbek, S.; Blanca, M.; Blanca-Lopez, N.; Bochenek, G.; Brockow, K.; Campo, P.; Celik, G.; Cernadas, J.; et al. Classification and practical approach to the diagnosis and management of hypersensitivity to nonsteroidal anti-inflammatory drugs. Allergy 2013, 68, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.L.; Makowska, J.S.; Blanca, M.; Bavbek, S.; Bochenek, G.; Bousquet, J.; Celik, B.G.; Demoly, P.; Gomes, E.R.; Niżankowska-Mogilnicka, E.; et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs)—Classification, diagnosis and management: Review of the EAACI/ENDA(#) and GA2LEN/HANNA*. Allergy 2011, 66, 818–829. [Google Scholar]
- Sachs, B.; Fatangare, A.; Sickmann, A.; Glässner, A. Lymphocyte transformation test: History and current approaches. J. Immunol. Methods. 2021, 493, 113036. [Google Scholar] [CrossRef]
- Kim, M.H.; Shim, E.J.; Jung, J.W.; Sohn, S.W.; Kang, H.R. A case of allopurinol-induced fixed drug eruption confirmed with a lymphocyte transformation test. Allergy Asthma Immunol. Res. 2012, 4, 309–310. [Google Scholar] [CrossRef]
- Böhm, R.; Cascorbi, I. Pharmacogenetics and Predictive Testing of Drug Hypersensitivity Reactions. Front Pharmacol. 2016, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- Kameswari, P.D.; Selvaraj, N.; Adhimoolam, M. Fixed drug eruptions caused by cross-reactive quinolones. J. Basic. Clin. Pharm. 2014, 5, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Thami, G.P. Fixed drug eruption caused by itraconazole: Reactivity and cross reactivity. J. Am. Acad. Dermatol. 2008, 58, 521–522. [Google Scholar] [CrossRef]
- Mithari, H.S.; Gole, P.V.; Kharkar, V.D.; Mahajan, S.A. Generalized Bullous Fixed Drug Eruption to Fluconazole; with Cross-Reactivity to Tinidazole. Indian J. Dermatol. 2019, 64, 335–337. [Google Scholar]
- Cravo, M.; Gonçalo, M.; Figueiredo, A. Fixed drug eruption to cetirizine with positive lesional patch tests to the three piperazine derivatives. Int. J. Dermatol. 2007, 46, 760–762. [Google Scholar] [CrossRef]
- Sarkar, R.; Kaur, C.; Kanwar, A.J. Extensive fixed drug eruption to nimesulide with cross-sensitivity to sulfonamides in a child. Pediatr. Dermatol. 2002, 19, 553–554. [Google Scholar] [CrossRef]
- Khan, D.A.; Banerji, A.; Blumenthal, K.G.; Phillips, E.J.; Solensky, R.; White, A.A.; Bernstein, J.A.; Chu, D.K.; Ellis, A.K.; Golden, D.B.; et al. Drug allergy: A 2022 practice parameter update. J. Allergy Clin. Immunol. 2022, 150, 1333–1393. [Google Scholar] [CrossRef]
- Strom, B.L.; Schinnar, R.; Apter, A.J.; Margolis, D.J.; Lautenbach, E.; Hennessy, S.; Bilker, W.B.; Pettitt, D. Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N. Engl. J. Med. 2003, 349, 1628–1635. [Google Scholar] [CrossRef]
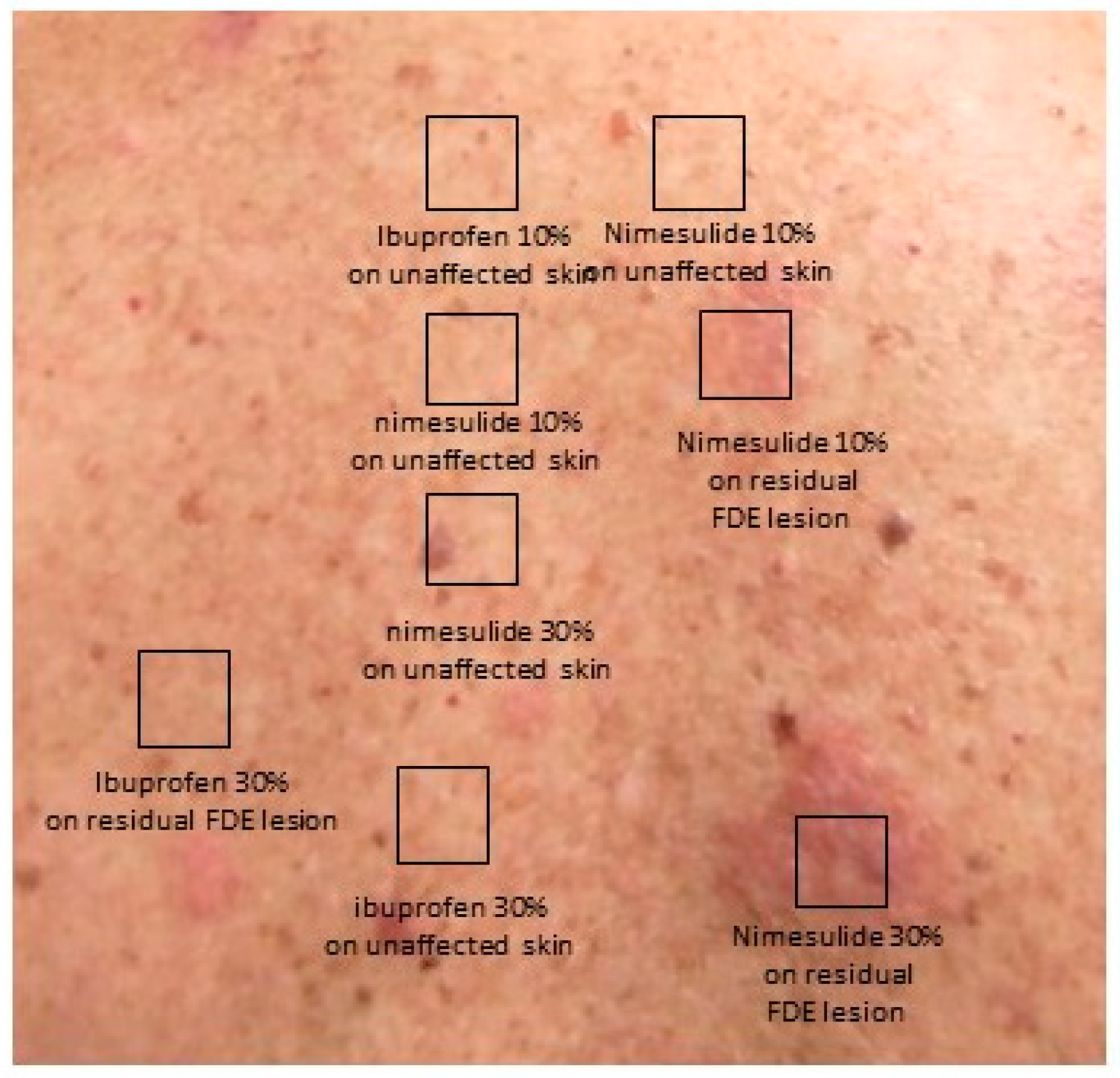
| Author, Year, Reference | Gender | Number of Patients (Male) | Age (Years) | Clinical Description of Initial Reaction | Affected Sites of Initial Reaction | Initial Suspected Agents | Interval Time from Agent Ingestion | Diagnostic Tests Performed | Cross-Reactivity (NSAIDs) |
|---|---|---|---|---|---|---|---|---|---|
| Ammar et al., 2022 [10] | 0 Males 2 Females | 2 (0) | Patient 1: 49 Patient 2: 52 | Patient 1: three itchy erythematous plaques that faded spontaneously, leaving residual hyperpigmented patches Patient 2: four erythematous plaques | Patient 1: upper lip, chin, and right hand Patient 2: right and left wrists | Patient 1: mefenamic acid, naproxen, acetaminophen Patient 2: mefenamic acid, piroxicam | Patient 1: 1 day after (first episode) 1 day after (second episode) Hours after (third episode) Patient 2: 1 day after (all episodes before first visit) | Patient 1: after first and second episodes patch testing for naproxen (−) and acetaminophen (−) After third episode, skin biopsy indicative of FDE; patch test for naproxen (−); OPT for naproxen (+), indomethacin (+), ketoprofen (+), tiaprofenic acid (+), piroxicam (−), celecoxib (−), diclofenac (−), and acetaminophen (−) Patient 2: after first visit patch testing for lysine acetylsalicylate (−), mefenamic acid (−), piroxicam (−), naproxen (−), and celecoxib (−) OPT for lysine acetylsalicylate (+), mefenamic acid (+), piroxicam (+), naproxen (−), and celecoxib (−) | Patient 1: naproxen, indomethacin, ketoprofen, and tiaprofenic acid Patient 2: lysine acetylsalicylate, mefenamic acid, and piroxicam |
| Ouni et al., 2021 [11] | 1 Male 0 Females | 1 (1) | 40 | Multiple hyperpigmented lesions with pruritic erythematous and violaceous features | Upper extremities and trunk | Mefenamic acid, lansoprazole, paracetamol (first episode), mefenamic acid (second and third episode) | 1 week (first episode), hours after (second episode) | Patch testing for diclofenac (+), celecoxib (−), and piroxicam (−), and OPT for diclofenac (+) | Mefenamic acid and diclofenac |
| Movsisyan et al., 2019 [12] | 0 Males 1 Female | 1 (0) | 46 | Pruritic maculopapular lesions on face and a single burning macular lesion twice at the same site on the left side of the abdomen | Face and abdomen | Etoricoxib | NA | LTT: etoricoxib (+), celecoxib (+), and sulfamethoxazole (+) | Etoricoxib and celecoxib |
| Carneiro-Leao et al., 2019 [13] | 0 Males 1 Female | 1 (0) | 51 | Multiple violaceous, pruritic, and painful plaques (shoulders and elbows); violaceous bullous lesion (right upper thigh) consistent with bullous FDE | Shoulders, elbows, and right upper thigh | Etoricoxib | NA | Patch testing for celecoxib (+) and etoricoxib (+) | Etoricoxib and celecoxib |
| Ben Romdhane et al., 2019 [14] | 2 Males 5 Females | 7 (2) | Mean: 40 (26–55) | Multiple (two to four lesions) in five patients and solitary lesions in two consistent with FDE. Bullous eruptions observed in two cases. Lesions were between 1 and 5 cm in size, were well demarcated, and left residual hyperpigmentation | The upper extremities were involved in five cases, followed by the face and the trunk | Piroxicam | 2 days (2 hours to 3 days) | Patch testing for piroxicam (+) in 6/6 patients evaluated and meloxicam (+) in 1/7 patients evaluated, and OPT for meloxicam (+) for 2/6 patients evaluated | Piroxicam and meloxicam |
| Andrade et al., 2011 [15] | 27 Males 25 Females | 52 (27) (total patients) | Mean: 53 (20–78) (for all patients evaluated) | Lesions clinically consistent with FDE | NA | NSAIDs were suspected clinically in 47/52 cases, followed by antibiotics 15/52, paracetamol 8/52, and allopurinol 4/52. | NA | Patch testing (+) in 21/52 patients. Of these, 20 were reactive to NSAIDs including nimesulide (n = 9), piroxicam (n = 9), and etoricoxib (n = 2). For nine patients with patch testing positive for piroxicam, eight were also reactive to tenoxicam and two to meloxicam, whereas no reaction was observed to lornoxicam. | From nine patients with positive test reactions to piroxicam, eight were also reactive to tenoxicam and two to meloxicam |
| Ozdemir et al., 2011 [16] | 0 Males 1 Female | 1 (0) | 44 | Brownish, sharply demarcated and round pruritic plaques, and vulvar pruritus | Forearm, right knee, and vulvar region | Piroxicam, ibuprofen, and flurbiprofen | 10–15 min | Patch testing for piroxicam (+), meloxicam (−), tenoxicam (−), and nimesulide (−) OPT meloxicam (+), naproxen (−), and nimesulide (−) | Piroxicam and meloxicam |
| Pérez-Calderon et al., 2011 [17] | 0 Males 1 Female | 1 (0) | 52 | Erythematous plaques with associated burning and itching sensation | Forehead and infraclavicular area | Naproxen | NA | Patch testing for naproxen (−), ibuprofen (−), ketoprofen (−), dexketoprofen (−), dexibuprofen (−), flurbiprofen (−), ketorolac (−), diclofenac (−), indomethacin (−), bufexamac (−), benzydamine (−), phenylbutazone (−), nabumetone (−), and piroxicam (−) OPT for naproxen (+), nabumetone (+), and dexketoprofen (−) | Naproxen and nabumetone |
| Ozkaya 2008 [18] | 0 Males 1 Female | 1 (0) | 18 | Circular indurated violaceous plaque, 10 mm in diameter | Left earlobe (sites of ear piercing) | Tenoxicam and piroxicam | Few hours | OPT for tenoxicam (+) and piroxicam (+) | Tenoxicam and piroxicam |
| Linares et al., 2007 [19] | 0 Males 1 Female | 1 | 55 | Erythematous and pruritic lesion with residual hyperpigmentation | Back of the neck | Aceclofenac | 10 h | Patch testing for aceclofenac (+) and diclofenac (+) OPT for acetylsalicylic acid (−) | Aceclofenac and diclofenac |
| Ozkaya-Bayazit 2003 [20] | 53 Males 52 Females | 105 | Mean: 35.2 (4–67) | Lesions clinically consistent with FDE | Genital mucosa (50.5%), trunk (38.1%), lips (37.1%), and hands (32.4%) while other sites were affected less commonly | Trimethoprim-sulfamethoxazole (cotrimoxazole), naproxen, dipyrone, tenoxicam, piroxicam, paracetamol, and dimenhydrinate | NA | OPT trimethoprim-sulfamethoxazole (+) in 67 patients (63.8%), followed by naproxen (+) in 25 patients (23.8%), dipyrone (+) in 6 patients (5.7%), oxicam derivatives (+) in 5 patients (tenoxicam (+) in 3 and piroxicam (+) in 2) (4.8%), paracetamol (+) in 1 patient (0.95%), and dimenhydrinate (+) in 1 patient (0.95%). One patient reacted both to piroxicam and tenoxicam. | Tenoxicam and piroxicam in one patient |
| Oliveira et al., 1999 [21] | 0 Males 1 Female | 1 | 55 | Erythematous, painful and edematous patches ranging from 2 to 8 cm | Trunk, buttocks, and thighs | Piroxicam | NA | Patch testing for piroxicam (+), tenoxicam (+), nabumetone (−), diclotenac (−), thiocolchicoside (−), antiglaucoma eye drops (−), NSAID series (−), and PCDRG series (−) | Piroxicam and tenoxicam |
| Ordoqui et al., 1995 [22] | 1 Male 1 Female | 2 | Patient 1: 45 Patient 2: 40 | Patient 1: Large edematous plaques with bullae Patient 2: Erythemato-violaceous macules | Patient 1: both elbows, distal hand phalanges, thighs, and the inguinal region Patient 2: left foot | Pt:1 piroxicam Pt:2 piroxicam | NA | Patient 1: Patch testing for piroxicam (+), tenoxicam (+), and droxicam (+) Patient 2: patch testing for piroxicam (−) and OPT for piroxicam (+), tenoxicam (+), and droxicam (+) | Patient 1: piroxicam, tenoxicam, and droxicam Patient 2: piroxicam, tenoxicam, and droxicam |
| Alanko 1994 [23] | 18 Males 12 Females | 30 | 16–76 | Lesions clinically consistent with FDE | NA | 16/30 phenazone salicylate | NA | Patch testing for phenazone salicylate (+) for all 16 patients Phenazone salicylate (per oral challenge performed in 3 out of 16 patients) | Cross-reactivity between phenazone derivatives was studied in eight patients with FDE caused by phenazone salicylate. Out of eight patients with a positive skin reaction to phenazone salicylate, eight showed a positive reaction to phenazone, three showed a positive reaction to aminophenazone, and four showed a positive reaction to propyphenazone. |
| Gastaminza 1993 [24] | 0 Males 1 Female | 1 | 57 | Burning and itching on the upper extensor forearms and on the back; a circular erythema (2–3 cm in diameter) | Upper extensor forearms and back | Piroxicam | Next day (morning) | Patch testing for piroxicam (+), droxicam (+), tenoxicam (+), and thiosalicylic acid (−) | Piroxicam, droxicam, and tenoxicam |
| Kanwar et al., 1988 [25] | 29 Males 69 Females | 98 | 21–40 (3 patients less that 10 years old) | Lesions clinically consistent with FDE; 55 patients had a few lesions, 36 were classified as moderate, while there were 7 patients with GBFDE | Cutaneous involvement (43 patients), mucous membrane (33 patients), cutaneous with mucous membrane involvement (22 patients) | Trimethoprim-sulphamethoxazole (45), acetylsalicylic acid (24), hyoscine butylbromide (8), ibuprofen (6), oxyphenbutazone (6), tetracycline hydrochloride (2), phenolphthalein (1), and phenobarbitone (1) | NA | OPT for acetylsalicylic acid (+) in 24; ibuprofen (+) in 6; and oxyphenbutazone (+) in 6 patients, with 1 patient positive for phenylbutazone | Out of six patients with a positive reaction to oxyphenbutazone, one showed a positive reaction to phenylbutazone |
| Alanko et al., 1987 [26] | 14 Males 10 Females | 24 | Mean 42.8 years, (21–71) | Lesions clinically consistent with FDE | NA | Phenazone salicylate | NA | Topical provocation testing for phenazone salicylate (+) in nine patients. Topical provocation with phenazone derivatives was performed in three patients, in which one FDE was originally caused by phenazone salicylate. A positive reaction to phenazone was obtained in all three patients. Provocation with propyphenazone and aminophenazone was observed in one patient. OPT in 2 of 9 patients with FDE due to phenazone salicylate and all 15 patients with FDE due to a drug other than phenazone salicylate had been challenged with the suspected drug and had given a positive reaction. One of the patients with a negative topical provocation test for propyphenazone and aminophenazone was later tested by oral provocation with the respective drugs with the results being negative. | In all three patients, a positive reaction was seen with phenazone, but only one patient showed positive results with propyphenazone and aminophenazone |
| Kanwar et al., 1984 [27] | M: F ratio = a little more than 2:1 | 106 | 21–40 (3 patients less that 10 years old) | Lesions clinically consistent with FDE | Patients with cutaneous involvement (44), mucous membrane involvement (23), and cutaneous and mucous membrane involvement (39) | Each patient was tested with the drugs commonly known to cause fixed eruptions as well as those being taken at the time of the reaction | NA | OPT was performed in 71 patients (not specified) (36 patients did not agree to provocation testing) Positive for acetylsalicylic acid (18), hyposcine butylbromide (15), oxyphenbutazone (14), sulphadiazine (7), tetracycline hydrochloride (6), metamizole (5), and ibuprofen (3) | Of 14 patients with a positive reaction to oxyphenbutazone, 6 patients were also tested with phenylbutazone, but only 2 showed a reaction |
| Pasricha 1979 [28] | NA | 40 | NA | Lesions clinically consistent with FDE | NA | Each patient was tested with the drugs commonly known to cause fixed eruptions and also those being taken at the time of the eruption | NA | Provocation test were not completed in 12 patients, and the causative drug could not be found. In the remaining patients, the causative drugs were shown to be tetracyclines (6), analgin (metamizole) (6), oxyphenbutazone (5), phenobarbitone (4), sulphadiazine (3), sulphaphenazole (2), penicillin (1), sulphadimethoxone (1), saridon (1), sulphadimidine (1), and sulphamethoxypyridazine (1) | Of five patients who showed a reaction with oxy-phenylbutazone, the one patient also tested with phenylbutazone showed evidence of cross-sensitivity |
| Pandhi and Dedi 1975 [29] | 1 Male 0 Females | 1 | 34 | Round, erythematous and itchy patches on the back, arms and right palm; swelling of the lips | Back, arms, right palm, lips | Oxyphenbutazone | 6 h | OPT testing for oxyphenbutazone (+) and phenylbutazone (+) | Oxyphenbutazone (+) and phenylbutazone (+) |
| Nayyar and Pasricha 1972 [30] | 0 Males 1 Female | 1 | 30 | Dark red, oval, itchy patches | Arms, thighs, legs, lips | Oxyphenbutazone, sulphadiazine, vitamin B, codopyrin, and acetylsalicylic acid | Few hours | OPT testing for Sulfatriad (sulphadiazine, sulphamcrazinc and sulphadimidine), Crocin (paracetamol), Aspirin, and Codopyrine (Aspirin, Phcnacctin, and Codein phosphate) was negative. Positive OPT for oxyphenbutazone and phenylbutazone | Oxyphenbutazone (+) and phenylbutazone (+) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makris, M.; Papapostolou, N.; Koumprentziotis, I.-A.; Pappa, G.; Katoulis, A.C. Nimesulide-Induced Fixed Drug Eruption Followed by Etoricoxib-Induced Fixed Drug Eruption: An Unusual Case Report and Review of the Literature. J. Clin. Med. 2024, 13, 1583. https://doi.org/10.3390/jcm13061583
Makris M, Papapostolou N, Koumprentziotis I-A, Pappa G, Katoulis AC. Nimesulide-Induced Fixed Drug Eruption Followed by Etoricoxib-Induced Fixed Drug Eruption: An Unusual Case Report and Review of the Literature. Journal of Clinical Medicine. 2024; 13(6):1583. https://doi.org/10.3390/jcm13061583
Chicago/Turabian StyleMakris, Michael, Niki Papapostolou, Ioannis-Alexios Koumprentziotis, Georgia Pappa, and Alexander C. Katoulis. 2024. "Nimesulide-Induced Fixed Drug Eruption Followed by Etoricoxib-Induced Fixed Drug Eruption: An Unusual Case Report and Review of the Literature" Journal of Clinical Medicine 13, no. 6: 1583. https://doi.org/10.3390/jcm13061583
APA StyleMakris, M., Papapostolou, N., Koumprentziotis, I.-A., Pappa, G., & Katoulis, A. C. (2024). Nimesulide-Induced Fixed Drug Eruption Followed by Etoricoxib-Induced Fixed Drug Eruption: An Unusual Case Report and Review of the Literature. Journal of Clinical Medicine, 13(6), 1583. https://doi.org/10.3390/jcm13061583








