Relationship between Permanent Catheter Patency and Nutrient Score in Patients Aged >75 Years Requiring Renal Replacement Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Definitions
2.2.1. Infection of the Permanent Catheter
- -
- When infection clues such as redness, tenderness, heating sense, and pus were suspected at the permanent catheter exit site, and infection was suspected simultaneously in laboratory tests;
- -
- When no evidence of infection before catheter insertion was noted; however, symptoms of infection such as fever emerged within 5 days after insertion, and infection was confirmed on blood culture (no prominent infection was in other organs).
2.2.2. Malfunction of the Permanent Catheter
- -
- Failure to attain and maintain an extracorporeal blood flow sufficient to perform HD (<300 mL/min);
- -
- Catheter dysfunction due to catheter-tip malposition and mechanical kinking of the catheter.
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.H.; Lv, J.C.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef]
- Hong, Y.A.; Ban, T.H.; Kang, C.Y.; Hwang, S.D.; Choi, S.R.; Lee, H.; Jung, H.Y.; Kim, K.; Kwon, Y.E.; Kim, S.H.; et al. Trends in epidemiologic characteristics of end-stage renal disease from 2019 Korean Renal Data System (KORDS). Kidney Res. Clin. Pract. 2021, 40, 52–61. [Google Scholar] [CrossRef]
- Diandra, J.C.; Lo, Z.J.; Ang, W.W.; Feng, J.F.; Narayanan, S.; Tan, G.W.L.; Chandrasekar, S. A Review of Arteriovenous Fistulae Creation in Octogenarians. Ann. Vasc. Surg. 2018, 46, 331–336. [Google Scholar] [CrossRef]
- Bossola, M.; Muscaritoli, M.; Tazza, L.; Giungi, S.; Tortorelli, A.; Rossi Fanelli, F.; Luciani, G. Malnutrition in hemodialysis patients: What therapy? Am. J. Kidney Dis. 2005, 46, 371–386. [Google Scholar] [CrossRef]
- Panichi, V.; Cupisti, A.; Rosati, A.; Di Giorgio, A.; Scatena, A.; Menconi, O.; Bozzoli, L.; Bottai, A. Geriatric nutritional risk index is a strong predictor of mortality in hemodialysis patients: Data from the Riscavid cohort. J. Nephrol. 2014, 27, 193–201. [Google Scholar] [CrossRef]
- Takagi, K.; Takahashi, H.; Miura, T.; Yamagiwa, K.; Kawase, K.; Muramatsu-Maekawa, Y.; Koie, T.; Mizuno, M. Prognostic Value of the Controlling Nutritional Status (CONUT) Score in Patients at Dialysis Initiation. Nutrients 2022, 14, 2317. [Google Scholar] [CrossRef]
- Maenosono, R.; Fukushima, T.; Kobayashi, D.; Matsunaga, T.; Yano, Y.; Taniguchi, S.; Fujiwara, Y.; Komura, K.; Uehara, H.; Kagitani, M.; et al. Unplanned hemodialysis initiation and low geriatric nutritional risk index scores are associated with end-stage renal disease outcomes. Sci. Rep. 2022, 12, 11101. [Google Scholar] [CrossRef]
- Jankowska, M.; Cobo, G.; Lindholm, B.; Stenvinkel, P. Inflammation and Protein-Energy Wasting in the Uremic Milieu. Contrib. Nephrol. 2017, 191, 58–71. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.M.; et al. Etiology of the Protein-Energy Wasting Syndrome in Chronic Kidney Disease: A Consensus Statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef]
- Ignacio de Ulibarri, J.; Gonzalez-Madrono, A.; de Villar, N.G.; Gonzalez, P.; Gonzalez, B.; Mancha, A.; Rodriguez, F.; Fernandez, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Abd-El-Gawad, W.M.; Abou-Hashem, R.M.; El Maraghy, M.O.; Amin, G.E. The validity of Geriatric Nutrition Risk Index: Simple tool for prediction of nutritional-related complication of hospitalized elderly patients. Comparison with Mini Nutritional Assessment. Clin. Nutr. 2014, 33, 1108–1116. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Gillespie, I.A.; Tunks, J.; Addison, J.; Kronenberg, F.; Drueke, T.B.; Marcelli, D.; Schernthaner, G.; Eckardt, K.U.; Floege, J.; et al. Inflammation Modifies the Paradoxical Association between Body Mass Index and Mortality in Hemodialysis Patients. J. Am. Soc. Nephrol. 2016, 27, 1479–1486. [Google Scholar] [CrossRef]
- Zhou, C.M.; Lin, X.; Ma, G.Y.; Yuan, J.; Zha, Y. Increased Predialysis Extracellular to Intracellular Water Ratio Is Associated with Sarcopenia in Hemodialysis Patients. J. Ren. Nutr. 2023, 33, 157–164. [Google Scholar] [CrossRef]
- Gracia-Iguacel, C.; González-Parra, E.; Mahillo, I.; Ortiz, A. Low Intracellular Water, Overhydration, and Mortality in Hemodialysis Patients. J. Clin. Med. 2020, 9, 3616. [Google Scholar] [CrossRef]
- Gracia-Iguacel, C.; González-Parra, E.; Mahillo, I.; Ortiz, A. Criteria for classification of protein-energy wasting in dialysis patients: Impact on prevalence. Br. J. Nutr. 2019, 121, 1271–1278. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeon, J.W.; Kim, H.R.; Park, H.; Han, S.; Hwang, Y.; Park, H.; Park, K.; Lee, E.J.; Ham, Y.R.; et al. Ratio of Extracellular to Intracellular Water Is Associated with Permanent Catheter Patency Survival in Patients Receiving Maintenance Hemodialysis. Diagnostics 2023, 13, 2545. [Google Scholar] [CrossRef]
- Tanriover, B.; Carlton, D.; Saddekni, S.; Hamrick, K.; Oser, R.; Westfall, A.O.; Allon, M. Bacteremia associated with tunneled dialysis catheters: Comparison of two treatment strategies. Kidney Int. 2000, 57, 2151–2155. [Google Scholar] [CrossRef]
- Levin, N.W.; Kotanko, P. Improving albumin levels among hemodialysis patients. Am. J. Kidney Dis. 2006, 48, 171–173. [Google Scholar] [CrossRef]
- Unver, S.; Atasoyu, E.M.; Evrenkaya, T.R.; Ardic, N.; Ozyurt, M. Risk factors for the infections caused by temporary double-lumen hemodialysis catheters. Arch. Med. Res. 2006, 37, 348–352. [Google Scholar] [CrossRef]
- Bergström, J.; Lindholm, B. Malnutrition, cardiac disease, and mortality: An integrated point of view. Am. J. Kidney Dis. 1998, 32, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Forauer, A.R.; Theoharis, C. Histologic changes in the human vein wall adjacent to indwelling central venous catheters. J. Vasc. Interv. Radiol. 2003, 14, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.R. Hemodialysis Central Venous Catheter Dysfunction. Semin. Dial. 2008, 21, 516–521. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Kaller, R.; Arbanasi, E.M.; Muresan, A.V.; Voidazan, S.; Arbanasi, E.M.; Horváth, E.; Suciu, B.A.; Hosu, I.; Halmaciu, I.; Brinzaniuc, K.; et al. The Predictive Value of Systemic Inflammatory Markers, the Prognostic Nutritional Index, and Measured Vessels’ Diameters in Arteriovenous Fistula Maturation Failure. Life 2022, 12, 1447. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, A.; Remuzzi, A.; Franzoni, M.; Misra, S. The molecular mechanisms of hemodialysis vascular access failure. Kidney Int. 2016, 89, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Roy-Chaudhury, P. Advances and new frontiers in the pathophysiology of venous neointimal hyperplasia and dialysis access stenosis. Adv. Chronic Kidney Dis. 2009, 16, 329–338. [Google Scholar] [CrossRef]
- Yamagata, K.; Hoshino, J.; Sugiyama, H.; Hanafusa, N.; Shibagaki, Y.; Komatsu, Y.; Konta, T.; Fujii, N.; Kanda, E.; Sofue, T.; et al. Clinical practice guideline for renal rehabilitation: Systematic reviews and recommendations of exercise therapies in patients with kidney diseases. Ren. Replace. Ther. 2019, 5, 28. [Google Scholar] [CrossRef]
- Ashby, D.; Borman, N.; Burton, J.; Corbett, R.; Davenport, A.; Farrington, K.; Flowers, K.; Fotheringham, J.; Fox, R.N.A.; Franklin, G.; et al. Renal Association Clinical Practice Guideline on Haemodialysis. BMC Nephrol. 2019, 20, 379. [Google Scholar] [CrossRef]
- Farrington, K.; Covic, A.; Nistor, I.; Aucella, F.; Clyne, N.; De Vos, L.; Findlay, A.; Fouque, D.; Grodzicki, T.; Iyasere, O.; et al. Clinical Practice Guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR < 45 mL/min/1.73 m2): A summary document from the European Renal Best Practice Group. Nephrol. Dial. Transpl. 2017, 32, 9–16. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef]
- Liu, Y.M.; Coresh, J.; Eustace, J.A.; Longenecker, J.C.; Jaar, B.; Fink, N.E.; Tracy, R.P.; Powe, N.R.; Klag, M.J. Association between cholesterol level and mortality in—Role of inflammation dialysis patients and malnutrition. JAMA—J. Am. Med. Assoc. 2004, 291, 451–459. [Google Scholar] [CrossRef]
- Kopple, J.D.; Zhu, X.F.; Lew, N.L.; Lowrie, E.G. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999, 56, 1136–1148. [Google Scholar] [CrossRef]


| Characteristic | Overall (N = 166) | Catheter Group | p | |
|---|---|---|---|---|
| Patent Group (N = 103) | Nonpatent Group (N = 63) | |||
| Age (median [range] years) | 82.0 [75–97] | 81.0 [75–97] | 83.0 [75–92] | 0.016 |
| Sex [n (%)] | ||||
| Men | 74 (44.6) | 55 (53.4) | 19 (30.2) | 0.003 |
| Women | 92 (55.4) | 48 (46.6) | 44 (69.8) | |
| Comorbidities [n (%)] | ||||
| Diabetes mellitus | 93 (56.0) | 62 (60.2) | 31 (49.2) | 0.168 |
| Hypertension | 141 (84.9) | 85 (82.5) | 56 (88.9) | 0.269 |
| Heart failure | 67 (40.4) | 42 (40.8) | 25 (39.7) | 0.890 |
| Ischemic heart disease | 45 (27.1) | 32 (31.1) | 13 (20.6) | 0.144 |
| Atrial fibrillation | 41 (24.7) | 23 (22.3) | 18 (28.6) | 0.369 |
| Cerebral infarction | 22 (13.3) | 17 (16.5) | 5 (7.9) | 0.115 |
| Liver cirrhosis | 11 (6.6) | 3 (2.9) | 8 (12.7) | 0.014 |
| Medication [n (%)] | ||||
| Aspirin | 50 (30.1) | 28 (27.2) | 22 (34.9) | 0.295 |
| Clopidogrel | 36 (21.7) | 23 (22.3) | 13 (20.6) | 0.799 |
| Warfarin | 9 (5.4) | 6 (5.8) | 3 (4.8) | 0.771 |
| Cilostazol | 15 (9.0) | 12 (11.7) | 3 (4.8) | 0.135 |
| NOAC | 12 (7.2) | 11 (10.7) | 1 (1.6) | 0.028 |
| Statin | 70 (42.2) | 48 (46.6) | 22 (34.9) | 0.141 |
| Laboratory data (median [range]) | ||||
| Hemoglobin (g/dL) | 9.7 [5.5–13.2] | 9.7 [5.5–13.2] | 9.5 [6.3–11.9] | 0.685 |
| Total lymphocyte count (103/µL) | 1100.0 [120–4620] | 1160.0 [120–3700] | 940.0 [130–4620] | 0.233 |
| Platelet (000s) | 166.0 [12–520] | 165.0 [12–445] | 140.0 [20–520] | 0.224 |
| CRP (mg/dL) | 2.5 [0.1–40] | 1.4 [0.1–40.0] | 6.8 [0.2–37.8] | <0.001 |
| Total protein (g/dL) | 6.0 [3.9–7.9] | 6.1 [4.2–7.6] | 5.9 [3.9–7.9] | 0.340 |
| Albumin (g/dL) | 3.0 [1.6–4.3] | 3.1 [2.2–4.3] | 2.8 [1.6–4.1] | <0.001 |
| BUN (mg/dL) | 51.9 [13.0–184.5] | 53.3 [13.0–184.5] | 51.0 [17.4–118.0] | 0.708 |
| Creatinine (mg/dL) | 4.3 [0.5–16.2] | 4.5 [0.7–16.2] | 3.8 [0.5–10.8] | 0.051 |
| Total cholesterol (mg/dL) | 127.5 [50–290] | 129.0 [50–239] | 121.0 [50–290] | 0.526 |
| Total calcium (mg/dL) | 8.0 [5.5–11.1] | 8.0 [5.5–10.2] | 8.0 [5.7–11.1] | 0.292 |
| Phosphorus (mg/dL) | 4.0 [0.3 –10.7] | 4.1 [1.2–10.7] | 3.9 [0.3–7.4] | 0.175 |
| Sodium (mEq/L) | 138.0 [121–153.6] | 138.0 [126–147.0] | 137.8 [121–153.6] | 0.821 |
| Potassium (mEq/L) | 4.4 [2.8–7.8] | 4.5 [2.8–7.0] | 4.3 [2.8–7.8] | |
| Body composition (median [range]) | ||||
| ECW (L) | 13.9 [7.8–31.1] | 14.4 [7.8–31.1] | 13.7 [8.4–23.7] | 0.137 |
| ICW (L) | 13.3 [7.9–30.4] | 14.3 [7.9–30.4] | 11.9 [8.2–20.7] | 0.012 |
| TBW (L) | 27.3 [16.3–53.5] | 28.4 [16.3–53.5] | 26.0 [18.3–43.7] | 0.028 |
| ECW/ICW ratio | 1.10 [0.53–1.90] | 1.07 [0.53–1.90] | 1.13 [0.75–1.56] | 0.101 |
| LTI (kg/m2) | 10.3 [5.3–26.1] | 11.3 [5.8–26.1] | 9.7 [5.3–23.3] | 0.089 |
| FTI (kg/m2) | 10.8 [0.1–33.8] | 10.2 [0.1–33.8] | 11.2 [4.3–25.0] | 0.180 |
| LTM (kg) | 25.16 [2.2–69.4] | 27.6 [2.2–69.4] | 23.2 [13.8–49.4] | 0.097 |
| ATM (kg) | 24.9 [0.2–56.2] | 24.2 [0.2–52.2] | 25.1 [2.4–56.2] | 0.162 |
| FAT (kg) | 19.0 [0.3–41.3] | 18.3 [0.3–38.4] | 19.1 [7.2–41.3] | 0.133 |
| Dry weight (kg) | 52.8 [24.8–92.5] | 53.3 [24.8–89.3] | 51.5 [32.8–92.5] | 0.641 |
| BMI (kg/m2) | 23.6 [15.1–44.3] | 23.5 [15.1–34.2] | 23.8 [15.3–44.3] | 0.255 |
| Information on initiating hemodialysis (median [range]) | ||||
| Ultrafiltration volume (L) | 1.5 [0.2–4.0] | 1.5 [0.3–4.0] | 1.5 [0.2–4.0] | 0.268 |
| Further management [n (%)] | ||||
| VA creation | 121 (72.9) | 89 (86.4) | 32 (50.8) | <0.001 |
| VA abandonment | 45 (27.1) | 14 (13.6) | 31 (50.2) | |
| Nutrient score (median [range]) | ||||
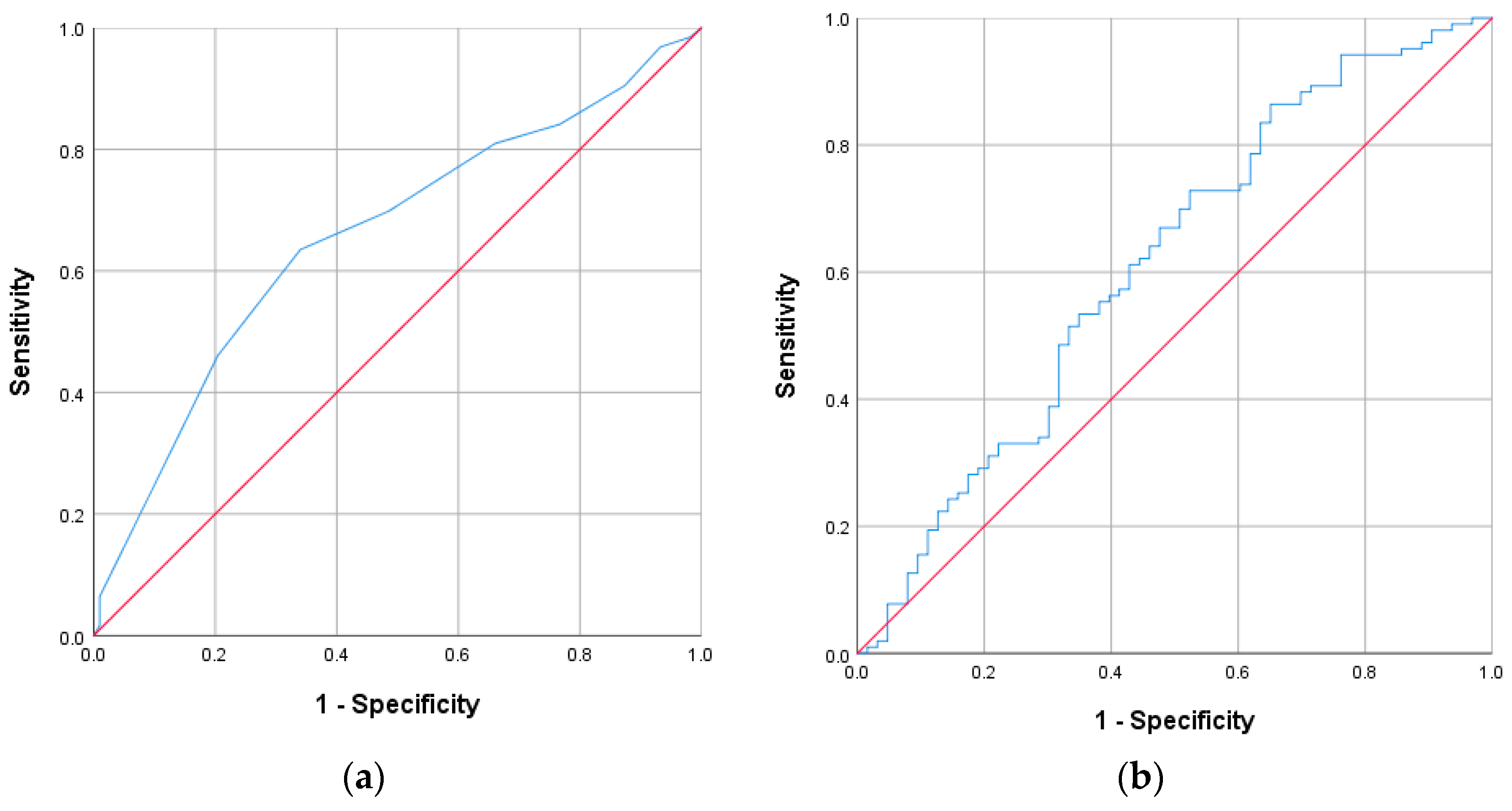
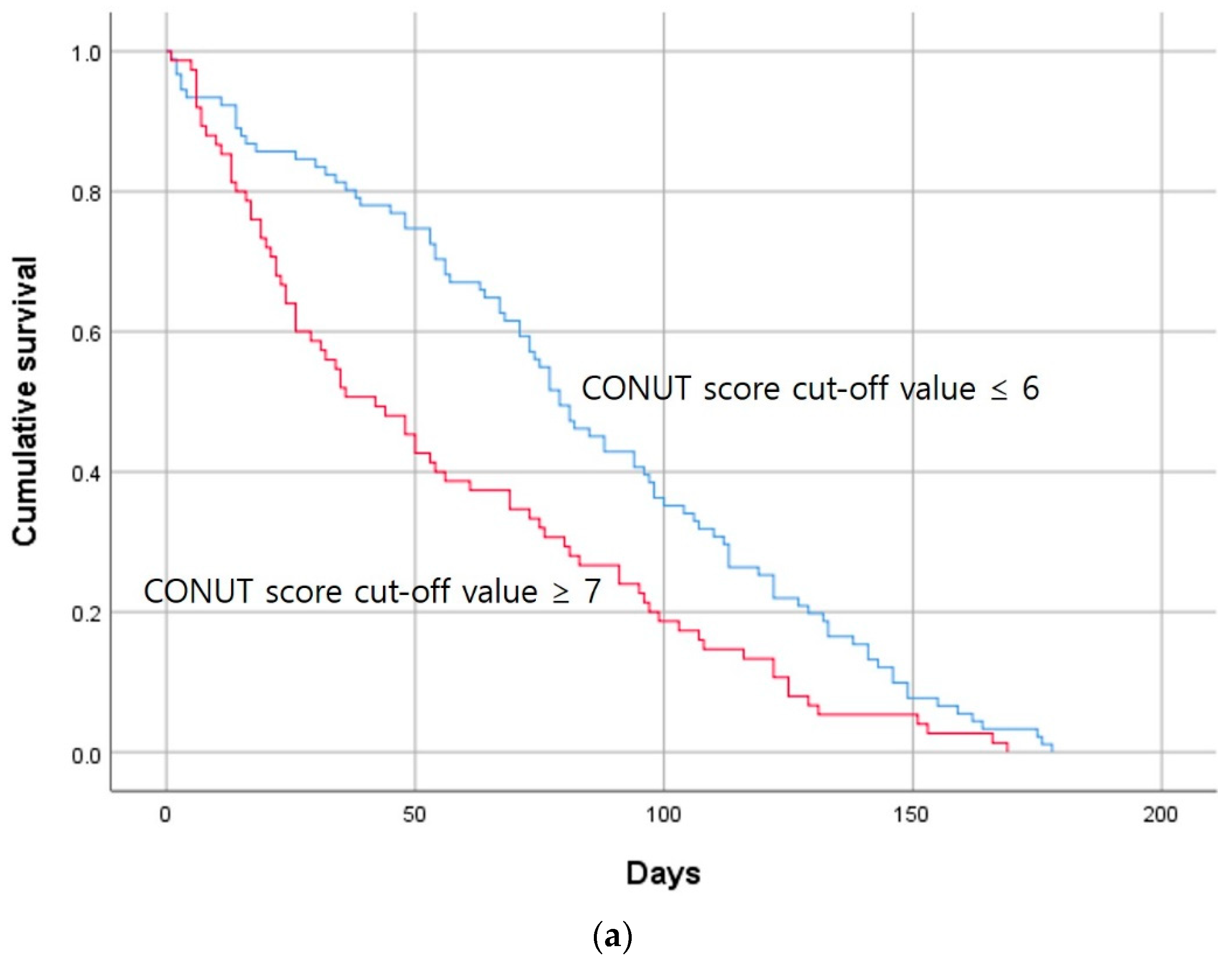
| CONUT score | 6.0 [0–12] | 5.0 [0–12] | 7.0 [0–12] | 0.001 |
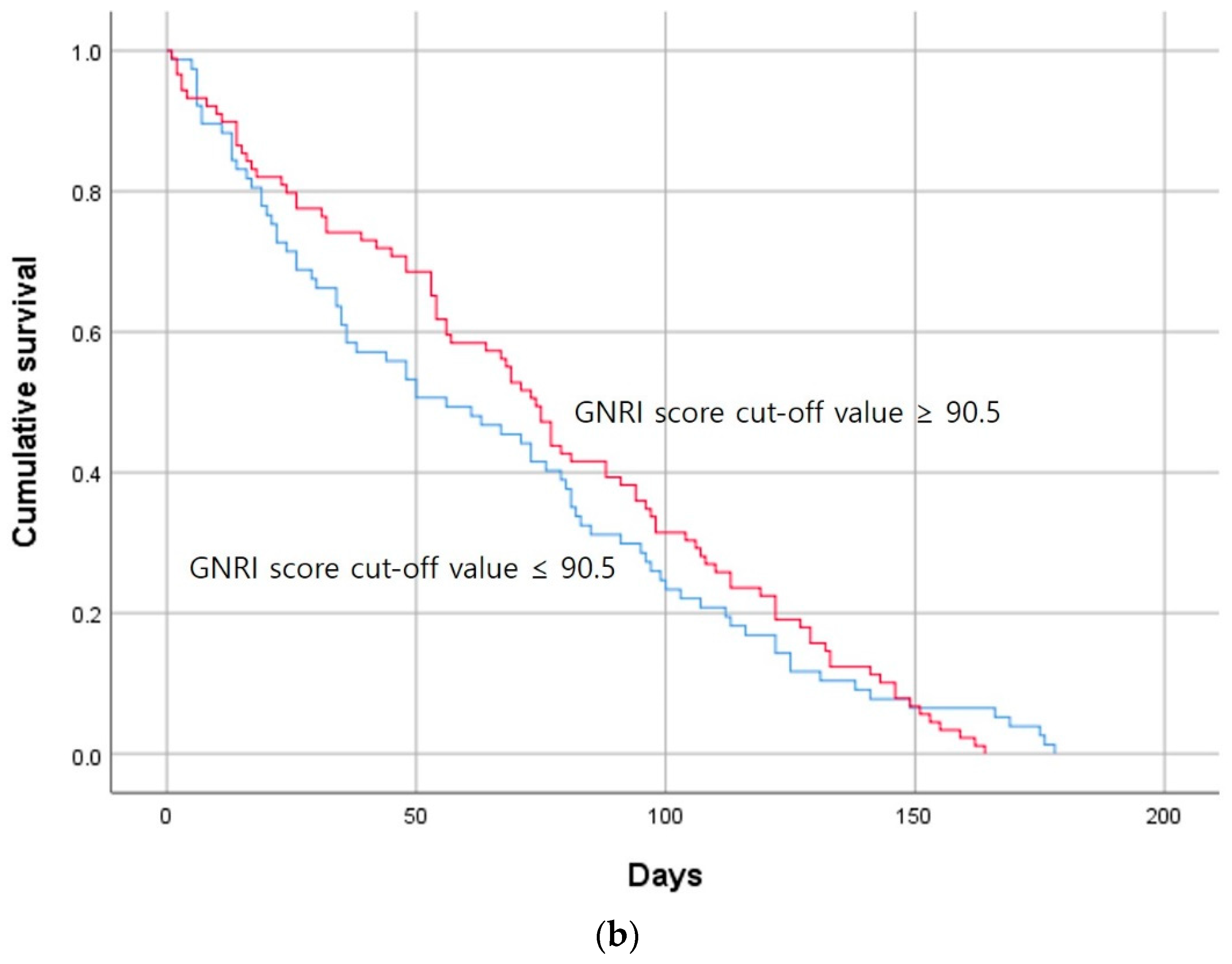
| GNRI score | 91.5 [64.6–125.0] | 93.7 [68.0–118.7] | 87.8 [64.6–125.0] | 0.018 |
| Permanent catheter use date (median [range]) | 69.0 [1–178] | 88.0 [6–178] | 63.0 [1–141] | <0.001 |
| Hazard Ratio | 95% CI of the Difference | p Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Sex | 0.828 | 0.609 | 1.126 | 0.229 |
| Age | 1.048 | 1.013 | 1.085 | 0.007 |
| DM | 0.876 | 0.642 | 1.194 | 0.402 |
| HTN | 0.901 | 0.587 | 1.383 | 0.634 |
| BMI | 1.029 | 0.996 | 1.063 | 0.084 |
| ECW | 0.997 | 0.961 | 1.034 | 0.871 |
| ICW | 0.980 | 0.943 | 1.019 | 0.311 |
| TBW | 0.994 | 0.974 | 1.014 | 0.528 |
| E/I ratio | 1.869 | 0.905 | 3.861 | 0.091 |
| LTI | 0.971 | 0.930 | 1.015 | 0.197 |
| FTI | 1.030 | 0.999 | 1.062 | 0.055 |
| LTM | 0.995 | 0.981 | 1.009 | 0.494 |
| ATM | 1.011 | 0.997 | 1.025 | 0.121 |
| FAT | 1.015 | 0.996 | 1.035 | 0.115 |
| Hemoglobin | 0.956 | 0.856 | 1.067 | 0.421 |
| Total lymphocyte count | 1.000 | 1.000 | 1.000 | 0.131 |
| Platelet | 0.998 | 0.996 | 1.000 | 0.071 |
| CRP | 1.049 | 1.027 | 1.071 | <0.001 |
| Total protein | 0.962 | 0.785 | 1.179 | 0.708 |
| Albumin | 0.664 | 0.485 | 0.910 | 0.011 |
| BUN | 0.999 | 0.993 | 1.006 | 0.865 |
| Creatinine | 0.959 | 0.900 | 1.021 | 0.190 |
| Total cholesterol | 0.999 | 0.995 | 1.002 | 0.467 |
| Total calcium | 1.042 | 0.869 | 1.249 | 0.656 |
| Phosphorus | 0.930 | 0.827 | 1.045 | 0.221 |
| Sodium | 1.001 | 0.968 | 1.036 | 0.936 |
| Potassium | 0.965 | 0.805 | 1.156 | 0.698 |
| CONUT score | 1.655 | 1.213 | 2.259 | 0.001 |
| GNRI score | 0.927 | 0.678 | 1.267 | 0.634 |
| Hazard Ratio | 95% CI of the Difference | p Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Model 1 | 1.655 | 1.213 | 2.259 | 0.001 |
| Model 2 | 1.665 | 1.215 | 2.281 | 0.002 |
| Model 3 | 1.690 | 1.232 | 2.319 | 0.001 |
| Model 4 | 1.714 | 1.234 | 2.381 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.J.; Hwang, Y.; Jeon, J.W.; Kim, H.R.; Han, S.; Park, H.; Lee, E.J.; Ham, Y.R.; Na, K.R.; Park, H.; et al. Relationship between Permanent Catheter Patency and Nutrient Score in Patients Aged >75 Years Requiring Renal Replacement Therapy. J. Clin. Med. 2024, 13, 1562. https://doi.org/10.3390/jcm13061562
Kim MJ, Hwang Y, Jeon JW, Kim HR, Han S, Park H, Lee EJ, Ham YR, Na KR, Park H, et al. Relationship between Permanent Catheter Patency and Nutrient Score in Patients Aged >75 Years Requiring Renal Replacement Therapy. Journal of Clinical Medicine. 2024; 13(6):1562. https://doi.org/10.3390/jcm13061562
Chicago/Turabian StyleKim, Moo Jun, Yunkyeong Hwang, Jae Wan Jeon, Hae Ri Kim, Suyeon Han, Heewon Park, Eu Jin Lee, Young Rok Ham, Ki Ryang Na, Hyerim Park, and et al. 2024. "Relationship between Permanent Catheter Patency and Nutrient Score in Patients Aged >75 Years Requiring Renal Replacement Therapy" Journal of Clinical Medicine 13, no. 6: 1562. https://doi.org/10.3390/jcm13061562
APA StyleKim, M. J., Hwang, Y., Jeon, J. W., Kim, H. R., Han, S., Park, H., Lee, E. J., Ham, Y. R., Na, K. R., Park, H., & Choi, D. E. (2024). Relationship between Permanent Catheter Patency and Nutrient Score in Patients Aged >75 Years Requiring Renal Replacement Therapy. Journal of Clinical Medicine, 13(6), 1562. https://doi.org/10.3390/jcm13061562






