Diagnostic Yield and Clinical Implications of Implantable Loop Recorders in Patients with Syncope in Germany: A National Database Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Patient Population
2.3. Statistics
3. Results
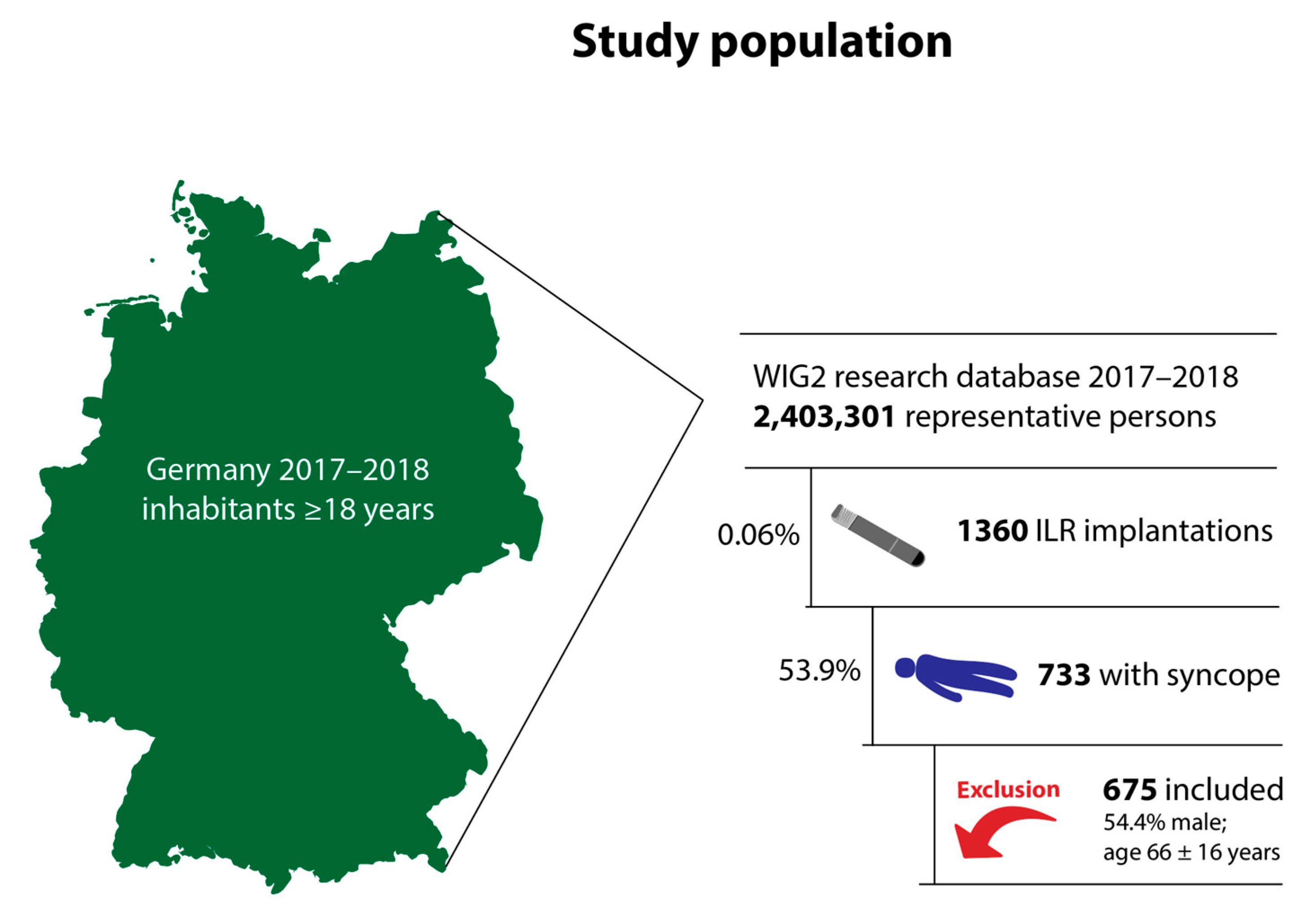
3.1. Study Population and Baseline Characteristics
3.2. Follow-Up
4. Discussion
- Overall yield of cardiac arrhythmia diagnoses in patients with syncope and ILR was high at 65% during a follow-up period of two years.
- Interventional antiarrhythmic therapies were established in 23.6%, including pacemaker implantations in 20.0%.
- Aside from therapies for the prevention of syncope, new anticoagulation therapy was also initiated in 21.5%.
4.1. Diagnostic Yield after ILR Implantation
4.2. Therapeutic Implications
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soteriades, E.S.; Evans, J.C.; Larson, M.G.; Chen, M.H.; Chen, L.; Benjamin, E.J.; Levy, D. Incidence and Prognosis of Syncope. N. Engl. J. Med. 2002, 347, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Farwell, D.J.; Freemantle, N.; Sulke, N. The Clinical Impact of Implantable Loop Recorders in Patients with Syncope. Eur. Heart J. 2006, 27, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Ammirati, F.; Arabia, F.; Quartieri, F.; Tomaino, M.; Ungar, A.; Lunati, M.; Russo, V.; Rosso, A.D.; Gaggioli, G.; et al. Assessment of a Standardized Algorithm for Cardiac Pacing in Older Patients Affected by Severe Unpredictable Reflex Syncopes. Eur. Heart J. 2015, 36, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Menozzi, C.; Moya, A.; Andresen, D.; Blanc, J.J.; Krahn, A.D.; Wieling, W.; Beiras, X.; Deharo, J.C.; Russo, V.; et al. Pacemaker Therapy in Patients with Neurally Mediated Syncope and Documented Asystole. Circulation 2012, 125, 2566–2571. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.-C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martín, A.; et al. 2018 ESC Guidelines for the Diagnosis and Management of Syncope. Eur. Heart J. 2018, 39, 1883–1948. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, F.; Biondi-Zoccai, G.; Reed, M.J.; Gabayan, G.Z.; Suzuki, M.; Costantino, G.; Furlan, R.; Rosso, A.D.; Sarasin, F.P.; Sun, B.C.; et al. Incidence, Etiology and Predictors of Adverse Outcomes in 43,315 Patients Presenting to the Emergency Department with Syncope: An International Meta-Analysis. Int. J. Cardiol. 2013, 167, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Bass, E.B.; Curtiss, E.I.; Arena, V.C.; Hanusa, B.H.; Cecchetti, A.; Karpf, M.; Kapoor, W.N. The Duration of Holter Monitoring in Patients with Syncope. Is 24 Hours Enough? Arch. Intern. Med. 1990, 150, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Svennberg, E.; Tjong, F.; Goette, A.; Akoum, N.; Biase, L.D.; Bordachar, P.; Boriani, G.; Burri, H.; Conte, G.; Deharo, J.C.; et al. How to Use Digital Devices to Detect and Manage Arrhythmias: An EHRA Practical Guide. Europace 2022, 24, 979–1005. [Google Scholar] [CrossRef]
- Svennberg, E.; Caiani, E.G.; Bruining, N.; Desteghe, L.; Han, J.K.; Narayan, S.M.; Rademakers, F.E.; Sanders, P.; Duncker, D. The Digital Journey: 25 Years of Digital Development in Electrophysiology from an Europace Perspective. Europace 2023, 25, euad176. [Google Scholar] [CrossRef]
- Bogossian, H.; Duncker, D. Unaufhaltsame Innovationen in Der Elektrophysiologie. Herzschrittmachertherapie Elektrophysiologie 2022, 33, 1–2. [Google Scholar] [CrossRef]
- Boriani, G.; Svennberg, E.; Guerra, F.; Linz, D.; Casado-Arroyo, R.; Malaczynska-Rajpold, K.; Duncker, D.; Boveda, S.; Merino, J.L.; Leclercq, C. Reimbursement Practices for Use of Digital Devices in Atrial Fibrillation and Other Arrhythmias: A European Heart Rhythm Association Survey. Europace 2022, 24, 1834–1843. [Google Scholar] [CrossRef]
- Lombardi, F.; Calosso, E.; Mascioli, G.; Marangoni, E.; Donato, A.; Rossi, S.; Pala, M.; Foti, F.; Lunati, M. Utility of Implantable Loop Recorder (Reveal Plus®) in the Diagnosis of Unexplained Syncope. Europace 2005, 7, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Edvardsson, N.; Frykman, V.; van Mechelen, R.; Mitro, P.; Mohii-Oskarsson, A.; Pasquié, J.-L.; Ramanna, H.; Schwertfeger, F.; Ventura, R.; Voulgaraki, D.; et al. Use of an Implantable Loop Recorder to Increase the Diagnostic Yield in Unexplained Syncope: Results from the PICTURE Registry. Europace 2011, 13, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Krahn, A.D.; Klein, G.J.; Yee, R.; Skanes, A.C. Randomized Assessment of Syncope Trial. Circulation 2001, 104, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.D.; Defaye, P.; Romeyer-Bouchard, C.; Roche, F.; Dauphinot, V.; Deharo, J.-C.; Jacon, P.; Lamaison, D.; Bathélemy, J.-C.; Isaaz, K.; et al. Clinical Impact of the Implantable Loop Recorder in Patients with Isolated Syncope, Bundle Branch Block and Negative Workup: A Randomized Multicentre Prospective Study. Arch. Cardiovasc. Dis. 2013, 106, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Podoleanu, C.; DaCosta, A.; Defaye, P.; Taieb, J.; Galley, D.; Bru, P.; Maury, P.; Mabo, P.; Boveda, S.; Cellarier, G.; et al. Early Use of an Implantable Loop Recorder in Syncope Evaluation: A Randomized Study in the Context of the French Healthcare System (FRESH Study). Arch. Cardiovasc. Dis. 2014, 107, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Sulke, N.; Sugihara, C.; Hong, P.; Patel, N.; Freemantle, N. The Benefit of a Remotely Monitored Implantable Loop Recorder as a First Line Investigation in Unexplained Syncope: The EaSyAS II Trial. Europace 2016, 18, 912–918. [Google Scholar] [CrossRef]
- Schuchert, A. Indikationen Zur Loop-Rekorder-Implantation Bei Synkope. Herzschrittmachertherapie Elektrophysiologie 2018, 29, 193–198. [Google Scholar] [CrossRef]
- Perings, C.; Wolff, C.; Wilk, A.; Witthohn, A.; Voss, R.; Rybak, K. Do Implantable Loop Recorders Impact the Survival of Patients with Recurrent Unexplained Syncope? J. Comp. Eff. Res. 2021, 10, 285–294. [Google Scholar] [CrossRef]
- Kapoor, W.N. Evaluation and Outcome of Patients with Syncope. Medicine 1990, 69, 160–175. [Google Scholar] [CrossRef]
- Altinsoy, M.; Sutton, R.; Kohno, R.; Sakaguchi, S.; Mears, R.K.; Benditt, D.G. Ambulatory ECG Monitoring for Syncope and Collapse in United States, Europe, and Japan: The Patients’ Viewpoint. J. Arrhythmia 2021, 37, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-K.; Sheldon, R.S.; Benditt, D.G.; Cohen, M.I.; Forman, D.E.; Goldberger, Z.D.; Grubb, B.P.; Hamdan, M.H.; Krahn, A.D.; Link, M.S.; et al. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients with Syncope: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017, 136, e60–e122. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, Z.D.; Petek, B.J.; Brignole, M.; Shen, W.-K.; Sheldon, R.S.; Solbiati, M.; Deharo, J.-C.; Moya, A.; Hamdan, M.H. ACC/AHA/HRS Versus ESC Guidelines for the Diagnosis and Management of Syncope JACC Guideline Comparison. J. Am. Coll. Cardiol. 2019, 74, 2410–2423. [Google Scholar] [CrossRef] [PubMed]
- Von Scheid, W.; Bosch, R.; Klingenheben, T.; Schuchert, A.; Stellbrink, C.; Stockburger, M. Deutsche Gesellschaft für Kardiologie—Herz-und Kreislaufforschung e.V. ESC Pocket Guidelines. Diagnose und Management von Synkopen, Version 2018; Börm Bruckmeier Verlag GmbH: Grünwald, Germany, 2018. [Google Scholar]
- Dan, G.-A.; Scherr, D.; Jubele, K.; Farkowski, M.M.; Iliodromitis, K.; Conte, G.; Jędrzejczyk-Patej, E.; Vitali-Serdoz, L.; Potpara, T.S. Contemporary Management of Patients with Syncope in Clinical Practice: An EHRA Physician-Based Survey. Europace 2020, 22, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Sciaraffia, E.; Chen, J.; Hocini, M.; Larsen, T.B.; Potpara, T.; Blomström-Lundqvist, C. Use of Event Recorders and Loop Recorders in Clinical Practice: Results of the European Heart Rhythm Association Survey. Europace 2014, 16, 1384–1386. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boriani, G.; Burri, H.; Svennberg, E.; Imberti, J.F.; Merino, J.L.; Leclercq, C. Current Status of Reimbursement Practices for Remote Monitoring of Cardiac Implantable Electrical Devices across Europe. Europace 2022, 24, euac118. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Imberti, J.F.; Leyva, F.; Casado-Arroyo, R.; Chun, J.; Braunschweig, F.; Zylla, M.M.; Duncker, D.; Farkowski, M.M.; Pürerfellner, H.; et al. Length of Hospital Stay for Elective Electrophysiological Procedures: A Survey from the European Heart Rhythm Association. Europace 2023, 25, euad297. [Google Scholar] [CrossRef]
- Kommata, V.; Deharo, J.-C.; Drossart, I.; Foldager, D.; Svennberg, E.; Vernooy, K.; Verstrael, A.; Duncker, D. The ‘Myrhythmdevice.Org’ Educational Website for Patients with Implanted Cardiac Devices from the European Heart Rhythm Association. Europace 2022, 24, 1713–1715. [Google Scholar] [CrossRef]
- Jilek, C.; Lewalter, T. Implantierbare EKG-Monitore. Herzschrittmachertherapie Elektrophysiologie 2020, 31, 254–259. [Google Scholar] [CrossRef]
- Veltmann, C.; Bosch, R.; Boer, J.; Endres, M.; Frankenstein, L.; Gröschel, K.; Hansen, C.; Straube, F. Empfehlung Zur Indikationsstellung Implantierbarer Ereignisrecorder. Kardiologie 2023, 17, 389–405. [Google Scholar] [CrossRef]
- Swart, E.; Gothe, H.; Geyer, S.; Jaunzeme, J.; Maier, B.; Grobe, T.G.; Ihle, P. Gute Praxis Sekundärdatenanalyse (GPS): Leitlinien Und Empfehlungen. Gesundheitswesen 2015, 77, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Andersohn, F.; Walker, J. Characteristics and External Validity of the German Health Risk Institute (HRI) Database. Pharmacoepidemiol. Drug Saf. 2016, 25, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Research Database of the German Scientific Institute of Health Economics and Health System Research (WIG2). Available online: https://www.wig2.de/analysetools/wig2-forschungsdatenbank.html (accessed on 6 January 2024).
- Lau, D.H.; Pierre, B.; Cabanas, P.; Martens, E.; Bisignani, G.; Hofer, D.; Berruezo, A.; Eschalier, R.; Mansourati, J.; Gaspar, T.; et al. Diagnostic Yield of an Insertable Cardiac Monitor in a Large Patient Population. Heart Rhythm. O2 2023, 4, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, T.-H.; Oh, Y.-S.; Oh, S.; Choi, J.-I.; Kim, J.-B.; Nah, J.-C.; Im, S.I.; Kang, K.-W.; Han, S.; et al. Usefulness of an Implantable Loop Recorder in Diagnosing Unexplained Syncope and Predictors for Pacemaker Implantation. J. Korean Med. Sci. 2019, 35, e11. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Auricchio, A.; Botto, G.L.; Joseph, J.M.; Roberts, G.J.; Grammatico, A.; Nabutovsky, Y.; Piccini, J.P. Insertable Cardiac Monitoring Results in Higher Rates of Atrial Fibrillation Diagnosis and Oral Anticoagulation Prescription after Ischaemic Stroke. Europace 2023, 25, euad212. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.; Dai, Q.; Flynn, D.B.; Bosch, N.A.; Helm, R.H.; Monahan, K.M.; Andersson, C.; Anderson, C.D.; Walkey, A.J. Meta-Analysis of Randomized Clinical Trials Comparing the Impact of Implantable Loop Recorder versus Usual Care After Ischemic Stroke for Detection of Atrial Fibrillation and Stroke Risk. Am. J. Cardiol. 2022, 162, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Buck, B.H.; Hill, M.D.; Quinn, F.R.; Butcher, K.S.; Menon, B.K.; Gulamhusein, S.; Siddiqui, M.; Coutts, S.B.; Jeerakathil, T.; Smith, E.E.; et al. Effect of Implantable vs. Prolonged External Electrocardiographic Monitoring on Atrial Fibrillation Detection in Patients with Ischemic Stroke. JAMA 2021, 325, 2160–2168. [Google Scholar] [CrossRef]
- Milstein, N.S.; Musat, D.L.; Allred, J.; Seiler, A.; Pimienta, J.; Oliveros, S.; Bhatt, A.G.; Preminger, M.; Sichrovsky, T.; Shaw, R.E.; et al. Detection of Atrial Fibrillation Using an Implantable Loop Recorder Following Cryptogenic Stroke: Implications for Post-Stroke Electrocardiographic Monitoring. J. Interv. Card. Electrophysiol. 2020, 57, 141–147. [Google Scholar] [CrossRef]
- Arcinas, L.A.; McIntyre, W.F.; Hayes, C.J.; Ibrahim, O.A.; Baranchuk, A.M.; Seifer, C.M. Atrial Fibrillation in Elderly Patients with Implantable Loop Recorders for Unexplained Syncope. Ann. Noninvasive Electrocardiol. 2019, 24, e12630. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Marinelli, E.A.; Arbelo, E.; Boriani, G.; Boveda, S.; Buckley, C.M.; Camm, A.J.; Casadei, B.; Chua, W.; Dagres, N.; et al. Early Diagnosis and Better Rhythm Management to Improve Outcomes in Patients with Atrial Fibrillation: The 8th AFNET/EHRA Consensus Conference. Europace 2022, 25, 6–27. [Google Scholar] [CrossRef]
- Svendsen, J.H.; Diederichsen, S.Z.; Højberg, S.; Krieger, D.W.; Graff, C.; Kronborg, C.; Olesen, M.S.; Nielsen, J.B.; Holst, A.G.; Brandes, A.; et al. Implantable Loop Recorder Detection of Atrial Fibrillation to Prevent Stroke (The LOOP Study): A Randomised Controlled Trial. Lancet 2021, 398, 1507–1516. [Google Scholar] [CrossRef]
| Diagnosis | n (%) | Mean Time to Diagnosis (Days) |
|---|---|---|
| Any cardiac diagnosis | 566 (83.9) | 116 ± 162 |
| AV block 1 | 59 (8.7) | 206 ± 197 |
| Cardiac conduction disease 2 | 135 (20) | 212 ± 213 |
| Other arrhythmias 3 | 275 (40.7) | 176 ± 184 |
| Atrial fibrillation 4 | 250 (37.0) | 212 ± 213 |
| Therapy | n (%) | Mean Time to Diagnosis (Days) |
|---|---|---|
| Pacemaker | 135 (20.0) | 225 ± 185 |
| Defibrillator | 10 (1.5) | 160 ± 136 |
| Ablation | 20 (3) | 338 ± 238 |
| Anticoagulation | 145 (21.5) | 160 ± 136 |
| Antiarrhythmic therapy | 32 (4.7) | 227 ± 180 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller-Leisse, J.; Hillmann, H.A.K.; Iserloh, L.; Fruehauf, B.; Duncker, D. Diagnostic Yield and Clinical Implications of Implantable Loop Recorders in Patients with Syncope in Germany: A National Database Analysis. J. Clin. Med. 2024, 13, 1564. https://doi.org/10.3390/jcm13061564
Mueller-Leisse J, Hillmann HAK, Iserloh L, Fruehauf B, Duncker D. Diagnostic Yield and Clinical Implications of Implantable Loop Recorders in Patients with Syncope in Germany: A National Database Analysis. Journal of Clinical Medicine. 2024; 13(6):1564. https://doi.org/10.3390/jcm13061564
Chicago/Turabian StyleMueller-Leisse, Johanna, Henrike Aenne Katrin Hillmann, Laura Iserloh, Bjoern Fruehauf, and David Duncker. 2024. "Diagnostic Yield and Clinical Implications of Implantable Loop Recorders in Patients with Syncope in Germany: A National Database Analysis" Journal of Clinical Medicine 13, no. 6: 1564. https://doi.org/10.3390/jcm13061564
APA StyleMueller-Leisse, J., Hillmann, H. A. K., Iserloh, L., Fruehauf, B., & Duncker, D. (2024). Diagnostic Yield and Clinical Implications of Implantable Loop Recorders in Patients with Syncope in Germany: A National Database Analysis. Journal of Clinical Medicine, 13(6), 1564. https://doi.org/10.3390/jcm13061564







