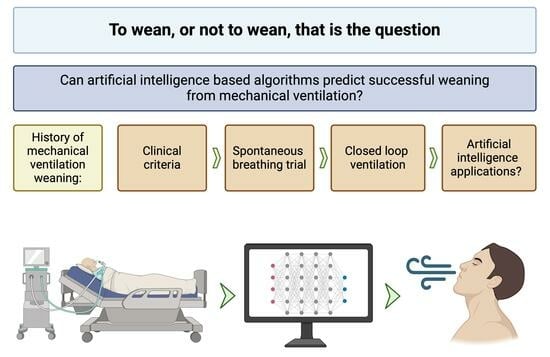
Using Artificial Intelligence to Predict Mechanical Ventilation Weaning Success in Patients with Respiratory Failure, Including Those with Acute Respiratory Distress Syndrome
Abstract
1. Introduction
2. Traditional Approaches to Weaning
3. Role of Predictive Modeling in Weaning
4. AI/ML in MV Weaning Prediction
4.1. Introduction to AI/ML
4.2. AI/ML MV Weaning Models
| Study | AI/ML Model | Type of Patients/Cases | Number of Participants in Training Phase | Factors That Correlate with Outcome | AUROC of Training Phase | External Validation? | AUROC of Validation Phase | Clinical Use | Model Effect on Clinical Practice |
|---|---|---|---|---|---|---|---|---|---|
| Liao et al. [44] | XGBoost | Chronic ventilation | 670 | PS, FiO2, T-piece trial, mPaw, PEEP, APACHE II score | 0.868 | No | NA | Yes |
|
| Menguy et al. [9] | ZGPD | Medical ICU | 108 | BMI, heart rate variation, P0.1 | 83% global performance | No | NA | No | NA |
| Chang et al. [38] | Naïve Bayes classifier algorithm | Surgical patients (lung resection) | 709 | Estimated postoperative lung function, exercise load | 0.912 | No | NA | Yes |
|
| Chen et al. [6] | LR | Cardiac ICU | 1439 | Expiratory minute ventilation, expiratory tidal volume, ventilation rate set, heart rate, peak pressure, pH, age | 0.86 | No | NA | No | NA |
| Jia et al. [2] | CNN | ICU | 2299 | Richmond Agitation-Sedation Scale, SBT, FiO2, ventilator mode, PIP, PEEP | 0.94 | No | NA | No | NA |
| Kim et al. [23] | GF-GAT | ICU | 832 | NA | 0.8 | Yes (temporal validation) | 0.8 | No | NA |
| Kim et al. [4] | VC | ICU | 23,242 | Lactate concentration, age, presence of cerebrovascular disease, and BUN | 0.861 | No | NA | No | NA |
| Otaguro et al. [56] | LightGBM | ICU | 117 | Duration of MV, age, PEEP, LDH, APTT, GCS, BUN, A-a gradient, CRP | 0.95 | No | NA | No | NA |
| Liu et al. [22] | LightGBM | ICU | 5873 in 1st stage 4172 in 2nd stage | First stage—FiO2, APACH II score, PEEP, mPaw Second stage—n of SBT, n of suctions | 1st stage 0.860 2nd stage 0.923 | No | NA | Yes |
|
| Hsieh et al. [50] | ANN | ICU | 3602 | Therapeutic intervention scoring system score, chronic hemodialysis, RSBI, heart rate, P/F ratio, MEP | 0.85 | No | NA | No | NA |
| Pai et al. [57] | XGBoost | ICU | 5940 | GCS, RASS, urine output, injected fluids, Ppeak, and MAP | 0.921 | No | NA | No | NA |
| Chen et al. [58] | lightGBM | ICU | 3636 | Duration [hours] of ventilation, PaO2, PaCO2 | 0.8198 | No | NA | No | NA |
| Zhao et al. [59] | CatBoost | ICU | 16,189 | Duration of ventilation, PS level | 0.835 | Yes | 0.803 | No | NA |
| Fleuren et al. [60] | XGBoost | ICU COVID-19 patients | 883 | FiO2, Vt, duration of controlled ventilation, CRP, WBC, PLT, BMI | 0.7 | No | NA | No | NA |
| Fabregat et al. [54] | SVM | ICU | 697 | Δt, GCS, BMI, ROX, and Pplat | 0.983 | No | NA | No | NA |
| Kuo et al. [52] | ANN | ICU | 121 | Mean inspiratory time, mean expiratory time, mean Vt, and mean breathing frequency | NA | Yes | 0.83 | No | NA |
| Huang et al. [55] | RF | ICU | 233 | FiO2, Ppeak, PEEP, Pmean, RR, Vt | 0.976 | No | NA | No | NA |
| Hsieh et al. [51] | ANN | ICU | 3602 | Older age, APACHE II, and comorbidities (mainly DM) | 0.849–0.942 | No | NA | No | NA |
5. Discussion
5.1. Limitations of Artificial Intelligence
5.2. Ethical Considerations
5.3. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinto, J.; González, H.; Arizmendi, C.; González, H.; Muñoz, Y.; Giraldo, B.F. Analysis of the Cardiorespiratory Pattern of Patients Undergoing Weaning Using Artificial Intelligence. Int. J. Environ. Res. Public Health 2023, 20, 4430. [Google Scholar] [CrossRef]
- Jia, Y.; Kaul, C.; Lawton, T.; Murray-Smith, R.; Habli, I. Prediction of Weaning from Mechanical Ventilation Using Convolutional Neural Networks. Artif. Intell. Med. 2021, 117, 102087. [Google Scholar] [CrossRef]
- Kwong, M.T.; Colopy, G.W.; Weber, A.M.; Ercole, A.; Bergmann, J.H.M. The Efficacy and Effectiveness of Machine Learning for Weaning in Mechanically Ventilated Patients at the Intensive Care Unit: A Systematic Review. Bio-Des. Manuf. 2019, 2, 31–40. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.K.; Kim, H.; Jung, H.; Koh, S.; Kim, Y.; Yoon, D.; Yi, H.; Kim, H.-J. Machine Learning Algorithms Predict Successful Weaning From Mechanical Ventilation Before Intubation: Retrospective Analysis From the Medical Information Mart for Intensive Care IV Database. JMIR Form. Res. 2023, 7, e44763. [Google Scholar] [CrossRef]
- Cheng, K.-H.; Tan, M.-C.; Chang, Y.-J.; Lin, C.-W.; Lin, Y.-H.; Chang, T.-M.; Kuo, L.-K. The Feasibility of a Machine Learning Approach in Predicting Successful Ventilator Mode Shifting for Adult Patients in the Medical Intensive Care Unit. Medicina 2022, 58, 360. [Google Scholar] [CrossRef]
- Chen, W.-T.; Huang, H.-L.; Ko, P.-S.; Su, W.; Kao, C.-C.; Su, S.-L. A Simple Algorithm Using Ventilator Parameters to Predict Successfully Rapid Weaning Program in Cardiac Intensive Care Unit Patients. JPM 2022, 12, 501. [Google Scholar] [CrossRef]
- Mancebo, J. Weaning from Mechanical Ventilation. Eur. Respir. J. 1996, 9, 1923–1931. [Google Scholar] [CrossRef]
- Boles, J.-M.; Bion, J.; Connors, A.; Herridge, M.; Marsh, B.; Melot, C.; Pearl, R.; Silverman, H.; Stanchina, M.; Vieillard-Baron, A.; et al. Weaning from Mechanical Ventilation. Eur. Respir. J. 2007, 29, 1033–1056. [Google Scholar] [CrossRef]
- Menguy, J.; De Longeaux, K.; Bodenes, L.; Hourmant, B.; L’Her, E. Defining Predictors for Successful Mechanical Ventilation Weaning, Using a Data-Mining Process and Artificial Intelligence. Sci. Rep. 2023, 13, 20483. [Google Scholar] [CrossRef]
- Torrini, F.; Gendreau, S.; Morel, J.; Carteaux, G.; Thille, A.W.; Antonelli, M.; Mekontso Dessap, A. Prediction of Extubation Outcome in Critically Ill Patients: A Systematic Review and Meta-Analysis. Crit. Care 2021, 25, 391. [Google Scholar] [CrossRef]
- Ferguson, N.D.; Fan, E.; Camporota, L.; Antonelli, M.; Anzueto, A.; Beale, R.; Brochard, L.; Brower, R.; Esteban, A.; Gattinoni, L.; et al. The Berlin Definition of ARDS: An Expanded Rationale, Justification, and Supplementary Material. Intensive Care Med. 2012, 38, 1573–1582. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Huang, T.; Yang, R.; Cheng, H.; Wang, H.; Yin, H.; Lyu, J. Developing an Explainable Machine Learning Model to Predict the Mechanical Ventilation Duration of Patients with ARDS in Intensive Care Units. Heart Lung 2023, 58, 74–81. [Google Scholar] [CrossRef]
- Passos, A.M.B.; Valente, B.C.S.; Machado, M.D.; Borges, M.R.; Paula, S.G.; Geraldo, L.-F.; Adib, K.R.; Daniel, D.; Carlos, M.; Roselaine, O.; et al. Effect of a Protective-Ventilation Strategy on Mortality in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 1998, 338, 347–354. [Google Scholar] [CrossRef]
- Thompson, B.T.; Bernard, G.R. ARDS Network (NHLBI) Studies: Successes and Challenges in ARDS Clinical Research. Crit. Care Clin. 2011, 27, 459–468. [Google Scholar] [CrossRef][Green Version]
- Amato, M.B.P.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.V.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.; Mercat, A.; et al. Driving Pressure and Survival in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef]
- Gattinoni, L.; Caironi, P.; Pelosi, P.; Goodman, L.R. What Has Computed Tomography Taught Us about the Acute Respiratory Distress Syndrome? Am. J. Respir. Crit. Care Med. 2001, 164, 1701–1711. [Google Scholar] [CrossRef]
- The National Heart, Lung, and Blood Institute PETAL Clinical Trials Network. Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2019, 380, 1997–2008. [Google Scholar] [CrossRef]
- Guérin, C.; Reignier, J.; Richard, J.-C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone Positioning in Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef]
- Wawrzeniak, I.C.; Regina Rios Vieira, S.; Almeida Victorino, J. Weaning from Mechanical Ventilation in ARDS: Aspects to Think about for Better Understanding, Evaluation, and Management. BioMed Res. Int. 2018, 2018, 5423639. [Google Scholar] [CrossRef]
- Marshall, G.; Sanguinet, J.; Batra, S.; Foreman, M.J.; Peruchini, J.; Lopez, S.; De Guzman, R.; Rivera, N.; Hightower, T.; Malone, C.; et al. Association between Ventilator-Associated Events and Implementation of Acute Respiratory Distress Syndrome (ARDS) Ventilator Weaning Protocol. Am. J. Infect. Control 2023, 51, 1321–1323. [Google Scholar] [CrossRef]
- Liu, C.-F.; Hung, C.-M.; Ko, S.-C.; Cheng, K.-C.; Chao, C.-M.; Sung, M.-I.; Hsing, S.-C.; Wang, J.-J.; Chen, C.-J.; Lai, C.-C.; et al. An Artificial Intelligence System to Predict the Optimal Timing for Mechanical Ventilation Weaning for Intensive Care Unit Patients: A Two-Stage Prediction Approach. Front. Med. 2022, 9, 935366. [Google Scholar] [CrossRef]
- Kim, G.-H.; Kim, J.-W.; Kim, K.H.; Kang, H.; Moon, J.Y.; Shin, Y.M.; Park, S. FT-GAT: Graph Neural Network for Predicting Spontaneous Breathing Trial Success in Patients with Mechanical Ventilation. Comput. Methods Programs Biomed. 2023, 240, 107673. [Google Scholar] [CrossRef]
- Kondili, E.; Makris, D.; Georgopoulos, D.; Rovina, N.; Kotanidou, A.; Koutsoukou, A. COVID-19 ARDS: Points to Be Considered in Mechanical Ventilation and Weaning. JPM 2021, 11, 1109. [Google Scholar] [CrossRef]
- Girard, T.D.; Bernard, G.R. Mechanical Ventilation in ARDS. Chest 2007, 131, 921–929. [Google Scholar] [CrossRef]
- Kallet, R.H.; Zhuo, H.; Yip, V.; Gomez, A.; Lipnick, M.S. Spontaneous Breathing Trials and Conservative Sedation Practices Reduce Mechanical Ventilation Duration in Subjects With ARDS. Respir. Care 2018, 63, 1–10. [Google Scholar] [CrossRef]
- Adaptive Support Ventilation: Evidence-based benefits|Hamilton Medical AG. Available online: https://www.hamilton-medical.com/dam/jcr:3346351e-5c5a-4f53-92ae-bbfd2f269e8a/ASV-benefits-white-paper-en-689214.00.pdf (accessed on 26 January 2024).
- Company Profile|Dräger. Available online: https://www.draeger.com/en_sea/About-Draeger (accessed on 26 January 2024).
- Örmgård, C. Inventing Therapies that Save Lives. Available online: https://news.getinge.com/uk/inventing-therapies-that-save-lives (accessed on 26 January 2024).
- Puritan BennettTM PAV+TM Software|Medtronic. Available online: https://www.medtronic.com/covidien/en-us/products/mechanical-ventilation/software/puritan-bennett-pav-plus-software.html (accessed on 26 January 2024).
- Lellouche, F.; Brochard, L. Advanced Closed Loops during Mechanical Ventilation (PAV, NAVA, ASV, SmartCare). Best Pract. Res. Clin. Anaesthesiol. 2009, 23, 81–93. [Google Scholar] [CrossRef]
- Botta, M.; Tsonas, A.M.; Sinnige, J.S.; De Bie, A.J.R.; Bindels, A.J.G.H.; Ball, L.; Battaglini, D.; Brunetti, I.; Buiteman–Kruizinga, L.A.; Van Der Heiden, P.L.J.; et al. Effect of Automated Closed-Loop Ventilation versus convenTional VEntilation on Duration and Quality of Ventilation in Critically Ill Patients (ACTiVE)–Study Protocol of a Randomized Clinical Trial. Trials 2022, 23, 348. [Google Scholar] [CrossRef]
- Kampolis, C.F.; Mermiri, M.; Mavrovounis, G.; Koutsoukou, A.; Loukeri, A.A.; Pantazopoulos, I. Comparison of Advanced Closed-Loop Ventilation Modes with Pressure Support Ventilation for Weaning from Mechanical Ventilation in Adults: A Systematic Review and Meta-Analysis. J. Crit. Care 2022, 68, 1–9. [Google Scholar] [CrossRef]
- Chen, S.-C.; Cheng, W.-E.; Shih, C.-M.; Chu, C.-C.; Liu, C.-J. Adaptive Support Ventilation: Review of the Literature and Clinical Applications. Crit Care 2008, 19, 465–471. [Google Scholar]
- Fernández, J.; Miguelena, D.; Mulett, H.; Godoy, J.; Martinón-Torres, F. Adaptive Support Ventilation: State of the Art Review. Indian J. Crit. Care Med. 2013, 17, 16–22. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, X.; Jiang, Y.; Guo, Y.; Zhang, W.; He, H.; Yin, Y. Comparison of Clinical Outcomes in Critical Patients Undergoing Different Mechanical Ventilation Modes: A Systematic Review and Network Meta-Analysis. Front. Med. 2023, 10, 1159567. [Google Scholar] [CrossRef]
- Linton, D.M.; Renov, G.; Lafair, J.; Vasiliev, L.; Friedman, G. Adaptive Support Ventilation as the Sole Mode of Ventilatory Support in Chronically Ventilated Patients. Crit. Care Resusc. 2006, 8, 11–14. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Hung, K.-C.; Wang, L.-K.; Yu, C.-H.; Chen, C.-K.; Tay, H.-T.; Wang, J.-J.; Liu, C.-F. A Real-Time Artificial Intelligence-Assisted System to Predict Weaning from Ventilator Immediately after Lung Resection Surgery. Int. J. Environ. Res. Public Health 2021, 18, 2713. [Google Scholar] [CrossRef]
- Khanzode, K.C.A. Advantages and Disadvantages of Artificial Intelligence and Machine Learning: A Literature Review. Int. J. Libr. Inf. Sci. (IJLIS) 2020, 9, 3. [Google Scholar]
- Khalpey, Z.; Wilson, P.; Suri, Y.; Culbert, H.; Deckwa, J.; Khalpey, A.; Rozell, B. Leveling Up: A Review of Machine Learning Models in the Cardiac ICU. Am. J. Med. 2023, 136, 979–984. [Google Scholar] [CrossRef]
- Yu, C.; Liu, J.; Zhao, H. Inverse Reinforcement Learning for Intelligent Mechanical Ventilation and Sedative Dosing in Intensive Care Units. BMC Med. Inform. Decis. Mak. 2019, 19, 57. [Google Scholar] [CrossRef]
- Stokes, K.; Castaldo, R.; Federici, C.; Pagliara, S.; Maccaro, A.; Cappuccio, F.; Fico, G.; Salvatore, M.; Franzese, M.; Pecchia, L. The Use of Artificial Intelligence Systems in Diagnosis of Pneumonia via Signs and Symptoms: A Systematic Review. Biomed. Signal Process. Control 2022, 72, 103325. [Google Scholar] [CrossRef]
- Yuan, K.-C.; Tsai, L.-W.; Lee, K.-H.; Cheng, Y.-W.; Hsu, S.-C.; Lo, Y.-S.; Chen, R.-J. The Development an Artificial Intelligence Algorithm for Early Sepsis Diagnosis in the Intensive Care Unit. Int. J. Med. Inform. 2020, 141, 104176. [Google Scholar] [CrossRef]
- Liao, K.-M.; Ko, S.-C.; Liu, C.-F.; Cheng, K.-C.; Chen, C.-M.; Sung, M.-I.; Hsing, S.-C.; Chen, C.-J. Development of an Interactive AI System for the Optimal Timing Prediction of Successful Weaning from Mechanical Ventilation for Patients in Respiratory Care Centers. Diagnostics 2022, 12, 975. [Google Scholar] [CrossRef]
- Topol, E.J. High-Performance Medicine: The Convergence of Human and Artificial Intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Hartmann, T.; Passauer, J.; Kneilling, M.; Volc, S.; Hartmann, J.; Schmidberger, L. Basic Principles of Artificial Intelligence in Dermatology Explained Using Melanoma. J. Dtsch. Dermatol. Ges. 2024, 1–9. [Google Scholar] [CrossRef]
- Handelman, G.S.; Kok, H.K.; Chandra, R.V.; Razavi, A.H.; Lee, M.J.; Asadi, H. eDoctor: Machine Learning and the Future of Medicine. J. Intern. Med. 2018, 284, 603–619. [Google Scholar] [CrossRef]
- Greco, M.; Caruso, P.F.; Cecconi, M. Artificial Intelligence in the Intensive Care Unit. Semin. Respir. Crit. Care Med. 2021, 42, 002–009. [Google Scholar] [CrossRef]
- Badillo, S.; Banfai, B.; Birzele, F.; Davydov, I.I.; Hutchinson, L.; Kam-Thong, T.; Siebourg-Polster, J.; Steiert, B.; Zhang, J.D. An Introduction to Machine Learning. Clin. Pharma Ther. 2020, 107, 871–885. [Google Scholar] [CrossRef]
- Hsieh, M.-H.; Hsieh, M.-J.; Chen, C.-M.; Hsieh, C.-C.; Chao, C.-M.; Lai, C.-C. An Artificial Neural Network Model for Predicting Successful Extubation in Intensive Care Units. J. Clin. Med. 2018, 7, 240. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Hsieh, M.J.; Cheng, A.-C.; Chen, C.-M.; Hsieh, C.-C.; Chao, C.-M.; Lai, C.-C.; Cheng, K.-C.; Chou, W. Predicting Weaning Difficulty for Planned Extubation Patients with an Artificial Neural Network. Medicine 2019, 98, e17392. [Google Scholar] [CrossRef]
- Kuo, H.-J.; Chiu, H.-W.; Lee, C.-N.; Chen, T.-T.; Chang, C.-C.; Bien, M.-Y. Improvement in the Prediction of Ventilator Weaning Outcomes by an Artificial Neural Network in a Medical ICU. Respir. Care 2015, 60, 1560–1569. [Google Scholar] [CrossRef]
- da Silva, R.B.; Neves, V.R.; Montarroyos, U.R.; Silveira, M.S.; Sobral Filho, D.C. Heart Rate Variability as a Predictor of Mechanical Ventilation Weaning Outcomes. Heart Lung J. Crit. Care 2023, 59, 33–36. [Google Scholar] [CrossRef]
- Fabregat, A.; Magret, M.; Ferré, J.A.; Vernet, A.; Guasch, N.; Rodríguez, A.; Gómez, J.; Bodí, M. A Machine Learning Decision-Making Tool for Extubation in Intensive Care Unit Patients. Comput. Methods Programs Biomed. 2021, 200, 105869. [Google Scholar] [CrossRef]
- Huang, K.-Y.; Hsu, Y.-L.; Chen, H.-C.; Horng, M.-H.; Chung, C.-L.; Lin, C.-H.; Xu, J.-L.; Hou, M.-H. Developing a Machine-Learning Model for Real-Time Prediction of Successful Extubation in Mechanically Ventilated Patients Using Time-Series Ventilator-Derived Parameters. Front. Med. 2023, 10, 1167445. [Google Scholar] [CrossRef]
- Otaguro, T.; Tanaka, H.; Igarashi, Y.; Tagami, T.; Masuno, T.; Yokobori, S.; Matsumoto, H.; Ohwada, H.; Yokota, H. Machine Learning for Prediction of Successful Extubation of Mechanical Ventilated Patients in an Intensive Care Unit: A Retrospective Observational Study. J. Nippon Med. School 2021, 88, 408–417. [Google Scholar] [CrossRef]
- Pai, K.-C.; Su, S.-A.; Chan, M.-C.; Wu, C.-L.; Chao, W.-C. Explainable Machine Learning Approach to Predict Extubation in Critically Ill Ventilated Patients: A Retrospective Study in Central Taiwan. BMC Anesthesiol. 2022, 22, 351. [Google Scholar] [CrossRef]
- Chen, T.; Xu, J.; Ying, H.; Chen, X.; Feng, R.; Fang, X.; Gao, H.; Wu, J. Prediction of Extubation Failure for Intensive Care Unit Patients Using Light Gradient Boosting Machine. IEEE Access 2019, 7, 150960–150968. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Wang, H.; Luo, J.C.; Luo, M.H.; Liu, L.P.; Yu, S.J.; Liu, K.; Zhang, Y.J.; Sun, P.; Tu, G.W.; et al. Development and Validation of a Machine-Learning Model for Prediction of Extubation Failure in Intensive Care Units. Front. Med. 2021, 8, 676343. [Google Scholar] [CrossRef]
- Fleuren, L.M.; Dam, T.A.; Tonutti, M.; de Bruin, D.P.; Lalisang, R.C.A.; Gommers, D.; Cremer, O.L.; Bosman, R.J.; Rigter, S.; Wils, E.J.; et al. Predictors for Extubation Failure in COVID-19 Patients Using a Machine Learning Approach. Crit. Care 2021, 25, 448. [Google Scholar] [CrossRef]
- Hashimoto, D.A.; Witkowski, E.; Gao, L.; Meireles, O.; Rosman, G. Artificial Intelligence in Anesthesiology. Anesthesiology 2020, 132, 379–394. [Google Scholar] [CrossRef]
- Wachter, S.; Mittelstadt, B.; Russell, C. Counterfactual Explanations Without Opening the Black Box: Automated Decisions and the GDPR. SSRN J. 2017, 31, 841–887. [Google Scholar] [CrossRef]
- Murphy, K.; Di Ruggiero, E.; Upshur, R.; Willison, D.J.; Malhotra, N.; Cai, J.C.; Malhotra, N.; Lui, V.; Gibson, J. Artificial Intelligence for Good Health: A Scoping Review of the Ethics Literature. BMC Med. Ethics 2021, 22, 14. [Google Scholar] [CrossRef]
| Proprietary Ventilation Mode | Company | Country of Origin |
|---|---|---|
| ASV | Hamilton Medical | Switzerland |
| SmartCare | Dräger | Germany |
| NAVA | GETINGE | Sweden |
| PAV | Medtronic | USA |
| Machine Learning Method | Description | Usage | Strengths | Weaknesses |
|---|---|---|---|---|
| Linear regression | Estimates the linear relationship between dependent and independent variables. | Regression | Simple model to implement and understand. | Outliers can affect the regression. Assumes independence between attributes. Not a complete description of relationships among variables. |
| Logistic regression | Sigmoid function to assign a probability for an event. | Classification | Simple model. Makes no assumptions about distributions. Measures the predictor’s coefficient size and its direction of association. Interpret model coefficients as indicators of feature importance. | Less suitable for complex situations. Assumption of linearity between the dependent and independent variables. Can only be used to predict discrete functions. Cannot solve non-linear problems because it has a linear decision surface. |
| Decision trees | A flowchart-like tree structure that splits the training data into subsets based on the values of the attributes until a stopping criterion is met. | Classification and regression | Simple to understand and interpret. Deals with unbalanced data. Variable Selection—can identify the most significant variables and the relation between variables. Handles missing values. Non-parametric nature—keeps the model simple and less prone to significant errors. | Overfitting. Sensitive to small variations and alterations in the input data that can drastically change the structure of the decision tree. Biased learning—without proper parameter tuning, decision trees can create bias if some classes dominate. |
| Random forest | Combination of many overfitted algorithm-generated deep decision trees outputs in order to deal with the bias and overfitting of a single decision tree. | Classification and regression | Reduced risk of overfitting Flexibility—can handle both regression and classification. Can determine feature importance. | Time-consuming process. Requires more resources. More complex model to interpret. |
| Boosting | A strong classifier model built by a series of weak classifiers in order to decrease the error. Each weak classifier tries to correct the errors present in the previous classifier. This continues till the training dataset is predicted correctly or the maximum number of models are added. Gradient Boosting—boosting technique that builds a final model from the sum of several weak learning algorithms that were trained on the same dataset (numerical or categorical data). XGBoost (v2.0.3) —a regularized version of the t gradient boosting technique. Outperforms the standard gradient boosting method in speed, and the dataset can contain both numerical and categorical variables. | Classification and regression | Improved Accuracy—reduced risk for bias. Reduce the risk of overfitting—reweighting the inputs that are classified wrongly. Better handling of imbalanced data—focusing more on the data points that are misclassified. Better Interpretability—breaking the model decision process into multiple processes. | Vulnerable to the outliers. Difficult to use boosting algorithms for real-time applications. Computationally expensive for large datasets. |
| K-nearest neighbors | The algorithm places new, unclassified data near its K-nearest neighbors in a field of labeled data points. | Classification | Interpretable results since it relies on proximity calculations. Simple method. | No learning steps. Does not identify the most relevant features to place new data—influenced by noise. Choosing the right K. Computing and time-consuming. |
| Neural networks | Model that mimics the complex functions of the human brain—activation of a group of neurons from one neural layer activates other neurons in the next layer until the output layer gives the final interpretation of the model. | Classification | Adaptability—the model can adapt to new situations and learn from data. Pattern recognition—excel in audio and image identification, as well as natural language processing. Parallel processing—can process numerous processes at once, improving computational efficiency. Non-linearity—can use non-linear activation functions in order to model and comprehend complicated data. | Computational intensity—training demands a lot of computing power. Black box Nature—difficult to understand how decisions were made. Overfitting. Large training datasets. |
| Support vector machines | Algorithm used for linear or nonlinear classification or regression. Algorithms find the maximum separating hyperplane in an N-dimensional space between the different classes available in the target feature. | Classification and regression | Perform well with high-dimensional data. Require less memory and use it effectively. Perform well when there is a large gap between classes. | Long training period— not practical for large datasets. Inability to handle overlapping classes and noise. Poorly performed when the number of features for each data point is greater than the number of training data samples. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stivi, T.; Padawer, D.; Dirini, N.; Nachshon, A.; Batzofin, B.M.; Ledot, S. Using Artificial Intelligence to Predict Mechanical Ventilation Weaning Success in Patients with Respiratory Failure, Including Those with Acute Respiratory Distress Syndrome. J. Clin. Med. 2024, 13, 1505. https://doi.org/10.3390/jcm13051505
Stivi T, Padawer D, Dirini N, Nachshon A, Batzofin BM, Ledot S. Using Artificial Intelligence to Predict Mechanical Ventilation Weaning Success in Patients with Respiratory Failure, Including Those with Acute Respiratory Distress Syndrome. Journal of Clinical Medicine. 2024; 13(5):1505. https://doi.org/10.3390/jcm13051505
Chicago/Turabian StyleStivi, Tamar, Dan Padawer, Noor Dirini, Akiva Nachshon, Baruch M. Batzofin, and Stephane Ledot. 2024. "Using Artificial Intelligence to Predict Mechanical Ventilation Weaning Success in Patients with Respiratory Failure, Including Those with Acute Respiratory Distress Syndrome" Journal of Clinical Medicine 13, no. 5: 1505. https://doi.org/10.3390/jcm13051505
APA StyleStivi, T., Padawer, D., Dirini, N., Nachshon, A., Batzofin, B. M., & Ledot, S. (2024). Using Artificial Intelligence to Predict Mechanical Ventilation Weaning Success in Patients with Respiratory Failure, Including Those with Acute Respiratory Distress Syndrome. Journal of Clinical Medicine, 13(5), 1505. https://doi.org/10.3390/jcm13051505