Abstract
Background: An unexplained condition that follows transcatheter aortic valve implantation (TAVI) is platelet count reduction (PR). According to published research, patients with balloon-expandable valves (BEVs) had a greater PR than those with self-expandable valves (SEVs). Objectives: The purpose of this study was to investigate the incidence and clinical effects of PR following TAVI. Methods: In total, 1.122 adult TAVI patients were enrolled. Propensity score matching was carried out in a 1:1 ratio between patients with BEVs and those with SEVs. The analysis included changes in platelet count, in-hospital mortality, and early postoperative adverse events. Results: Notably, 632 patients were matched (BEV:316; SEV:316). All patients’ post-procedural platelet counts changed according to a parabolic curve, using a mixed regression model for repeated analyses (estimate = −0.931; standard error = 0.421; p = 0.027). The platelet count varied comparably in patients with BEVs and SEVs (estimate = −4.276, standard error = 4.760, p = 0.369). The average time for obtaining the nadir platelet count value was three days after implantation (BEV: 146 (108–181) vs. SEV: 149 (120–186); p = 0.142). Overall, 14.6% of patients (92/632) had post-procedural platelet count <100,000/µL. There was no difference between the two prosthesis types (BEV:51/316; SEV:41/316; p = 0.266). Thrombocytopenia was found to be significantly linked to blood product transfusions, lengthier stays in the intensive care unit and hospital, and in-hospital mortality. Conclusions: TAVI, irrespective of the type of implanted valve, is linked to a significant but temporary PR. Thrombocytopenia increases the risk of serious complications and in-hospital death in TAVI patients. To explore and clarify the causes and associated effects, further prospective research is necessary.
1. Introduction
Transcatheter aortic valve implantation (TAVI) completely changed the way we deal with high-risk patients with severe aortic stenosis, becoming a valuable daily therapeutic option. Lately, the role of TAVI has evolved worldwide thanks to the expansion of guideline recommendations to include patients with lower surgical risk [1,2]. TAVI technology improvement and process simplification occurred thanks to new prostheses (e.g., smaller delivery sheets or the ability to reposition) and operator experience, resulting in an improved safety profile and fewer procedure-related adverse events such as stroke, pacemaker implantation, paravalvular leaks, and access site complications [3,4].
Among periprocedural TAVI complications, platelet count reduction (PR) has always been neglected, and its incidence and relevance were not well known until the recent interest it raised among researchers [5,6,7]. Post-TAVI PR has been explained by several general mechanisms, such as inflammation, drug toxicity (e.g., heparin, aspirin or other antiplatelet drugs, warfarin, and novel oral anticoagulants), mechanical damage from shear stress (e.g., in the event of a paravalvular leak), the activation of the coagulation cascade, decreased platelet production, impaired platelet renewal, and dilution pseudo-thrombocytopenia. Although the causes of these events remain unclear, theories are based on research involving surgical bioprostheses. While the etiology seemed multifactorial, PR after TAVI is linked to poor clinical outcomes. Dvir and colleagues found that patients with a high reduction in platelet count (≥50%) had a worse 1-year survival rate compared to individuals with a lesser platelet count decline (p < 0.001) (1-year survival: 65.8% vs. 83.9%) [8]. PR was found to be associated with acute kidney failure, vascular complications, bleeding complications, and a high mortality rate [5,6]. Moreover, limited data are available on the occurrence of PR after balloon-expandable valve (BEV) and self-expandable valve (SEV) implantation [5,7]. Only a few studies have demonstrated that the use of BEVs was linked to a higher drop in the post-procedural platelet count than the use of SEVs [5,7].
The aim of this study was to analyze the platelet count variation after TAVI and the prognostic implications for the early clinical outcomes related to this phenomenon.
2. Materials and Methods
The Post-Operative Thrombocytopenia After Bio-Prosthesis Implantation Study in TAVI Patients (PORTRAIT-TAVI) is a retrospective, international, multicenter study that involved adult patients receiving a transcatheter bioprosthesis at 9 centers of cardiac surgery in Italy (5 centers), the Netherlands (1 center), Switzerland (1 center), Poland (1 center), and Germany (1 center) from July 2009 to January 2020.
2.1. Ethical Statement
This study is registered in clinicaltrial.gov (Identifier: NCT03835598). The study was approved by the Institutional Review Board of each participating center. The need for individual patient consent for the study was waived by the committee.
2.2. Inclusion and Exclusion Criteria
Patients older than 18 years who required a transcatheter biological aortic bioprosthesis for severe aortic stenosis were considered for the analysis. All patients were evaluated by a multidisciplinary heart team who determined TAVI indications, approach, and the type of transcatheter valves used. Patients were treated either with a balloon-expandable or a self-expandable valve. All patients gave written informed consent before the procedure. Patient demographics, symptoms, and comorbidities were documented, and individual risk was calculated by the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE). Transthoracic echocardiography was the initial screening examination used to evaluate the severity of aortic stenosis. TAVI access route and valve size were selected using computer tomography measurements.
Platelet counts were studied retrospectively, with available data analyzed from pre-implantation and on a daily basis (from day 0 to day 5) until discharge. Patients with a baseline platelet count below 150,000/µL were excluded from the analysis. Subjects with an oncologic disease, a concurrent infection or inflammatory disorder, or those who needed a concurrent percutaneous coronary intervention were also excluded from the study.
Following the procedure, all patients were given 300 mg of clopidogrel, and they were started on a double anti-aggregation treatment regimen that included 75 mg of clopidogrel and 100 mg of acetylsalicylic acid each day. Also, low-molecular-weight heparin was administered as a prophylactic deep vein thrombosis during the hospital stay.
Baseline characteristics, procedural data, and clinical outcomes were collected in a dedicated database after a robust check of its completeness and quality.
2.3. Definitions and Endpoints
The lowest recorded platelet count during hospitalization was defined as the nadir platelet count.
Thrombocytopenia occurs when platelet counts are less than 150 × 103/µL. It is further classified as moderate (59–99 × 103/µL), severe (<50 × 103/µL), and mild (100–149 × 103/µL). The adoption of a cut-off value of 100 × 103/µL was considered the most appropriate to identify a pathologic condition related to thrombocytopenia [9,10]. The early adverse events associated with a platelet count below 100 × 103/µL were investigated in the study population. Moreover, platelet count was analyzed by comparing SEV and BEV groups. The Valve Academic Research Consortium (VARC-3) criteria were used to define periprocedural events and mortality [11].
The primary aim of this study was to evaluate the frequency of the aforementioned outcomes in the overall population and the BEV and SEV groups. The secondary endpoint was to determine the risk factors for the development of periprocedural thrombocytopenia.
2.4. Statistical Analysis
Continuous variables are expressed as mean and standard deviation, or median and quartiles, respectively, for normally or non-normally distributed variables (as tested by the Shapiro–Wilk test) and were compared using Student’s t-test (or Wilcoxon–Mann–Whitney U test, as appropriate) and ANOVA (followed by the Tukey post hoc test) for multiple comparisons. Proportions are expressed as percentages and compared using the χ2 test or Fisher’s exact test, as appropriate.
Variables with a missing variable rate of more than 30% were excluded; otherwise, missing data were handled as follows: The mechanisms underlying missing data were investigated with sensitivity analysis and multiple imputation, generating five different datasets. The result of multiple treatment effects was pooled using the Rubin rules.
Propensity score matching was used to balance the distributions of the measured confounding baseline covariates between the SEV and BEV groups. The propensity score was obtained using logistic regression. Overlapping was assessed with common support plots. In addition, 1:1 matching was analyzed with different calipers ranging from 0.05 to 0.65, choosing the best one (0.20). The variables included in the propensity model are reported in Supplementary Figure S1.
The balance between the two matched groups was assessed with a standardized mean difference (SMD), considered optimal below 0.10. For the analysis of platelet counts over time, a mixed regression model was used, with time points as repeated measurements and patients as subjects. Logistic regression was used to assess the impact of the minimum post-procedural platelet count (<100.000/µL) on outcomes.
The odds ratios (ORs) and 95% confidence limits were reported for both the unmatched and matched groups and adjusted for those variables showing SMD > 0.10. Generalized linear models with a logarithmic link function were used to evaluate the association between risk and the minimum post-procedural platelet count cut-off with the intensive care unit (ICU) and in-hospital length of stay (LoS).
Moreover, both ICU and in-hospital LoS were transformed into nominal variables using the median value as a cut-off, and then logistic regression was performed.
Univariate and multivariate analyses were performed to identify factors that might predict a platelet count value <100 × 103/µL.
R-studio version 1.1.463 (2009–2018) was used for all statistical analyses. The significance of differences was considered at a p value < 0.05.
3. Results
3.1. Study Population
Supplementary Table S1 summarizes the baseline and procedural characteristics of the 1.122 patients enrolled in the study, comprising 395 patients (35.2%) who were treated with SEVs and 727 patients (64.8%) who received BEVs (Supplementary Table S1). Supplementary Tables S2–S4 show the results for the unmatched population. Propensity score matching yielded 316 patient pairs (Table 1). The average age of the overall matched population was 81 years old, and 83% had NYHA class III/IV symptoms at the time of the procedure. The mean aortic valve area index was 0.4 cm2/m2. Aspirin alone or dual antiplatelet therapy was taken by less than 4% of the overall population prior to valve implantation. Most TAVI procedures were elective, and the femoral artery was the preferred access route in both groups.

Table 1.
Baseline and procedural characteristics of the matched study population according to the implanted valve.
3.2. Changes in Blood Elements after TAVI
Pre-operative platelet count was >150.000/µL in all patients, as per inclusion criteria. A mixed regression model for repeated-measure analysis showed that the post-procedural platelet count changed according to a parabolic curve in all patients (estimate = −0.931, standard error = 0.421, p = 0.027, Figure 1).

Figure 1.
Platelet count variation after transcatheter aortic valve implantation. Abbreviation: PLT, platelet; Pre, pre-implantation; Disch, discharge.
The platelet count varied similarly in both BEV and SEV patients (estimate = −4.276, standard error = 4.760, p = 0.369, Table 2, Figure 2). On average, the nadir platelet count value was recorded three days after implantation (BEV:146 (108–181) vs. SEV:149 (120–186), p = 0.142).

Table 2.
Periprocedural laboratory values after transcatheter aortic implantation in the matched population.
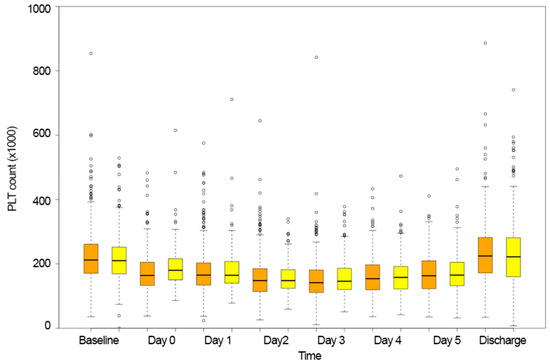
Figure 2.
Platelet count variation over time in the balloon-expandable group (orange bars) and self-expandable valve group (yellow bars).
Post-procedural thrombocythemia was recorded in 14.6% of patients (92/632) without any difference between the two types of prostheses (BEV:51/316, SEV:41/316, p = 0.266, Figure 3). The RBC count and hemoglobin value decreased over time after TAVI implantation; however, no difference was found between the groups (Table 2).
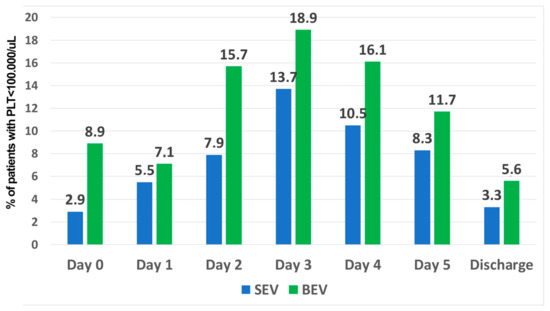
Figure 3.
Rate of patients with thrombocytopenia in the balloon-expandable group (blue bars) and self-expandable valve group (green bars).
3.3. Early Clinical Outcomes after TAVI
Nearly 22% of patients received RBC transfusions, but only 7% required more than two units (Table 3). Vascular problems and bleeding events affected 16% and 14% of the population, respectively (Table 3). The in-hospital mortality rate was similar between the groups (p = 0.102) and reached 6% in the overall population (Table 3).

Table 3.
Clinical outcomes after transcatheter aortic implantation in the matched population.
The platelet count <100 × 103/µL was significantly associated with a higher need for blood product transfusions (RBC, p < 0.001; FFP, p = 0.007; PLT, p = 0.001, Table 4). Likewise, ICU LoS, in-hospital LoS, and in-hospital mortality were significantly associated with thrombocytopenia (Table 4). Given that the main objectives of this research were to investigate the impact of TAVI on the platelet count and identify the contributing factors to a drop below 100 × 103/µL platelets, the results presented in Table 4 were corrected for variables with a statistically significant difference (SMD < 0.1) when matched, as previously mentioned in the Methods section.

Table 4.
The impact of minimum post-procedural platelet count (<100,000/µL) on outcomes.
Moreover, the univariate analyses revealed that a post-procedural platelet count <100 × 103/µL was significantly associated with the male sex (OR: 1.69; p = 0.002); the 26 mm prosthesis size (OR: 1.667, p = 0.014); the 29 mm prosthesis size (OR: 1.81, p = 0.017); the baseline platelet count (OR: 0.972; p < 0.001); prior liver cirrhosis (OR: 15.261, p = 0.012); and prior atrial fibrillation (OR: 2.132, p = 0.023) (Supplementary Table S5). Multivariate analysis showed that the BEV procedure (OR: 3.292, p = 0.045); dyslipidemia (OR:2.148, p = 0.048); the baseline platelet count (OR:0.975, p < 0.001); and prior liver cirrhosis (OR:12.109, p = 0.047) were predictors of a post-procedural platelet count <100 × 103/µL (Supplementary Table S5).
4. Discussion
The main findings of the present international multicenter study revealed that (1) patients receiving TAVI were exposed to PR immediately after implantation; (2) PR occurred comparably in BEV and SEV patients; and (3) periprocedural thrombocytopenia was significantly associated with the need for RBC transfusions, prolonged ICU, and in-hospital LoS, as well as in-hospital mortality.
Several studies have shown that PR is a common phenomenon after both surgical and transcatheter aortic bioprosthesis implantation [5,12,13,14]. This phenomenon seems to be determined by the interaction of patients’ related factors and TAVI’s related factors. Even if we excluded patients with pre-operative thrombocytopenia, we found that the baseline platelet count value could be a predictor of a periprocedural low platelet count. Similarly, predisposing risk factors (such as liver cirrhosis) could increase the possibility of thrombocytopenia after TAVI. However, the procedure itself seems to be associated with a risk for platelet count decrease. Inflammatory reactions due to blood interaction with the artificial valve and mechanical platelet destruction due to shear stress modification have been considered to be concomitant causes of PR [5,6,15]. However, a few less credible hypotheses have been suggested to elucidate the phenomenon. Similar to the speculation advanced to explain PR after a stentless surgical bioprosthesis, a recent observational in vitro study analyzing platelet apoptosis biomarkers revealed that a formaldehyde-based storage solution of the prosthesis caused platelet injury [16,17]. However, the study was underpowered, and its results require further confirmation from large randomized trials. Thus, the use of iodinated contrast agents was aimed as another possible etiologic factor for PR [6,18].
While the underlying mechanisms are still to be clarified, mechanical platelet destruction could occur after the administration of hypo-osmolar iodinated contrast agents [18]. Several papers [6,8,19] described BEV patients as more vulnerable to PR because the delivery procedures typically required a large number of low-osmolar contrast agents to confirm appropriate valve positioning. However, this hypothesis was never confirmed by solid evidence. Furthermore, as physicians’ skills and technology for delivering prostheses improved over time, the need for a large number of iodinated contrast agents decreased, undermining this theory [20].
As a result, prosthesis design and delivery systems were believed to be important factors in PR. In the literature, several reports described the occurrence of PR more frequently after BEV implantation than after SEV implantation [7,8,13,17]. A recent systematic review and meta-analysis confirmed that more than 80% of patients receiving BEVs had thrombocytopenia, compared to almost 50% of patients undergoing SEV implantation [5]. Our multivariate analysis showed that BEV implantation was a significant predictor of a platelet count below 100 × 103/µL. During BEV implantation, the use of the balloon could cause great endothelial damage and shear stress, triggering a higher decrease in the platelet count than in patients with SEVs [7]. Yet, this hypothesis is discordant with the different inflammatory responses elicited in BEV and SEV patients. We found a significantly increased WBC in SEV patients in the first three days after implantation. Abu Khadija and colleagues [21] reported a higher inflammatory response after SEV implantation than after the BEV procedure, which is consistent with our findings. The underlying explanation for the different immune responses could be ascribed to the distinct biocompatibility and materials between the prostheses (e.g., cobalt–chromium frame and bovine pericardial leaflets for BEVs vs. nitinol scaffold and porcine pericardial tissue for SEVs) [21]. Despite the different inflammatory responses, our results suggest that the platelet count varied regardless of the type of delivery system and prosthesis, contrasting what had previously been reported in smaller single-center retrospective studies [7,8,13,17]. Furthermore, Abu Khadija and colleagues confirmed that periprocedural thrombocytopenia was not associated with the SEV or BEV delivery systems, also comparing earlier and contemporary TAVI generations [21].
Beyond the numbers, platelet reduction is clinically relevant in TAVI patients. The association between the need for blood product transfusions, prolonged hospitalization, and in-hospital mortality is in line with other studies [7,8,21,22,23,24]. Hernandez-Enriquez and coauthors described a high rate of life-threatening bleeding events, major vascular complications, a greater need for RBC transfusions, and a high rate of sepsis and mortality in patients with a platelet count decrease over 30% at a 30-day follow-up [7]. Furthermore, Dvir and colleagues also reported prolonged ICU LoS and acute kidney injury as the drawbacks related to thrombocytopenia in TAVI patients [19]. Abu Khadija and associates reported that post-procedural PR was associated with higher rates of major bleeding, vascular complications, and mortality [21].
Zahid and colleagues found a greater incidence of bleeding complications associated with baseline hematological issues related to platelets and coagulation factors [25]. Significant bleeding episodes and blood problems are strictly correlated. It is important to emphasize the link between thrombocytopenia and life-threatening bleeding. Although there is a distinct correlation between these two phenomena, more research is necessary to fully understand the relationship. There are two potential outcomes: Either the patient experiences a bleeding episode followed by thrombocytopenia, or the patient experiences thrombocytopenia first and a hemorrhagic episode later. It is challenging to differentiate between the two situations because of the low rate of this event. Additional research in a prospective manner should be conducted to discriminate between the two possibilities.
However, taking into account the unfavorable effects of PR on clinical outcomes, TAVI implantation should be carefully examined in patients who are thrombocytopenic or who have a considerable risk of bleeding or other comorbidities. Moreover, currently, hospital stays are often shorter, and a drop in the platelet count might go unnoticed. A longer in-hospital length of stay should be considered for these patients in order to avoid overlooking a significant platelet drop with clinical impact.
Study Limitations
Although the present study is the first to analyze platelet count variation in a large population of TAVI patients, it has limitations. First, due to its observational and retrospective nature, both selection bias and unmeasured confounders cannot be excluded. Second, due to the multicenter design, events were adjudicated by investigators at each center. Therefore, a certain degree of under-reporting of events cannot be completely ruled out. Furthermore, the prosthetics technology changed during the research period. Platelet decrease may have had a greater impact on early prosthetic generations than on more recent ones. Similar to this, in the early years of the study, multiple procedures were carried out using a trans-apical approach, but more recently, the femoral artery was the favored access route. There is a chance that the access route contributed to the platelet count’s lowering rates.
In addition, biomarkers for platelet activation, inflammation, or hemolysis were not considered in this analysis. Lastly, heparin-induced thrombocytopenia was not investigated. However, heparin was found to have a limited role in post-TAVI thrombocytopenia [19,23,24].
5. Conclusions
Transcatheter aortic valve implantation is associated with a significant but transient PR, regardless of the type of prosthesis. TAVI patients who experience a PR below 100,000/µL are exposed to a high rate of early clinical adverse events and an increased in-hospital mortality rate. Prospective studies are needed to investigate and explain mechanisms and outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13061579/s1. Figure S1. Variables included in the propensity model. Table S1. Type of transcatheter prostheses. Table S2. Baseline and procedural characteristics of the unmatched study population according to the type of the implanted valve. Table S3. Periprocedural laboratory values after transcatheter aortic implantation in the unmatched population. Table S4. Clinical outcomes after transcatheter aortic implantation in the unmatched population. Table S5. Univariate and multivariate analyses.
Author Contributions
Conceptualization, F.J. and R.L.; methodology, M.D.M. and G.F.S.; software, M.K. and M.M.; validation, all authors; formal analysis, M.D.M. investigation, all authors; resources, all authors; data curation, F.J., G.F.S. and M.D.M.; writing—original draft preparation, all authors.; writing—review and editing, all authors; visualization, F.J.; supervision, R.L.; project administration, F.J.; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the MUMC (23 January 2019, METC 2018-0923).
Informed Consent Statement
Patient consent was waived by the Ethical Committee due to the retrospective design of the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
R.L. is a consultant for Medtronic and LivaNova and a member of the Medical Advisory Board of Eurosets and PulseCath (all honoraria are paid to the University); T.F. is a consultant at LivaNova and BioStable. The other authors declare no conflicts of interest.
References
- Writing Committee Members; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Thorac. Cardiovasc. Surg. 2021, 162, e183–e353. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Bourantas, C.V.; Modolo, R.; Baumbach, A.; Søndergaard, L.; Prendergast, B.D.; Ozkor, M.; Kennon, S.; Mathur, A.; Mullen, M.J.; Serruys, P.W. The evolution of device technology in transcatheter aortic valve implantation. EuroIntervention 2019, 14, e1826–e1833. [Google Scholar] [CrossRef] [PubMed]
- Cribier, A. The development of transcatheter aortic valve replacement (TAVR). Glob. Cardiol. Sci. Pr. 2017, 2016, e201632. [Google Scholar] [CrossRef] [PubMed]
- Jiritano, F.; Santarpino, G.; Serraino, G.F.; Ten Cate, H.; Matteucci, M.; Fina, D.; Mastroroberto, P.; Lorusso, R. Peri-procedural thrombocytopenia after aortic bioprosthesis implant: A sys-tematic review and meta-analysis comparison among conventional, stentless, rapid-deployment, and transcatheter valves. Int. J. Cardiol. 2019, 296, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mitrosz, M.; Chlabicz, M.; Hapaniuk, K.; Kaminski, K.A.; Sobkowicz, B.; Piszcz, J.; Dobrzycki, S.; Musial, W.J.; Hirnle, T.; Tycinska, A.M. Thrombocytopenia associated with TAVI—The summary of possible causes. Adv. Med. Sci. 2017, 62, 378–382. [Google Scholar] [CrossRef]
- Hernández-Enríquez, M.; Chollet, T.; Bataille, V.; Campelo-Parada, F.; Boudou, N.; Bouisset, F.; Grunenwald, E.; Porterie, J.; Freixa, X.; Regueiro, A.; et al. Comparison of the Frequency of Thrombocytopenia After Transfemoral Transcatheter Aortic Valve Implantation Between Balloon-Expandable and Self-Expanding Valves. Am. J. Cardiol. 2019, 123, 1120–1126. [Google Scholar] [CrossRef]
- Dvir, D.; Généreux, P.; Barbash, I.M.; Kodali, S.; Ben-Dor, I.; Williams, M.; Torguson, R.; Kirtane, A.J.; Minha, S.; Badr, S.; et al. Acquired thrombocytopenia after transcatheter aortic valve replacement: Clinical correlates and association with outcomes. Eur. Heart J. 2014, 35, 2663–2671. [Google Scholar] [CrossRef]
- Erkurt, M.A.; Kaya, E.; Berber, I.; Koroglu, M.; Kuku, I. Thrombocytopenia in Adults: Review Article. J. Hematol. 2012, 1, 44–53. [Google Scholar] [CrossRef]
- Santoshi, R.K.; Patel, R.; Patel, N.S.; Bansro, V.; Chhabra, G. A Comprehensive Review of Thrombocytopenia With a Spotlight on Intensive Care Patients. Cureus 2022, 14, e27718. [Google Scholar] [CrossRef]
- VARC-3 WRITING COMMITTEE; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef]
- Lorusso, R.; Jiritano, F.; Roselli, E.; Shrestha, M.; Folliguet, T.; Meuris, B.; Pollari, F.; Fischlein, T.; the PERSIST-AVR Investigators. Perioperative platelet reduction after sutureless or stented valve implantation: Results from the PERSIST-AVR controlled randomized trial. Eur. J. Cardio-Thorac. Surg. 2021, 60, 1359–1365. [Google Scholar] [CrossRef]
- Vogt, F.; Moscarelli, M.; Pollari, F.; Kalisnik, J.M.; Pfeiffer, S.; Fittkau, M.; Sirch, J.; Pförringer, D.; Jessl, J.; Eckner, D.; et al. Two approaches—One phenomenon—Thrombocytopenia after surgical and transcatheter aortic valve replacement. J. Card. Surg. 2020, 35, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Miura, D.; Takamori, A.; Nogami, E.; Yunoki, J.; Sakaguchi, Y. Predictors of short-term thrombocytopenia after transcatheter aortic valve implantation: A retrospective study at a single Japanese center. BMC Res. Notes 2020, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mondal, N.K.; Ding, J.; Koenig, S.C.; Slaughter, M.S.; Wu, Z.J. Paradoxical Effect of Nonphysiological Shear Stress on Platelets and von Willebrand Factor. Artif. Organs 2015, 40, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Yerebakan, C.; Kaminski, A.; Westphal, B.; Kundt, G.; Ugurlucan, M.; Steinhoff, G.; Liebold, A. Thrombocytopenia after aortic valve replacement with the Freedom Solo stentless bioprosthesis. Interact. Cardiovasc. Thorac. Surg. 2008, 7, 616–620. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corcione, N.; Romano, S.; Morello, A.; Ferraro, P.; Cimmino, M.; Albanese, M.; Tufano, M.; Capasso, D.; Buonpane, S.; Giordano, S.; et al. Thrombocytopenia Complicating Transcatheter Aortic Valve Implantation: Differences Between Two New-Generation Devices. J. Cardiovasc. Transl. Res. 2021, 14, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Gallet, R.; Seemann, A.; Yamamoto, M.; Hayat, D.; Mouillet, G.; Monin, J.-L.; Gueret, P.; Couetil, J.-P.; Dubois-Randé, J.-L.; Teiger, E.; et al. Effect of transcatheter (via femoral artery) aortic valve implantation on the platelet count and its consequences. Am. J. Cardiol. 2013, 111, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Cubero-Gómez, J.M.; Guerrero Márquez, F.J.; Diaz-de la-Llera, L.; Fernández-Quero, M.; Guisado-Rasco, A.; Villa-Gil-Ortega, M. Severe thrombocytopenia induced by iodinated contrast after coronary angiography: The use of gadolinium contrast and intravascular ultrasound as an alternative to guide percutaneous coronary intervention. Rev. Port. Cardiol. 2017, 36, e1–e61. [Google Scholar] [CrossRef]
- Spagnolo, P.; Giglio, M.; Di Marco, D.; Latib, A.; Besana, F.; Chieffo, A.; Montorfano, M.; Sironi, S.; Alfieri, O.; Colombo, A. Feasibility of ultra-low contrast 64-slice computed tomography angiography before transcatheter aortic valve implantation: A real-world experience. Eur. Heart J. -Cardiovasc. Imaging 2016, 17, 24–33. [Google Scholar] [CrossRef][Green Version]
- Abu Khadija, H.; Gandelman, G.; Ayyad, O.; Jaber, M.; Poles, L.; Jonas, M.; Paz, O.; Abu Sbaih, F.; Sella, G.; Shimoni, S.; et al. Differential systemic inflammatory responses after TAVI: The role of self versus balloon expandable devices. PLoS ONE 2021, 16, e0258963. [Google Scholar] [CrossRef] [PubMed]
- Jilaihawi, H.; Doctor, N.; Chakravarty, T.; Kashif, M.; Mirocha, J.; Cheng, W.; Lill, M.; Nakamura, M.; Gheorghiu, M.; Makkar, R.R. Major thrombocytopenia after balloon-expandable transcatheter aortic valve replacement: Prognostic implications and comparison to surgical aortic valve replacement. Catheter. Cardiovasc. Interv. 2015, 85, 130–137. [Google Scholar] [CrossRef]
- McCabe, J.M.; Huang, P.; Riedl, L.A.; Devireddy, S.R.; Grondell, J.; Connors, A.C.; Davidson, M.J.; Eisenhauer, A.C.; Welt, F.G.P. Incidence and implications of idiopathic thrombocytopenia following transcatheter aortic valve replacement with the Edwards Sapien© valves: A single center experience. Catheter. Cardiovasc. Interv. 2014, 83, 633–641. [Google Scholar] [CrossRef]
- Flaherty, M.P.; Mohsen, A.; Moore, J.B., 4th; Bartoli, C.R.; Schneibel, E.; Rawasia, W.; Williams, M.L.; Grubb, K.J.; Hirsch, G.A. Predictors and clinical impact of pre-existing and acquired thrombocytopenia following transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2015, 85, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Zahid, S.; Ullah, W.; Khan, M.U.; Abbas, S.; Din, M.T.U.; Uddin, M.F.; Inayat, A.; Ubaid, A.; Salman, F.; Thakkar, S.; et al. Trends, predictors, and outcomes of major bleeding after transcatheter aortic valve implantation, from national inpatient sample (2011–2018). Expert Rev. Cardiovasc. Ther. 2021, 19, 557–563. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).