Association between PET/CT Scan Findings, Treatment, and Cancer Incidence in a Cohort of AAA Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations and Reporting Standard
2.2. Cohort
2.3. Baseline Characteristics and Risk Factors
2.4. Definitions
2.5. Outcomes
2.6. Statistical Analysis
3. Results
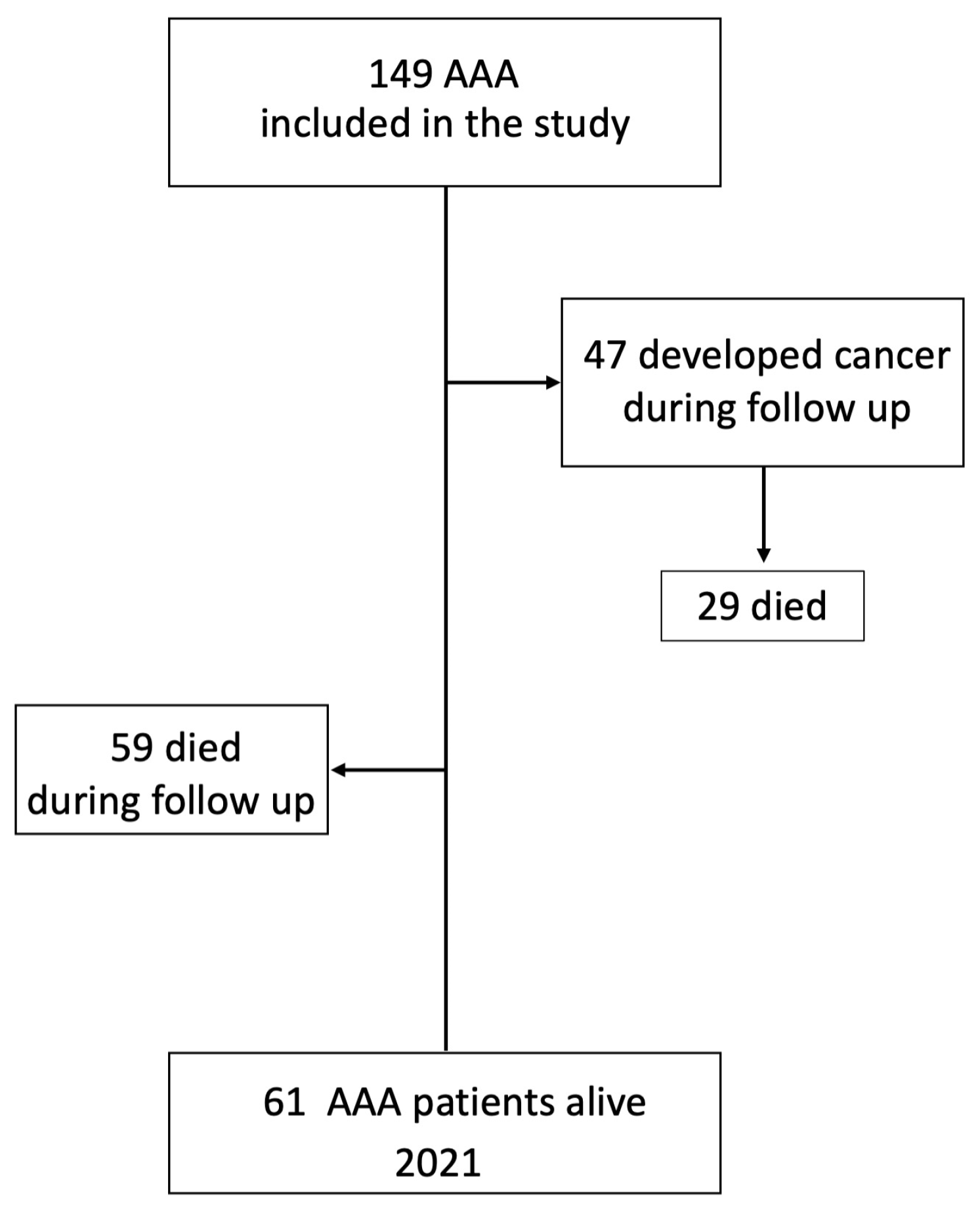
3.1. Patient Characteristics
3.2. Cancer Incidence during Follow-Up
3.3. PET Positive Status of a Patient Is Associated with Cancer Development
3.4. Risk Factors Associated with Cancer Incidence
3.5. AAA Surgery and Cancer Incidence
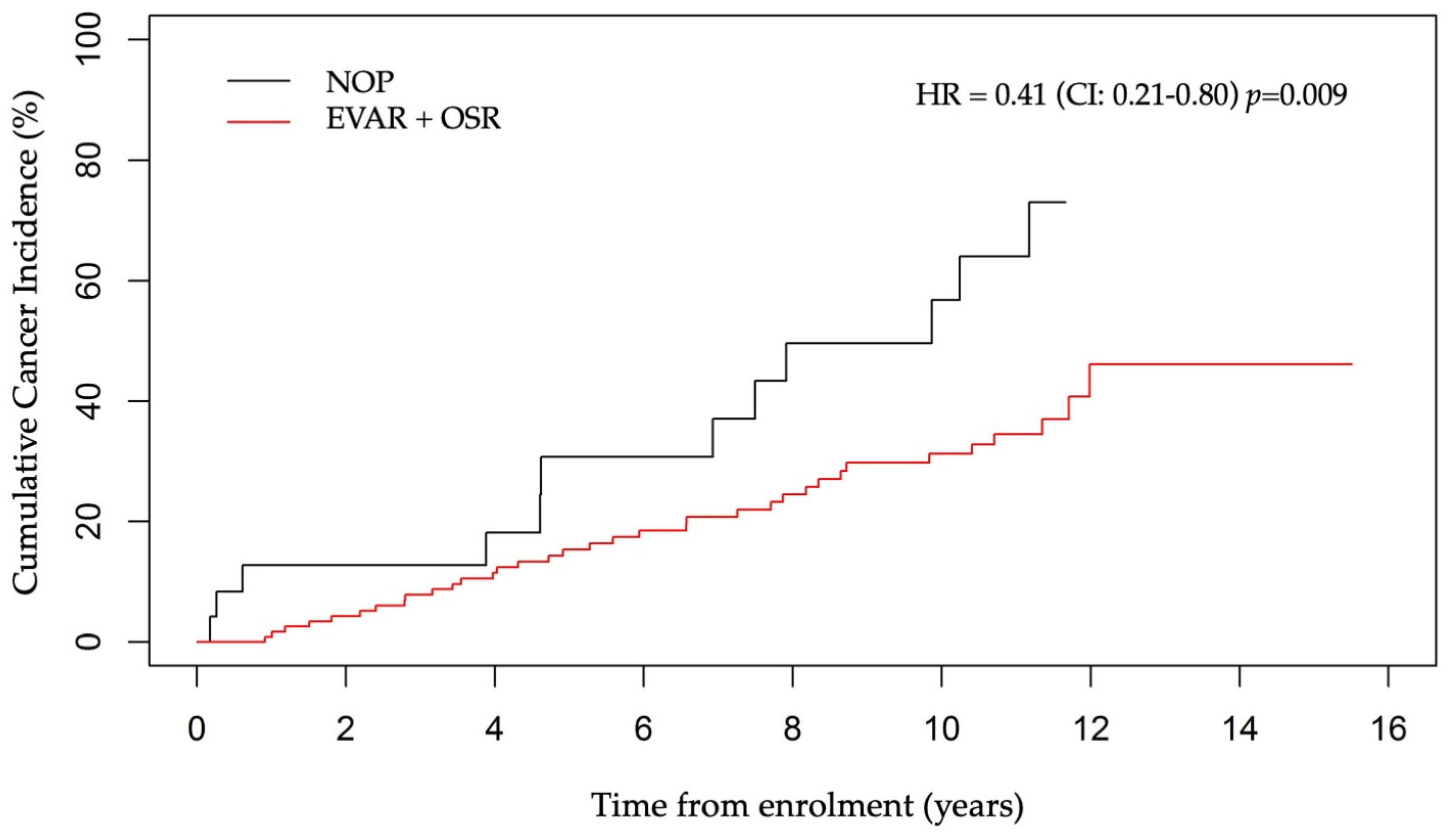
3.6. Cancer Incidence According to PET Status and AAA Treatment
3.7. Overall Survival
3.8. Risk Factors Associated with Survival
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakalihasan, N.; Michel, J.-B.; Katsargyris, A.; Kuivaniemi, H.; Defraigne, J.-O.; Nchimi, A.; Powell, J.T.; Yoshimura, K.; Hultgren, R. Abdominal Aortic Aneurysms. Nat. Rev. Dis. Primers 2018, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European Cancer Burden in 2020: Incidence and Mortality Estimates for 40 Countries and 25 Major Cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, L.; Daßler-Plenker, J.; Sun, L.; Egeblad, M. Innate Immunity and Cancer Pathophysiology. Annu. Rev. Pathol. 2022, 17, 425–457. [Google Scholar] [CrossRef] [PubMed]
- Lega, I.C.; Lipscombe, L.L. Review: Diabetes, Obesity, and Cancer-Pathophysiology and Clinical Implications. Endocr. Rev. 2020, 41, bnz014. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, T.F. Cardio-Oncology: A New Specialty Moves to Centre Stage. Eur. Heart J. 2019, 40, 1743–1746. [Google Scholar] [CrossRef] [PubMed]
- Blaes, A.H.; Shenoy, C. Is It Time to Include Cancer in Cardiovascular Risk Prediction Tools? Lancet 2019, 394, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Handy, C.E.; Quispe, R.; Pinto, X.; Blaha, M.J.; Blumenthal, R.S.; Michos, E.D.; Lima, J.A.C.; Guallar, E.; Ryu, S.; Cho, J.; et al. Synergistic Opportunities in the Interplay Between Cancer Screening and Cardiovascular Disease Risk Assessment: Together We Are Stronger. Circulation 2018, 138, 727–734. [Google Scholar] [CrossRef]
- Sundbøll, J.; Veres, K.; Horváth-Puhó, E.; Adelborg, K.; Sørensen, H.T. Risk and Prognosis of Cancer after Lower Limb Arterial Thrombosis. Circulation 2018, 138, 669–677. [Google Scholar] [CrossRef]
- Banke, A.; Schou, M.; Videbaek, L.; Møller, J.E.; Torp-Pedersen, C.; Gustafsson, F.; Dahl, J.S.; Køber, L.; Hildebrandt, P.R.; Gislason, G.H. Incidence of Cancer in Patients with Chronic Heart Failure: A Long-Term Follow-up Study. Eur. J. Heart Fail. 2016, 18, 260–266. [Google Scholar] [CrossRef]
- Meijers, W.C.; Maglione, M.; Bakker, S.J.L.; Oberhuber, R.; Kieneker, L.M.; de Jong, S.; Haubner, B.J.; Nagengast, W.B.; Lyon, A.R.; van der Vegt, B.; et al. Heart Failure Stimulates Tumor Growth by Circulating Factors. Circulation 2018, 138, 678–691. [Google Scholar] [CrossRef]
- Bertero, E.; Canepa, M.; Maack, C.; Ameri, P. Linking Heart Failure to Cancer: Background Evidence and Research Perspectives. Circulation 2018, 138, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, D.E.; Elliott, J.P.; Berguer, R. Coincidental Malignancy and Abdominal Aortic Aneurysm. Problems of Management. Arch. Surg. 1967, 95, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Tilson, M.D.; Fieg, E.L.; Harvey, M. Malignant Neoplasia in Patients with Abdominal Aortic Aneurysms. Arch. Surg. 1984, 119, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Schermerhorn, M.L.; Buck, D.B.; O’Malley, A.J.; Curran, T.; McCallum, J.C.; Darling, J.; Landon, B.E. Long-Term Outcomes of Abdominal Aortic Aneurysm in the Medicare Population. N. Engl. J. Med. 2015, 373, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Sakalihasan, N.; Van Damme, H.; Gomez, P.; Rigo, P.; Lapiere, C.M.; Nusgens, B.; Limet, R. Positron Emission Tomography (PET) Evaluation of Abdominal Aortic Aneurysm (AAA). Eur. J. Vasc. Endovasc. Surg. 2002, 23, 431–436. [Google Scholar] [CrossRef]
- Truijers, M.; Kurvers, H.A.J.M.; Bredie, S.J.H.; Oyen, W.J.G.; Blankensteijn, J.D. In Vivo Imaging of Abdominal Aortic Aneurysms: Increased FDG Uptake Suggests Inflammation in the Aneurysm Wall. J. Endovasc. Ther. 2008, 15, 462–467. [Google Scholar] [CrossRef]
- Sarda-Mantel, L.; Coutard, M.; Rouzet, F.; Raguin, O.; Vrigneaud, J.-M.; Hervatin, F.; Martet, G.; Touat, Z.; Merlet, P.; Le Guludec, D.; et al. 99mTc-Annexin-V Functional Imaging of Luminal Thrombus Activity in Abdominal Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2153–2159. [Google Scholar] [CrossRef]
- Defawe, O.D.; Hustinx, R.; Defraigne, J.O.; Limet, R.; Sakalihasan, N. Distribution of F-18 Fluorodeoxyglucose (F-18 FDG) in Abdominal Aortic Aneurysm: High Accumulation in Macrophages Seen on PET Imaging and Immunohistology. Clin. Nucl. Med. 2005, 30, 340–341. [Google Scholar] [CrossRef]
- Kotze, C.W.; Menezes, L.J.; Endozo, R.; Groves, A.M.; Ell, P.J.; Yusuf, S.W. Increased Metabolic Activity in Abdominal Aortic Aneurysm Detected by 18F-Fluorodeoxyglucose (18F-FDG) Positron Emission Tomography/Computed Tomography (PET/CT). Eur. J. Vasc. Endovasc. Surg. 2009, 38, 93–99. [Google Scholar] [CrossRef]
- Marini, C.; Morbelli, S.; Armonino, R.; Spinella, G.; Riondato, M.; Massollo, M.; Sarocchi, F.; Pane, B.; Augeri, C.; Abete, L.; et al. Direct Relationship between Cell Density and FDG Uptake in Asymptomatic Aortic Aneurysm Close to Surgical Threshold: An in Vivo and in Vitro Study. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 91–101. [Google Scholar] [CrossRef]
- Courtois, A.; Nusgens, B.V.; Hustinx, R.; Namur, G.; Gomez, P.; Somja, J.; Defraigne, J.-O.; Delvenne, P.; Michel, J.-B.; Colige, A.C.; et al. 18F-FDG Uptake Assessed by PET/CT in Abdominal Aortic Aneurysms Is Associated with Cellular and Molecular Alterations Prefacing Wall Deterioration and Rupture. J. Nucl. Med. 2013, 54, 1740–1747. [Google Scholar] [CrossRef]
- Reeps, C.; Bundschuh, R.A.; Pellisek, J.; Herz, M.; van Marwick, S.; Schwaiger, M.; Eckstein, H.-H.; Nekolla, S.G.; Essler, M. Quantitative Assessment of Glucose Metabolism in the Vessel Wall of Abdominal Aortic Aneurysms: Correlation with Histology and Role of Partial Volume Correction. Int. J. Cardiovasc. Imaging 2013, 29, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Jalalzadeh, H.; Indrakusuma, R.; Planken, R.N.; Legemate, D.A.; Koelemay, M.J.W.; Balm, R. Inflammation as a Predictor of Abdominal Aortic Aneurysm Growth and Rupture: A Systematic Review of Imaging Biomarkers. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Barwick, T.D.; Lyons, O.T.A.; Mikhaeel, N.G.; Waltham, M.; O’Doherty, M.J. 18F-FDG PET-CT Uptake Is a Feature of Both Normal Diameter and Aneurysmal Aortic Wall and Is Not Related to Aneurysm Size. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2310–2318. [Google Scholar] [CrossRef]
- Palombo, D.; Morbelli, S.; Spinella, G.; Pane, B.; Marini, C.; Rousas, N.; Massollo, M.; Cittadini, G.; Camellino, D.; Sambuceti, G. A Positron Emission Tomography/Computed Tomography (PET/CT) Evaluation of Asymptomatic Abdominal Aortic Aneurysms: Another Point of View. Ann. Vasc. Surg. 2012, 26, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Tegler, G.; Ericson, K.; Sörensen, J.; Björck, M.; Wanhainen, A. Inflammation in the Walls of Asymptomatic Abdominal Aortic Aneurysms Is Not Associated with Increased Metabolic Activity Detectable by 18-Fluorodeoxglucose Positron-Emission Tomography. J. Vasc. Surg. 2012, 56, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Courtois, A.; Nusgens, B.; Garbacki, N.; Hustinx, R.; Gomez, P.; Defraigne, J.-O.; Colige, A.C.; Sakalihasan, N. Circulating microRNAs Signature Correlates with Positive [18F]Fluorodeoxyglucose-Positron Emission Tomography in Patients with Abdominal Aortic Aneurysm. J. Vasc. Surg. 2018, 67, 585–595.e3. [Google Scholar] [CrossRef] [PubMed]
- Courtois, A.; Makrygiannis, G.; El Hachemi, M.; Hultgren, R.; Allaire, E.; Namur, G.; Hustinx, R.; Defraigne, J.-O.; Sakalihasan, N. Positron Emission Tomography/Computed Tomography Predicts and Detects Complications After Endovascular Repair of Abdominal Aortic Aneurysms. J. Endovasc. Ther. 2019, 26, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Reinikainen, J.; Adeleke, K.A.; Pieterse, M.E.; Groothuis-Oudshoorn, C.G.M. Time-Varying Covariates and Coefficients in Cox Regression Models. Ann. Transl. Med. 2018, 6, 121. [Google Scholar] [CrossRef]
- Reeps, C.; Essler, M.; Pelisek, J.; Seidl, S.; Eckstein, H.-H.; Krause, B.-J. Increased 18F-Fluorodeoxyglucose Uptake in Abdominal Aortic Aneurysms in Positron Emission/Computed Tomography Is Associated with Inflammation, Aortic Wall Instability, and Acute Symptoms. J. Vasc. Surg. 2008, 48, 417–423. [Google Scholar] [CrossRef]
- Hussain, S.P.; Harris, C.C. Inflammation and Cancer: An Ancient Link with Novel Potentials. Int. J. Cancer 2007, 121, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Courtois, A.; Nusgens, B.V.; Hustinx, R.; Namur, G.; Gomez, P.; Kuivaniemi, H.; Defraigne, J.-O.; Colige, A.C.; Sakalihasan, N. Gene Expression Study in Positron Emission Tomography-Positive Abdominal Aortic Aneurysms Identifies CCL18 as a Potential Biomarker for Rupture Risk. Mol. Med. 2015, 20, 697–706. [Google Scholar] [CrossRef]
- Treska, V.; Kocova, J.; Boudova, L.; Neprasova, P.; Topolcan, O.; Pecen, L.; Tonar, Z. Inflammation in the Wall of Abdominal Aortic Aneurysm and Its Role in the Symptomatology of Aneurysm. Cytokines Cell Mol. Ther. 2002, 7, 91–97. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New Functions for the Matrix Metalloproteinases in Cancer Progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Germolec, D.R.; Shipkowski, K.A.; Frawley, R.P.; Evans, E. Markers of Inflammation. Methods Mol. Biol. 2018, 1803, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Min, J.-Y.; Kim, H.G.; Mo, H.; Min, S.-K.; Min, S.; Ha, J.; Min, K.-B. Outcomes after Aortic Aneurysm Repair in Patients with History of Cancer: A Nationwide Dataset Analysis. BMC Surg. 2020, 20, 85. [Google Scholar] [CrossRef] [PubMed]

| Variable | Mean ± SD Number (%) |
|---|---|
| Age (years) | 71.5 ± 8.8 |
| Smokers | 128 (85.9) |
| Diabetes | 20 (13.4) |
| Hypertension | 91 (61.1) |
| COPD | 52 (34.9) |
| RI | 22 (14.8) |
| Stroke | 20 (13.4) |
| HLD | 97 (65.1) |
| AMI | 46 (30.9) |
| PAD | 41 (27.5) |
| Angina pectoris | 22 (14.8) |
| Variable | Number of Patients | Number of Cases (%) |
|---|---|---|
| Cancer Incidence | 149 | 47 (31.5) |
| Metastasis | 47 | 17 (36.2) |
| Type of Cancer | 47 | |
| Urologic | 14 (29.8) | |
| Lung | 13 (27.7) | |
| Digestive | 9 (19.1) | |
| Skin | 5 (10.6) | |
| Acute myeloid leukemia | 2 (4.3) | |
| Other | 4 (8.5) | |
| Cancer treatment | 47 | |
| Surgical | 20 (42.6) | |
| Chemotherapy | 5 (10.6) | |
| Radiotherapy | 4 (8.5) | |
| Surgical + Radiotherapy | 3 (6.4) | |
| Surgical + Immunotherapy | 1 (2.1) | |
| Surgical + Chemotherapy | 1 (2.1) | |
| Informed Refusal | 10 (21.3) | |
| Unknown | 3 (6.4) |
| Univariate Model | Multivariate Model | |||||
|---|---|---|---|---|---|---|
| Covariate | HR | HR (95%CI) | p-Value | HR | HR (95%CI) | p-Value |
| Age at baseline (years) | 1.03 | 1.00–1.07 | 0.047 | 1.04 | 1.00–1.08 | 0.042 |
| AAA diagnosis since entry (years) | 0.99 | 0.91–1.08 | 0.86 | 0.99 | 0.91–1.09 | 0.89 |
| Smoking (Yes vs. No) | 0.86 | 0.40–1.86 | 0.70 | 0.90 | 0.40–2.03 | 0.80 |
| Diabetes (Yes vs. No) | 1.02 | 0.45–2.32 | 0.96 | 0.89 | 0.37–2.13 | 0.78 |
| Hypertension (Yes vs. No) | 0.88 | 0.49–1.57 | 0.66 | 0.92 | 0.50–1.68 | 0.78 |
| COPD (Yes vs. No) | 1.06 | 0.56–2.02 | 0.86 | 1.03 | 0.51–2.05 | 0.94 |
| RI (Yes vs. No) | 0.72 | 0.26–2.01 | 0.53 | 0.64 | 0.22–1.87 | 0.41 |
| Stroke (Yes vs. No) | 0.61 | 0.22–1.71 | 0.35 | 0.55 | 0.19–1.61 | 0.28 |
| HLD (Yes vs. No) | 0.81 | 0.44–1.48 | 0.49 | 0.86 | 0.46–1.61 | 0.49 |
| AMI (Yes vs. No) | 0.82 | 0.42–1.63 | 0.57 | 0.72 | 0.33–1.57 | 0.40 |
| PAD (Yes vs. No) | 1.04 | 0.54–2.01 | 0.91 | 1.19 | 0.57–2.51 | 0.64 |
| Angina Pectoris (Yes vs. No) | 1.14 | 0.48–2.71 | 0.76 | 1.24 | 0.46–3.36 | 0.67 |
| Time-varying PET (+ vs. −) | 2.34 | 1.18–4.64 | 0.015 | 2.34 | 1.12–4.92 | 0.025 |
| Univariate Model | Multivariate Model | |||||
|---|---|---|---|---|---|---|
| Covariate | HR | HR (95%CI) | p-Value | HR | HR (95%CI) | p-Value |
| Age at baseline (years) | 1.07 | 1.04–1.10 | <0.0001 | 1.07 | 1.04–1.11 | <0.0001 |
| AAA diagnosis to entry (years) | 0.96 | 0.89–1.03 | 0.21 | 0.93 | 0.87–1.00 | 0.062 |
| Smoking (Yes vs. No) | 0.96 | 0.53–1.74 | 0.90 | 0.81 | 0.42–1.56 | 0.53 |
| Diabetes (Yes vs. No) | 0.74 | 0.38–1.45 | 0.38 | 0.71 | 0.35–1.44 | 0.34 |
| Hypertension (Yes vs. No) | 1.07 | 0.69–1.64 | 0.77 | 1.12 | 0.71–1.78 | 0.63 |
| COPD (Yes vs. No) | 2.02 | 1.32–3.09 | 0.0011 | 2.12 | 1.34–3.35 | 0.0014 |
| RI (Yes vs. No) | 1.77 | 1.02–3.06 | 0.042 | 1.36 | 0.74–2.50 | 0.32 |
| Stroke (Yes vs. No) | 1.40 | 0.79–2.50 | 0.25 | 1.39 | 0.75–2.56 | 0.29 |
| HLD (Yes vs. No) | 0.71 | 0.46–1.10 | 0.13 | 0.80 | 0.51–1.27 | 0.34 |
| AMI (Yes vs. No) | 1.63 | 1.05–2.52 | 0.029 | 1.52 | 0.93–2.47 | 0.097 |
| PAD (Yes vs. No) | 1.20 | 0.76–1.90 | 0.43 | 1.44 | 0.87–2.40 | 0.16 |
| Angina Pectoris (Yes vs. No) | 1.98 | 1.17–3.33 | 0.010 | 1.37 | 0.76–2.45 | 0.29 |
| Time-varying PET (+ vs. −) | 1.56 | 0.92–2.66 | 0.099 | 2.33 | 1.30–4.20 | 0.0048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakalihasan, N.; Bruls, S.; Hustinx, R.; Tchana-Sato, V.; Sakalihasan, S.; Hultgren, R.; Labropoulos, N.; Colige, A.; Durieux, R.; Drion, P.; et al. Association between PET/CT Scan Findings, Treatment, and Cancer Incidence in a Cohort of AAA Patients. J. Clin. Med. 2024, 13, 1569. https://doi.org/10.3390/jcm13061569
Sakalihasan N, Bruls S, Hustinx R, Tchana-Sato V, Sakalihasan S, Hultgren R, Labropoulos N, Colige A, Durieux R, Drion P, et al. Association between PET/CT Scan Findings, Treatment, and Cancer Incidence in a Cohort of AAA Patients. Journal of Clinical Medicine. 2024; 13(6):1569. https://doi.org/10.3390/jcm13061569
Chicago/Turabian StyleSakalihasan, Natzi, Samuel Bruls, Roland Hustinx, Vincent Tchana-Sato, Sarah Sakalihasan, Rebecka Hultgren, Nicos Labropoulos, Alain Colige, Rodolphe Durieux, Pierre Drion, and et al. 2024. "Association between PET/CT Scan Findings, Treatment, and Cancer Incidence in a Cohort of AAA Patients" Journal of Clinical Medicine 13, no. 6: 1569. https://doi.org/10.3390/jcm13061569
APA StyleSakalihasan, N., Bruls, S., Hustinx, R., Tchana-Sato, V., Sakalihasan, S., Hultgren, R., Labropoulos, N., Colige, A., Durieux, R., Drion, P., Albert, A., Defraigne, J.-O., & Musumeci, L. (2024). Association between PET/CT Scan Findings, Treatment, and Cancer Incidence in a Cohort of AAA Patients. Journal of Clinical Medicine, 13(6), 1569. https://doi.org/10.3390/jcm13061569