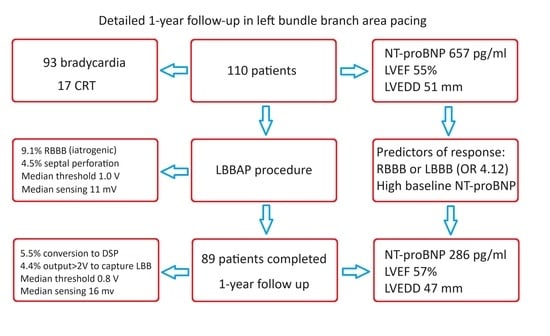
Detailed One-Year Follow-Up in Left Bundle Branch Area Pacing: Echocardiography, Natriuretic Peptide, Electrical Parameters and Complications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. LBBAP Lead Implantation
2.3. Device Programming
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Completion of Follow-Up Period
3.3. NT-proBNP Serum Level
3.4. Echocardiographic Parameters
3.5. Factors Correlated with Greater Improvement
3.6. Predictors of Response in Heart Function
3.7. Electrical Parameters
3.8. Complications
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heckman, L.I.B.; Luermans, J.G.L.M.; Jastrzębski, M.; Weijs, B.; Van Stipdonk, A.M.W.; Westra, S.; Uijl, D.D.; Linz, D.; Mafi-Rad, M.; Prinzen, F.W.; et al. A single-centre prospective evaluation of left bundle branch area pacemaker implantation characteristics. Neth. Heart J. 2022, 30, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Raymond-Paquin, A.; Verma, A.; Kolominsky, J.; Sanchez-Somonte, P.; Gul, E.E.; Pillai, A.; Kron, J.; Shepard, R.; Kalahasty, G.; Tsang, B.; et al. Left bundle branch area pacing in patients with atrioventricular conduction disease: A prospective multicenter study. Heart Rhythm. 2022, 19, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.K.; Master, V.M.; Terricabras, M.; Chiocchini, A.; Garg, A.; Kron, J.; Shepard, R.; Kalahasty, G.; Azizi, Z.; Tsang, B.; et al. Initial Experience, Safety, and Feasibility of Left Bundle Branch Area Pacing: A Multicenter Prospective Study. JACC Clin Electrophysiol. 2020, 6, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Fularz, M.; Mitkowski, P. The impact of operator experience on the success rate, time aspects, and electrocardiographic features in left bundle branch area pacing. Kardiol. Pol. 2023, 81, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębski, M.; Kiełbasa, G.; Cano, O.; Curila, K.; Heckman, L.; De Pooter, J.; Chovanec, M.; Rademakers, L.; Huybrechts, W.; Grieco, D.; et al. Left bundle branch area pacing outcomes: The multicentre European MELOS study. Eur. Heart J. 2022, 43, 4161–4173. [Google Scholar] [CrossRef] [PubMed]
- Heckman, L.I.B.; Luermans, J.G.L.M.; Curila, K.; Van Stipdonk, A.M.; Westra, S.; Smisek, R.; Prinzen, F.W.; Vernooy, K. Comparing Ventricular Synchrony in Left Bundle Branch and Left Ventricular Septal Pacing in Pacemaker Patients. J. Clin. Med. 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; Patel, N.R.; Ravi, V.; Zalavadia, D.V.; Dommaraju, S.; Garg, V.; Larsen, T.R.; Naperkowski, A.M.; Wasserlauf, J.; Krishnan, K.; et al. Clinical outcomes of left bundle branch area pacing compared to right ventricular pacing: Results from the Geisinger-Rush Conduction System Pacing Registry. Heart Rhythm. 2022, 19, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Q.; Wu, J.; Zhang, Y.; You, L.; Xie, R. Left bundle branch area pacing improving the left atrial outcomes in pace-dependent patients compared with right ventricular outflow tract septal pacing. Clin. Cardiol. 2023, 47, e24185. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.I.; Kim, T.H.; Cho, Y.H.; Bae, J.; Ahn, J.; Jang, J.Y.; Park, Y.W.; Kwak, C.H. Left bundle branch area pacing in mildly reduced heart failure: A systematic literature review and meta-analysis. Clin. Cardiol. 2023, 46, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, A.; Kiełbasa, G.; Moskal, P.; Ostrowska, A.; Bednarski, A.; Sondej, T.; Kusiak, A.; Rajzer, M.; Jastrzębski, M. Left bundle branch area pacing prevents pacing induced cardiomyopathy in long-term observation. Pacing Clin. Electrophysiol. 2023, 46, 629–638. [Google Scholar] [CrossRef]
- Su, L.; Wang, S.; Wu, S.; Xu, L.; Huang, Z.; Chen, X.; Zheng, R.; Jiang, L.; Ellenbogen, K.A.; Whinnett, Z.I.; et al. Long-Term Safety and Feasibility of Left Bundle Branch Pacing in a Large Single-Center Study. Circ. Arrhythm. Electrophysiol. 2021, 14, e009261. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Sharma, P.S.; Cano, Ó.; Ponnusamy, S.S.; Herweg, B.; Zanon, F.; Jastrzebski, M.; Zou, J.; Chelu, M.G.; Vernooy, K.; et al. Comparison of Left Bundle Branch Area Pacing and Biventricular Pacing in Candidates for Resynchronization Therapy. J. Am. Coll. Cardiol. 2023, 82, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Ponnusamy, S.; Cano, Ó.; Sharma, P.S.; Naperkowski, A.; Subsposh, F.A.; Moskal, P.; Bednarek, A.; Forno, A.R.D.; Young, W.; et al. Left Bundle Branch Area Pacing for Cardiac Resynchronization Therapy: Results From the International LBBAP Collaborative Study Group. JACC Clin. Electrophysiol. 2021, 7, 135–147. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Cano, O.; Ponnusamy, S.S.; Molina-Lerma, M.; Chan, J.Y.; Padala, S.K.; Sharma, P.S.; Whinnett, Z.I.; Herweg, B.; Upadhyay, G.A.; et al. Left bundle branch area pacing in patients with heart failure and right bundle branch block: Results from International LBBAP Collaborative-Study Group. Heart Rhythm. O2 2022, 3, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Lin, M.; Sun, Y.; Zhang, J.; Jiang, H.; Fu, G.; Zhang, W.; Wang, M. The specific value of upgrading to left bundle branch area pacing in patients with pacing-induced cardiomyopathy or non-pacing-induced cardiomyopathy related upgrade status: A retrospective study. Pacing Clin. Electrophysiol. 2023, 46, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębski, M.; Moskal, P.; Huybrechts, W.; Curila, K.; Sreekumar, P.; Rademakers, L.M.; Ponnusamy, S.S.; Herweg, B.; Sharma, P.S.; Bednarek, A.; et al. Left bundle branch-optimized cardiac resynchronization therapy (LOT-CRT): Results from an international LBBAP collaborative study group. Heart Rhythm. 2022, 19, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Z.; Zu, L.; Cheng, L.; Su, R.; Wang, X.; Liang, Z.; Chen, J.; Hang, F.; Du, J.; et al. Simplifying Physiological Left Bundle Branch Area Pacing Using a New Nine-Partition Method. Can. J. Cardiol. 2021, 37, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębski, M.; Kiełbasa, G.; Moskal, P.; Bednarek, A.; Kusiak, A.; Sondej, T.; Bednarski, A.; Rajzer, M.; Vijayaraman, P. Fixation beats: A novel marker for reaching the left bundle branch area during deep septal lead implantation. Heart Rhythm. 2021, 18, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Burri, H.; Jastrzebski, M.; Cano, Ó.; Čurila, K.; de Pooter, J.; Huang, W.; Israel, C.; Joza, J.; Romero, J.; Vernooy, K.; et al. EHRA clinical consensus statement on conduction system pacing implantation: Endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS). Europace 2023, 25, 1208–1236. [Google Scholar] [CrossRef] [PubMed]
- Somma, V.; Ha, F.J.; Palmer, S.; Mohamed, U.; Agarwal, S. Pacing-induced cardiomyopathy: A systematic review and meta-analysis of definition, prevalence, risk factors, and management. Heart Rhythm. 2023, 20, 282–290. [Google Scholar] [CrossRef] [PubMed]
| General | |
| Age, years, mean (SD) | 74.9 (8.7) |
| Female sex, n (%) | 43 (39.1) |
| Primary implantation, n (%) | 101 (91.8) |
| Pacing indications | |
| Atrioventricular block, n (%) | 56 (50.9) |
| Sick sinus syndrome, n (%) | 37 (33.6) |
| Cardiac resynchronization therapy, n (%) | 17 (15.5) |
| Device type | |
| LBBAP, n (%) | 98 (89.1) |
| LBBAP + ICD, n (%) | 10 (9.1) |
| LBBAP + RVP, n (%) | 2 (1.8) |
| Comorbidities | |
| Heart failure, n (%) | 33 (30) |
| Coronary artery disease, n (%) | 29 (26.4) |
| Hypertension, n (%) | 89 (80.1) |
| Atrial fibrillation, n (%) | 47 (42.7) |
| Diabetes, n (%) | 37 (28.2) |
| Chronic kidney disease, n (%) | 15 (13.6) |
| COPD, n (%) | 10 (9.1) |
| Parameter | NT-proBNP, pg/mL, Median (IQR) | LVEF, %, Median (IQR) | LVEDD, mm, Median (IQR) | LAD, mm, Mean (SD) | |
|---|---|---|---|---|---|
| Entire group | Pre-implant | 657 (250–1432) | 55 (49–60) | 51 (47–54) | 42.1 (5.3) |
| After 12 months | 286 (162–806) | 57 (52–62) | 47 (44–51) | 38.7 (5.7) | |
| p-value | <0.001 | 0.02 | <0.001 | <0.001 | |
| Bradycardia | Pre-implant | 450 (228–1193) | 58 (55–60) | 50 (46–53) | 41.4 (4.8) |
| After 12 months | 267 (149–719) | 59 (54–64) | 46 (43–51) | 38.4 (5.0) | |
| p-value | 0.008 | 0.24 | <0.001 | <0.001 | |
| CRT | Pre-implant | 1397 (1258–3934) | 30 (25–34) | 57 (55–70) | 46.0 (6.1) |
| After 12 months | 731 (286–1744) | 40 (32–43) | 57 (48–65) | 40.4 (8.4) | |
| p-value | 0.02 | 0.008 | 0.18 | 0.002 | |
| Parameter | Delta a NT-proBNP, pg/mL, Median (IQR) | Delta a LVEF, %, Mean (SD) | Delta a LVEDD, mm, Mean (SD) | Delta a LAD, mm, Mean (SD) |
|---|---|---|---|---|
| Bradycardia | −77 (−473 to 73) | 1.1 (7.4) | −2.4 (4.7) | −2.9 (3.4) |
| CRT | −990 (−2607 to −485) | 8.8 (9.1) | −2.6 (6.7) | −5.6 (4.8) |
| p-value | 0.006 | 0.005 | 0.79 | 0.04 |
| Factor | Parameters with Greater Improvement (Statistics) |
|---|---|
| CRT indication | NT-proBNP; LVEF; LAD (see in Table 3) |
| Lower baseline LVEF | NT-proBNP (rs = 0.25, p = 0.04); LVEF (rs = 0.58, p <0.001) |
| Presence of native LBBB | LVEF (increase: 6.1% vs. 1.2%, p = 0.03); LAD (decrease: 5.2 mm vs. 2.9 mm, p = 0.03) |
| Presence of native RBBB | LVEDD (decrease: 5.3 vs. 1.8 mm, p = 0.02 in entire group; 5.2 vs. 2.0 mm, p = 0.03 in patients with LVEF > 50%) |
| Higher baseline NT-proBNP | NT-proBNP (rs = 0.57, p < 0.001) |
| Higher baseline LVEDD | LVEDD (rs = 0.35, p = 0.002) |
| Higher baseline LAD | LAD (r = 0.24; p = 0.04) |
| Wider native QRS | LAD (rs = 0.23, p = 0.047) |
| More basal location of a tip | LVEF (r = 0.69; p = 0.02)—in CRT indication |
| Confirmed LBB capture | LVEDD (decrease 3.5 mm vs. increase 0.9 mm; p = 0.02)—in bradycardia patients with percentage of VP > 40% |
| Greater QRS narrowing | LAD (r = 0.25; p = 0.03) |
| Shorter paced V6-RWPT | LVEDD (r = 0.34; p = 0.03)—in bradycardia patients with percentage of VP > 40% |
| Parameter | NT-proBNP, pg/mL, Median (IQR) | LVEF, %, Median (IQR) | LVEDD, mm, Median (IQR) | LAD, mm, Mean (SD) | Number of Patients with VP > 40%, (%) | |
|---|---|---|---|---|---|---|
| Narrow native QRS (<120 ms) | Pre-implant | 532 (167–1536) | 59 (55–61) | 49 (46–53) | 42.4 (4.7) | 19 out of 40 (47.5) |
| After 12 months | 342 (156–849) | 59 (56–62) | 47 (44–51) | 40.1 (5.0) | ||
| p-value | 0.12 | 0.57 | 0.08 | <0.001 | ||
| Broad native QRS (≥120 ms) | Pre-implant | 414 (247–991) | 55 (54–60) | 51 (47–53) | 40.3 (4.8) | 29 out of 36 (80.6) |
| After 12 months | 244 (137–541) | 57 (52–65) | 46 (43–50) | 36.6 (4.4) | ||
| p-value | 0.03 | 0.35 | 0.001 | <0.001 | ||
| RBBB a | Pre-implant | 364 (243–1193) | 58 (55–60) | 51 (48–54) | 39.3 (4.5) | 13 out of 18 (72.2) |
| After 12 months | 206 (126–304) | 60 (56–65) | 45 (44–48) | 35.1 (4.2) | ||
| p-value | 0.02 | 0.32 | 0.004 | <0.001 | ||
| Factor | Univariable Analysis OR (95% CI); p-Value | Multivariable Analysis OR (95% CI); p-Value |
|---|---|---|
| CRT indication | 6.61 (1.35–32.37); 0.02 | - |
| Baseline NT-proBNP (per 500 pg/mL) | 1.31 (1.01–1.10); 0.01 | 1.37 (1.08–1.74); 0.009 |
| Baseline LVEF (per 5%) | 0.69 (0.54–0.89); 0.004 | - |
| Baseline LVEDD (per 5 mm) | 1.29 (0.91–1.82); 0.15 | - |
| Baseline LAD (per 5 mm) | 1.54 (0.97–2.44); 0.07 | - |
| Presence of native LBBB | 2.51 (0.83–7.64); 0.10 | - |
| Presence of native RBBB | 2.2 (0.72–6.78); 0.17 | - |
| Presence of native NICD | 0.84 (0.17–4.00); 0.82 | - |
| Presence of RBBB or LBBB | 3.19 (1.27–8.04); 0.01 | 4.12 (1.46–11.65); 0.008 |
| Native QRS duration (per 10 ms) | 1.18 (1.01–1.37); 0.03 | - |
| Delta QRS a (per 10 ms) | 0.83 (0.69–0.99); 0.04 | - |
| Parameter | Pacing Threshold, V, Median (IQR) | Sensing, mV, Median (IQR) | Impedance, Ohms, Mean (SD) |
|---|---|---|---|
| Intraprocedural | 1 (0.8–1.2) | 11 (7–14) | 881 (187) |
| 1 day after procedure | 0.4 (0.4–0.5) | 16 (8–22) | 664 (117) |
| 1 month after procedure | 0.6 (0.5–0.8) | 16 (11–22) | 603 (105) |
| 6 months after procedure | 0.6 (0.6–0.9) | 16 (11–22) | 592 (108) |
| 1 year after procedure | 0.8 (0.6–0.9) | 16 (11–16) | 589 (96) |
| p-value | <0.001 a | <0.001 b | <0.001 c |
| Acute Phase a | Follow-Up b | ||
|---|---|---|---|
| Major complications (life-threatening or requiring intervention) | |||
| Dislodgement of non-LBBAP lead | 2.7% | Twiddler syndrome | 1.1% |
| Pneumothorax | 0.9% | Infectious endocarditis | 1.1% |
| Pericardial effusion | 0.9% | Pericardial effusion | 1.1% |
| Pacing-induced cardiomyopathy | 1.1% | ||
| Patients with any major complication | 4.5% | Patients with any major complication | 4.4% |
| Minor complications and other conditions that required attention | |||
| Intraprocedural iatrogenic RBBB | 9.1% | Persistent iatrogenic RBBB | 3.3% |
| Intraprocedural perforation to the left ventricle cavity | 4.5% | Conversion to DSP (loss of LBBAP) | 5.5% |
| Mild dysfunction of non-LBBAP lead | 3.3% | ||
| High output (>2 V) required to maintain non-selective LBB capture | 4.4% | ||
| LBBAP lead capture threshold > 2 V | 1.1% | ||
| Patients with any of aforementioned | 13.6% | Patients with any of aforementioned | 15.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fularz, M.; Mitkowski, P. Detailed One-Year Follow-Up in Left Bundle Branch Area Pacing: Echocardiography, Natriuretic Peptide, Electrical Parameters and Complications. J. Clin. Med. 2024, 13, 1532. https://doi.org/10.3390/jcm13061532
Fularz M, Mitkowski P. Detailed One-Year Follow-Up in Left Bundle Branch Area Pacing: Echocardiography, Natriuretic Peptide, Electrical Parameters and Complications. Journal of Clinical Medicine. 2024; 13(6):1532. https://doi.org/10.3390/jcm13061532
Chicago/Turabian StyleFularz, Maciej, and Przemysław Mitkowski. 2024. "Detailed One-Year Follow-Up in Left Bundle Branch Area Pacing: Echocardiography, Natriuretic Peptide, Electrical Parameters and Complications" Journal of Clinical Medicine 13, no. 6: 1532. https://doi.org/10.3390/jcm13061532
APA StyleFularz, M., & Mitkowski, P. (2024). Detailed One-Year Follow-Up in Left Bundle Branch Area Pacing: Echocardiography, Natriuretic Peptide, Electrical Parameters and Complications. Journal of Clinical Medicine, 13(6), 1532. https://doi.org/10.3390/jcm13061532