Multicomponent Exercise Intervention for Preventing Falls and Improving Physical Functioning in Older Nursing Home Residents: A Single-Blinded Pilot Randomised Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Recruitment and Eligibility
2.3. Randomisation
2.4. Intervention Group Activities
2.5. Control Group Activities
2.6. Primary and Secondary Outcome Measures
2.7. Adherence and Adverse Events
2.8. Sample Size Estimation
2.9. Statistical Analyses
3. Results
3.1. Adherence and Adverse Events
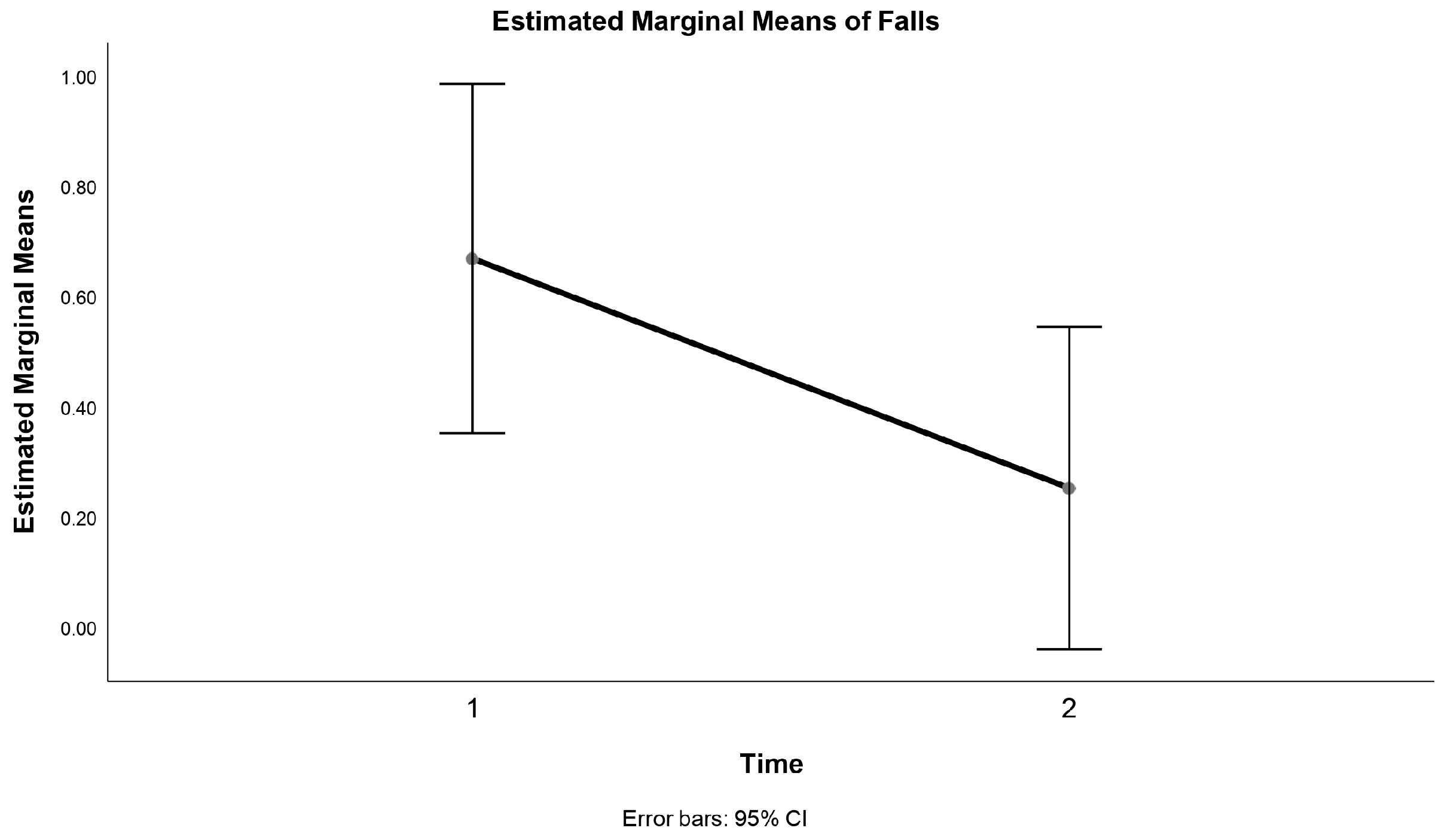
3.2. Falls
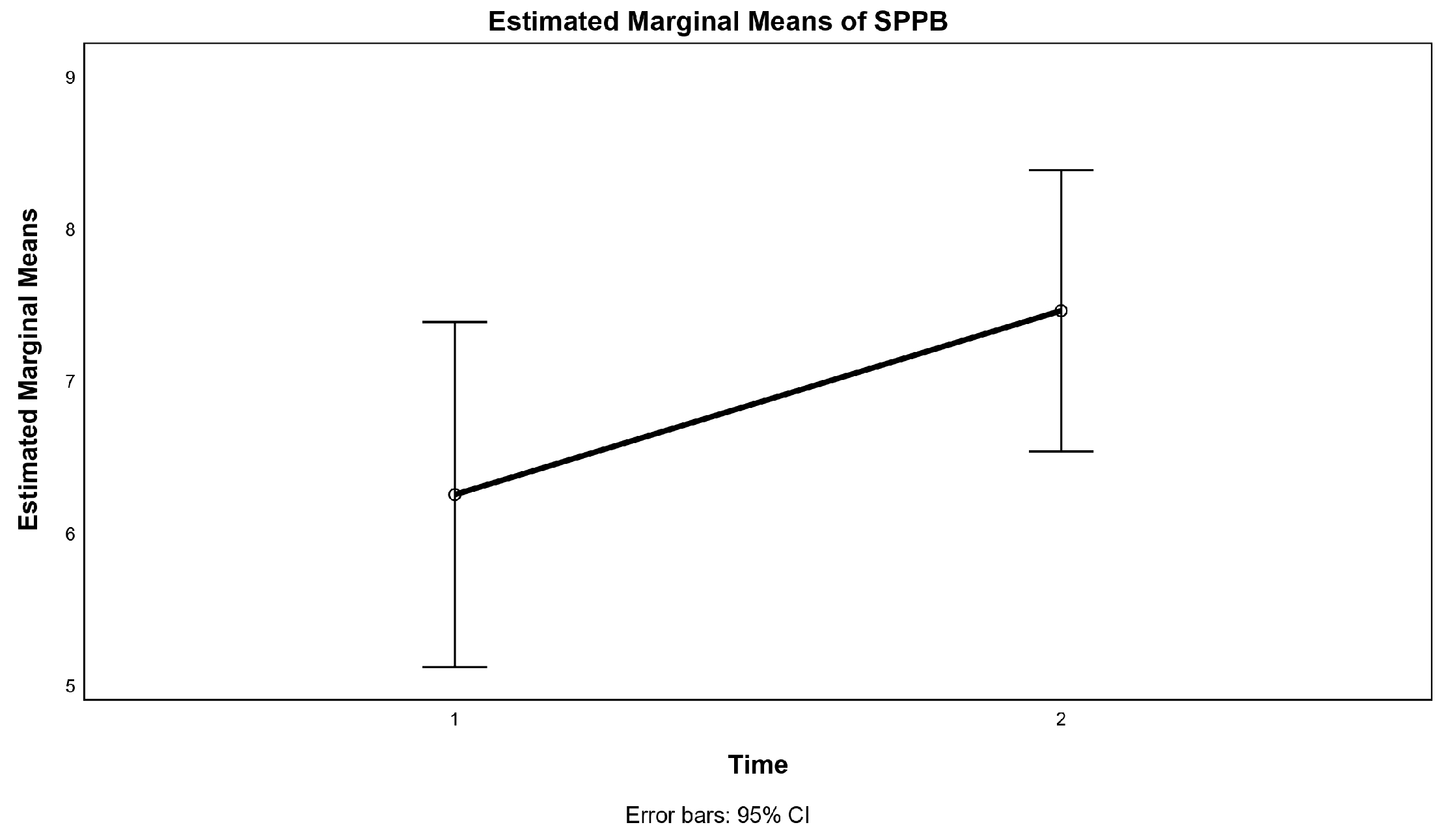
3.3. Physical Functioning
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for Clinical Practice and Public Health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Falls Prevention in Older Age; World Health Organization: Geneva, Switzerland, 2008; pp. 1–4. [Google Scholar]
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk Factors for Falls among Elderly Persons Living in the Community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef]
- Rapp, K.; Becker, C.; Lamb, S.E.; Icks, A.; Klenk, J. Hip Fractures in Institutionalized Elderly People: Incidence Rates and Excess Mortality. J. Bone Miner. Res. 2008, 23, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.B.; Eslick, G.D.; Duque, G. Exercise for Falls and Fracture Prevention in Long Term Care Facilities: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2013, 14, 685–689.e2. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Sievänen, H.; Palvanen, M.; Järvinen, T.; Parkkari, J. Prevention of Falls and Consequent Injuries in Elderly People. Lancet 2005, 366, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Sáez de Asteasu, M.L.; Izquierdo, M. Multicomponent Exercise and the Hallmarks of Frailty: Considerations on Cognitive Impairment and Acute Hospitalization. Exp. Gerontol. 2019, 122, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Mañas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Muir, S.W.; Gopaul, K.; Montero Odasso, M.M. The Role of Cognitive Impairment in Fall Risk among Older Adults: A Systematic Review and Meta-Analysis. Age Ageing 2012, 41, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Josephson, K.R. The Epidemiology of Falls and Syncope. Clin. Geriatr. Med. 2002, 18, 141–158. [Google Scholar] [CrossRef]
- Sorock, G.S.; Quigley, P.A.; Rutledge, M.K.; Taylor, J.; Luo, X.; Foulis, P.; Wang, M.-C.; Varadhan, R.; Bellantoni, M.; Baker, S.P. Central Nervous System Medication Changes and Falls in Nursing Home Residents. Geriatr. Nurs. 2009, 30, 334–340. [Google Scholar] [CrossRef]
- De Souto Barreto, P.; Demougeot, L.; Vellas, B.; Rolland, Y. How Much Exercise Are Older Adults Living in Nursing Homes Doing in Daily Life? A Cross-Sectional Study. J. Sports Sci. 2015, 33, 116–124. [Google Scholar] [CrossRef]
- Hopewell, S.; Adedire, O.; Copsey, B.J.; Boniface, G.J.; Sherrington, C.; Clemson, L.; Close, J.C.; Lamb, S.E. Multifactorial and Multiple Component Interventions for Preventing Falls in Older People Living in the Community. Cochrane Database Syst. Rev. 2018, 2018, CD012221. [Google Scholar] [CrossRef]
- Matthews, F.E.; Bennett, H.; Wittenberg, R.; Jagger, C.; Dening, T.; Brayne, C.; Cognitive Function, Ageing Studies (CFAS) Collaboration. Who Lives Where and Does It Matter? Changes in the Health Profiles of Older People Living in Long Term Care and the Community over Two Decades in a High Income Country. PLoS ONE 2016, 11, e0161705. [Google Scholar] [CrossRef]
- Deandrea, S.; Bravi, F.; Turati, F.; Lucenteforte, E.; La Vecchia, C.; Negri, E. Risk Factors for Falls in Older People in Nursing Homes and Hospitals. A Systematic Review and Meta-Analysis. Arch. Gerontol. Geriatr. 2013, 56, 407–415. [Google Scholar] [CrossRef]
- Akosile, C.O.; Anukam, G.O.; Johnson, O.E.; Fabunmi, A.A.; Okoye, E.C.; Iheukwumere, N.; Akinwola, M.O. Fear of Falling and Quality of Life of Apparently-Healthy Elderly Individuals from a Nigerian Population. J. Cross-Cult. Gerontol. 2014, 29, 201–209. [Google Scholar] [CrossRef]
- Jeon, M.; Gu, M.O.; Yim, J. Comparison of Walking, Muscle Strength, Balance, and Fear of Falling Between Repeated Fall Group, One-Time Fall Group, and Nonfall Group of the Elderly Receiving Home Care Service. Asian Nurs. Res. 2017, 11, 290–296. [Google Scholar] [CrossRef]
- Hartholt, K.A.; van Beeck, E.F.; Polinder, S.; van der Velde, N.; van Lieshout, E.M.M.; Panneman, M.J.M.; van der Cammen, T.J.M.; Patka, P. Societal Consequences of Falls in the Older Population: Injuries, Healthcare Costs, and Long-Term Reduced Quality of Life. J. Trauma Acute Care Surg. 2011, 71, 748. [Google Scholar] [CrossRef] [PubMed]
- Florence, C.S.; Bergen, G.; Atherly, A.; Burns, E.; Stevens, J.; Drake, C. Medical Costs of Fatal and Nonfatal Falls in Older Adults. J. Am. Geriatr. Soc. 2018, 66, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.; Whitney, J.C.; Lord, S.R.; Herbert, R.D.; Cumming, R.G.; Close, J.C.T. Effective Exercise for the Prevention of Falls: A Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2008, 56, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Debes, W.A.; Sadaqa, M.; Németh, Z.; Aldardour, A.; Prémusz, V.; Hock, M. Effect of Resistance Exercise on Body Composition and Functional Capacity in Older Women with Sarcopenic Obesity—A Systematic Review with Narrative Synthesis. J. Clin. Med. 2024, 13, 441. [Google Scholar] [CrossRef] [PubMed]
- Rétsági, E.; Prémusz, V.; Makai, A.; Melczer, C.; Betlehem, J.; Lampek, K.; Ács, P.; Hock, M. Association with Subjective Measured Physical Activity (GPAQ) and Quality of Life (WHOQoL-BREF) of Ageing Adults in Hungary, a Cross-Sectional Study. BMC Public Health 2020, 20, 1061. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Miyamato, T.; Okamae, A.; Tamaki, A.; Fujioka, H.; Wada, Y.; Uchiyama, Y.; Shinmura, K.; Domen, K. Physical Activity Combined with Resistance Training Reduces Symptoms of Frailty in Older Adults: A Randomized Controlled Trial. Arch. Gerontol. Geriatr. 2018, 76, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Tarazona-Santabalbina, F.J.; Gómez-Cabrera, M.C.; Pérez-Ros, P.; Martínez-Arnau, F.M.; Cabo, H.; Tsaparas, K.; Salvador-Pascual, A.; Rodriguez-Mañas, L.; Viña, J. A Multicomponent Exercise Intervention That Reverses Frailty and Improves Cognition, Emotion, and Social Networking in the Community-Dwelling Frail Elderly: A Randomized Clinical Trial. J. Am. Med. Dir. Assoc. 2016, 17, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Sadaqa, M.; Németh, Z.; Makai, A.; Prémusz, V.; Hock, M. Effectiveness of Exercise Interventions on Fall Prevention in Ambulatory Community-Dwelling Older Adults: A Systematic Review with Narrative Synthesis. Front. Public Health 2023, 11, 1209319. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.; Fairhall, N.J.; Wallbank, G.K.; Tiedemann, A.; Michaleff, Z.A.; Howard, K.; Clemson, L.; Hopewell, S.; Lamb, S.E. Exercise for Preventing Falls in Older People Living in the Community. Cochrane Database Syst. Rev. 2019, 1, CD012424. [Google Scholar] [CrossRef] [PubMed]
- Rezola-Pardo, C.; Irazusta, J.; Mugica-Errazquin, I.; Gamio, I.; Sarquis-Adamson, Y.; Gil, S.M.; Ugartemendia, M.; Montero-Odasso, M.; Rodriguez-Larrad, A. Effects of Multicomponent and Dual-Task Exercise on Falls in Nursing Homes: The AgeingOn Dual-Task Study. Maturitas 2022, 164, 15–22. [Google Scholar] [CrossRef]
- Montero-Odasso, M.M.; Kamkar, N.; Pieruccini-Faria, F.; Osman, A.; Sarquis-Adamson, Y.; Close, J.; Hogan, D.B.; Hunter, S.W.; Kenny, R.A.; Lipsitz, L.A.; et al. Evaluation of Clinical Practice Guidelines on Fall Prevention and Management for Older Adults. JAMA Netw. Open 2021, 4, e2138911. [Google Scholar] [CrossRef]
- Arrieta, H.; Rezola-Pardo, C.; Gil, S.M.; Virgala, J.; Iturburu, M.; Antón, I.; González-Templado, V.; Irazusta, J.; Rodriguez-Larrad, A. Effects of Multicomponent Exercise on Frailty in Long-Term Nursing Homes: A Randomized Controlled Trial. J. Am. Geriatr. Soc. 2019, 67, 1145–1151. [Google Scholar] [CrossRef]
- Wang, F.; Tian, B. The Effectiveness of Physical Exercise Type and Length to Prevent Falls in Nursing Homes: A Systematic Review and Meta-Analysis. J. Clin. Nurs. 2022, 31, 32–42. [Google Scholar] [CrossRef]
- Senderovich, H.; Tsai, P.M. Do Exercises Prevent Falls Among Older Adults: Where Are We Now? A Systematic Review. J. Am. Med. Dir. Assoc. 2020, 21, 1197–1206.e2. [Google Scholar] [CrossRef]
- Jahanpeyma, P.; Kayhan Koçak, F.Ö.; Yıldırım, Y.; Şahin, S.; Şenuzun Aykar, F. Effects of the Otago Exercise Program on Falls, Balance, and Physical Performance in Older Nursing Home Residents with High Fall Risk: A Randomized Controlled Trial. Eur. Geriatr. Med. 2021, 12, 107–115. [Google Scholar] [CrossRef]
- Taylor, L.M.; Parsons, J.; Moyes, S.A.; Binns, E.; Cavadino, A.; Taylor, D.; Lord, S.; Del Din, S.; Klenk, J.; Rochester, L.; et al. Effects of an Exercise Program to Reduce Falls in Older People Living in Long-Term Care: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2023, 25, 201–208.e6. [Google Scholar] [CrossRef]
- Cameron, I.D.; Dyer, S.M.; Panagoda, C.E.; Murray, G.R.; Hill, K.D.; Cumming, R.G.; Kerse, N. Interventions for Preventing Falls in Older People in Care Facilities and Hospitals. Cochrane Database Syst. Rev. 2018, 9, CD005465. [Google Scholar] [CrossRef] [PubMed]
- Toots, A.; Wiklund, R.; Littbrand, H.; Nordin, E.; Nordström, P.; Lundin-Olsson, L.; Gustafson, Y.; Rosendahl, E. The Effects of Exercise on Falls in Older People With Dementia Living in Nursing Homes: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2019, 20, 835–842.e1. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Zhao, Q.; Xiao, M.; Kong, L.; Xiao, L. The Effectiveness of Exercise for Fall Prevention in Nursing Home Residents: A Systematic Review Meta-Analysis. J. Adv. Nurs. 2018, 74, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- de Souto Barreto, P.; Cesari, M.; Denormandie, P.; Armaingaud, D.; Vellas, B.; Rolland, Y. Exercise or Social Intervention for Nursing Home Residents with Dementia: A Pilot Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2017, 65, E123–E129. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Izumi, K.; Hiramatsu, T.; Shogenji, M. Development of an Exercise Program for Fall Prevention for Elderly Persons in a Long-Term Care Facility. Jpn. J. Nurs. Sci. 2006, 3, 107–117. [Google Scholar] [CrossRef]
- Sahin, U.K.; Kirdi, N.; Bozoglu, E.; Meric, A.; Buyukturan, G.; Ozturk, A.; Doruk, H. Effect of Low-Intensity versus High-Intensity Resistance Training on the Functioning of the Institutionalized Frail Elderly. Int. J. Rehabil. Res. 2018, 41, 211. [Google Scholar] [CrossRef] [PubMed]
- Papalia, G.F.; Papalia, R.; Diaz Balzani, L.A.; Torre, G.; Zampogna, B.; Vasta, S.; Fossati, C.; Alifano, A.M.; Denaro, V. The Effects of Physical Exercise on Balance and Prevention of Falls in Older People: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2595. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, H.; Rezola-Pardo, C.; Zarrazquin, I.; Echeverria, I.; Yanguas, J.J.; Iturburu, M.; Gil, S.M.; Rodriguez-Larrad, A.; Irazusta, J. A Multicomponent Exercise Program Improves Physical Function in Long-Term Nursing Home Residents: A Randomized Controlled Trial. Exp. Gerontol. 2018, 103, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Courel-Ibáñez, J.; Buendía-Romero, Á.; Pallarés, J.G.; García-Conesa, S.; Martínez-Cava, A.; Izquierdo, M. Impact of Tailored Multicomponent Exercise for Preventing Weakness and Falls on Nursing Home Residents’ Functional Capacity. J. Am. Med. Dir. Assoc. 2022, 23, 98–104.e3. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Idoate, F.; Millor, N.; Gómez, M.; Rodriguez-Mañas, L.; Izquierdo, M. Multicomponent Exercises Including Muscle Power Training Enhance Muscle Mass, Power Output, and Functional Outcomes in Institutionalized Frail Nonagenarians. AGE 2014, 36, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Saco-Ledo, G.; Morales, J.S.; Gallardo-Gómez, D.; Morales-Palomo, F.; López-Ortiz, S.; Rivas-Baeza, B.; Castillo-García, A.; Jiménez-Pavón, D.; Santos-Lozano, A.; et al. Effects of Physical Exercise on Physical Function in Older Adults in Residential Care: A Systematic Review and Network Meta-Analysis of Randomised Controlled Trials. Lancet Healthy Longev. 2023, 4, e247–e256. [Google Scholar] [CrossRef] [PubMed]
- Barreto, P.d.S.; Morley, J.E.; Chodzko-Zajko, W.; Pitkala, K.H.; Weening-Djiksterhuis, E.; Rodriguez-Mañas, L.; Barbagallo, M.; Rosendahl, E.; Sinclair, A.; Landi, F.; et al. Recommendations on Physical Activity and Exercise for Older Adults Living in Long-Term Care Facilities: A Taskforce Report. J. Am. Med. Dir. Assoc. 2016, 17, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.-W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 Statement: Extension to Randomised Pilot and Feasibility Trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef]
- Robinovitch, S.N.; Feldman, F.; Yang, Y.; Schonnop, R.; Leung, P.M.; Sarraf, T.; Sims-Gould, J.; Loughin, M. Video Capture of the Circumstances of Falls in Elderly People Residing in Long-Term Care: An Observational Study. Lancet 2013, 381, 47–54. [Google Scholar] [CrossRef]
- Littbrand, H.; Rosendahl, E.; Lindelöf, N.; Lundin-Olsson, L.; Gustafson, Y.; Nyberg, L. A High-Intensity Functional Weight-Bearing Exercise Program for Older People Dependent in Activities of Daily Living and Living in Residential Care Facilities: Evaluation of the Applicability With Focus on Cognitive Function. Phys. Ther. 2006, 86, 489–498. [Google Scholar] [CrossRef]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical Activity and Public Health in Older Adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1435. [Google Scholar] [CrossRef]
- Farlie, M.K.; Robins, L.; Keating, J.L.; Molloy, E.; Haines, T.P. Intensity of Challenge to the Balance System Is Not Reported in the Prescription of Balance Exercises in Randomised Trials: A Systematic Review. J. Physiother. 2013, 59, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. Exercise and Physical Activity for Older Adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef] [PubMed]
- Chin A Paw, M.J.M.; van Poppel, M.N.M.; Twisk, J.W.R.; van Mechelen, W. Once a Week Not Enough, Twice a Week Not Feasible?: A Randomised Controlled Exercise Trial in Long-Term Care Facilities [ISRCTN87177281]. Patient Educ. Couns. 2006, 63, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Ashburn, A.; Stack, E.; Ballinger, C.; Fazakarley, L.; Fitton, C. The Circumstances of Falls among People with Parkinson’s Disease and the Use of Falls Diaries to Facilitate Reporting. Disabil. Rehabil. 2008, 30, 1205–1212. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. The Reliability and Validity of a 6-Minute Walk Test as a Measure of Physical Endurance in Older Adults. J. Aging Phys. Act. 1998, 6, 363–375. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Vellas, B.J.; Wayne, S.J.; Romero, L.; Baumgartner, R.N.; Rubenstein, L.Z.; Garry, P.J. One-Leg Balance Is an Important Predictor of Injurious Falls in Older Persons. J. Am. Geriatr. Soc. 1997, 45, 735–738. [Google Scholar] [CrossRef]
- Duncan, P.W.; Weiner, D.K.; Chandler, J.; Studenski, S. Functional Reach: A New Clinical Measure of Balance. J. Gerontol. 1990, 45, M192–M197. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.K.; Duncan, P.W.; Chandler, J.; Studenski, S.A. Functional Reach: A Marker of Physical Frailty. J. Am. Geriatr. Soc. 1992, 40, 203–207. [Google Scholar] [CrossRef] [PubMed]
- McGibbon, C.A.; Slayter, J.T.; Yetman, L.; McCollum, A.; McCloskey, R.; Gionet, S.G.; Oakley, H.; Jarrett, P. An Analysis of Falls and Those Who Fall in a Chronic Care Facility. J. Am. Med. Dir. Assoc. 2019, 20, 171–176. [Google Scholar] [CrossRef]
- Kang, H. Sample Size Determination and Power Analysis Using the G*Power Software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- McCarney, R.; Warner, J.; Iliffe, S.; van Haselen, R.; Griffin, M.; Fisher, P. The Hawthorne Effect: A Randomised, Controlled Trial. BMC Med. Res. Methodol. 2007, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Allen, F.; Darby, J.; Fox, C.; Gordon, A.L.; Horne, J.C.; Leighton, P.; Sims, E.; Logan, P.A. Contamination in Complex Healthcare Trials: The Falls in Care Homes (FinCH) Study Experience. BMC Med. Res. Methodol. 2020, 20, 46. [Google Scholar] [CrossRef]
- Magill, N.; Knight, R.; McCrone, P.; Ismail, K.; Landau, S. A Scoping Review of the Problems and Solutions Associated with Contamination in Trials of Complex Interventions in Mental Health. BMC Med. Res. Methodol. 2019, 19, 4. [Google Scholar] [CrossRef]
- Schnelle, J.F.; Kapur, K.; Alessi, C.; Osterweil, D.; Beck, J.G.; Al-Samarrai, N.R.; Ouslander, J.G. Does an Exercise and Incontinence Intervention Save Healthcare Costs in a Nursing Home Population? J. Am. Geriatr. Soc. 2003, 51, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Josephson, K.R.; Osterweil, D. Falls and Fall Prevention in the Nursing Home. Clin. Geriatr. Med. 1996, 12, 881–902. [Google Scholar] [CrossRef]
- Susilowati, I.; Nugraha, S.; Sabarinah, S.; Peltzer, K.; Pengpid, S.; Hasiholan, B. Prevalence and Risk Factors Associated with Falls among Community-Dwelling and Institutionalized Older Adults in Indonesia. Malays. Fam. Physician Off. J. Acad. Fam. Physicians Malays. 2020, 15, 30–38. [Google Scholar]
- Romli, M.H.; Tan, M.P.; Mackenzie, L.; Lovarini, M.; Suttanon, P.; Clemson, L. Falls amongst Older People in Southeast Asia: A Scoping Review. Public Health 2017, 145, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Inacio, M.C.; Moldovan, M.; Whitehead, C.; Sluggett, J.K.; Crotty, M.; Corlis, M.; Visvanathan, R.; Wesselingh, S.; Caughey, G.E. The Risk of Fall-Related Hospitalisations at Entry into Permanent Residential Aged Care. BMC Geriatr. 2021, 21, 686. [Google Scholar] [CrossRef]
- Shao, L.; Shi, Y.; Xie, X.-Y.; Wang, Z.; Wang, Z.-A.; Zhang, J.-E. Incidence and Risk Factors of Falls Among Older People in Nursing Homes: Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2023, 24, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; Bremner, W.J.; McKinlay, J.B. Age Trends in the Level of Serum Testosterone and Other Hormones in Middle-Aged Men: Longitudinal Results from the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.; Ross, R. Skeletal Muscle Mass and Distribution in 468 Men and Women Aged 18–88 Yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B.; et al. The Loss of Skeletal Muscle Strength, Mass, and Quality in Older Adults: The Health, Aging and Body Composition Study. J. Gerontol. Ser. A 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.M.D.B.M.; Ribeiro, K.M.O.B.D.F.; Jerez-Roig, J.; Araújo, J.R.T.; Lima, K.C.D. Recurrent Falls and Risk Factors among Institutionalized Older People. Ciênc. Saúde Coletiva 2019, 24, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, A.; Giordano, A.; Antonelli Incalzi, R.; Lusignani, M. Risk Factors Associated with Accidental Falls among Italian Nursing Home Residents: A Longitudinal Study (FRAILS). Geriatr. Nur. (Lond.) 2020, 41, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.; Lopes, P.; Lara, J.; Moreira, N.; Pereira, G.; Rodacki, A. Nursing Home Residence, Overweight and Cognitive Status Are Related to Falls in Older Adults: A Cross-Sectional Study. Curr. Aging Sci. 2023, 16, 125–132. [Google Scholar] [CrossRef]
- da Câmara, S.M.A.; Alvarado, B.E.; Guralnik, J.M.; Guerra, R.O.; Maciel, Á.C.C. Using the Short Physical Performance Battery to Screen for Frailty in Young-Old Adults with Distinct Socioeconomic Conditions. Geriatr. Gerontol. Int. 2013, 13, 421–428. [Google Scholar] [CrossRef]
- Cano-Escalera, G.; Graña, M.; Irazusta, J.; Labayen, I.; Gonzalez-Pinto, A.; Besga, A. Mortality Risks after Two Years in Frail and Pre-Frail Older Adults Admitted to Hospital. J. Clin. Med. 2023, 12, 3103. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Gutierrez Avila, G.; Alfaro-Acha, A.; Amor Andres, M.S.; De Los Angeles de la Torre Lanza, M.; Escribano Aparicio, M.V.; Humanes Aparicio, S.; Larrion Zugasti, J.L.; Gomez-Serranillo Reus, M.; Rodriguez-Artalejo, F.; et al. The Prevalence of Frailty Syndrome in an Older Population from Spain. The Toledo Study for Healthy Aging. J. Nutr. Health Aging 2011, 15, 852–856. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, J.; Chen, X.; Hou, L.; Lin, X.; Yang, M. Prevalence and Associated Factors of Sarcopenia in Nursing Home Residents: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2019, 20, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Croisier, J.-L.; Reginster, J.-Y.; Lenaerts, C.; Brunois, T.; Rygaert, X.; Petermans, J.; Bruyère, O. Prediction of the Incidence of Falls and Deaths Among Elderly Nursing Home Residents: The SENIOR Study. J. Am. Med. Dir. Assoc. 2018, 19, 18–24. [Google Scholar] [CrossRef] [PubMed]

| Variable | Intervention Group (n = 12) | Control Group (n = 12) | p-Value |
|---|---|---|---|
| Age (years) | 78.3 ± 7.0 | 78.5 ± 7.4 | 0.93 |
| Sex n (%) | |||
| Male | 3 (25) | 4 (33.3) | 1.00 |
| Female | 9 (75) | 8 (66.7) | |
| BMI (kg/m2) | 24.1 ± 5.3 | 25.7 ± 3.8 | 0.41 |
| Falls in previous 3 months | 0.4 ± 0.6 | 0.9 ± 0.8 | 0.14 |
| MMSE score | 26.1 ± 3.1 | 25.3 ± 3.9 | 0.68 |
| Variable | Intervention Group (n = 12) | Control Group (n = 12) | Time × Group Effect | ||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| Report of falls | 0.4 ± 0.6 | 0.3 ± 0.9 | 0.9 ± 0.8 | 0.3 ± 0.5 § | 0.23 |
| SPPB score | 5.9 ± 2.7 | 7.7 ± 1.9 ** | 6.6 ± 2.6 | 7.3 ± 2.4 | 0.20 |
| 6MWT (m) | 223.1 ± 137.9 | 235.8 ± 133.8 | 251.3 ± 92.2 | 200.9 ± 123.3 | 0.08 |
| TUG (s) | 18.6 ± 9.2 | 16.9 ± 9.5 | 15.8 ± 4.1 | 18.7 ± 8.1 | 0.97 |
| SLS dominant (s) | 2.5 ± 1.7 | 2.0 ± 1.3 | 3.5 ± 3.1 | 2.4 ± 1.4 | 0.16 |
| SLS non-dominant (s) | 3.4 ± 4.9 | 2.8 ± 2.9 | 3.3 ± 3.6 | 1.1 ± 0.9 | 0.26 |
| FRT (cm) | 34.5 ± 4.3 | 36.5 ± 8.1 | 33.9 ± 11.8 | 32.4 ± 6.0 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadaqa, M.; Debes, W.A.; Németh, Z.; Bera-Baka, Z.; Vachtler-Szepesi, M.; Nácziné Földes, L.; Prémusz, V.; Hock, M. Multicomponent Exercise Intervention for Preventing Falls and Improving Physical Functioning in Older Nursing Home Residents: A Single-Blinded Pilot Randomised Controlled Trial. J. Clin. Med. 2024, 13, 1577. https://doi.org/10.3390/jcm13061577
Sadaqa M, Debes WA, Németh Z, Bera-Baka Z, Vachtler-Szepesi M, Nácziné Földes L, Prémusz V, Hock M. Multicomponent Exercise Intervention for Preventing Falls and Improving Physical Functioning in Older Nursing Home Residents: A Single-Blinded Pilot Randomised Controlled Trial. Journal of Clinical Medicine. 2024; 13(6):1577. https://doi.org/10.3390/jcm13061577
Chicago/Turabian StyleSadaqa, Munseef, Wesam A. Debes, Zsanett Németh, Zsófia Bera-Baka, Marianna Vachtler-Szepesi, Loretta Nácziné Földes, Viktória Prémusz, and Márta Hock. 2024. "Multicomponent Exercise Intervention for Preventing Falls and Improving Physical Functioning in Older Nursing Home Residents: A Single-Blinded Pilot Randomised Controlled Trial" Journal of Clinical Medicine 13, no. 6: 1577. https://doi.org/10.3390/jcm13061577
APA StyleSadaqa, M., Debes, W. A., Németh, Z., Bera-Baka, Z., Vachtler-Szepesi, M., Nácziné Földes, L., Prémusz, V., & Hock, M. (2024). Multicomponent Exercise Intervention for Preventing Falls and Improving Physical Functioning in Older Nursing Home Residents: A Single-Blinded Pilot Randomised Controlled Trial. Journal of Clinical Medicine, 13(6), 1577. https://doi.org/10.3390/jcm13061577









