Co-Morbidity Clusters in Post-COVID-19 Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Lung Function
2.3. CAT Score Measurement
2.4. Measurement of Quality of Life
2.5. Statistics
3. Results
3.1. Lung Function Tests
3.2. CAT Score
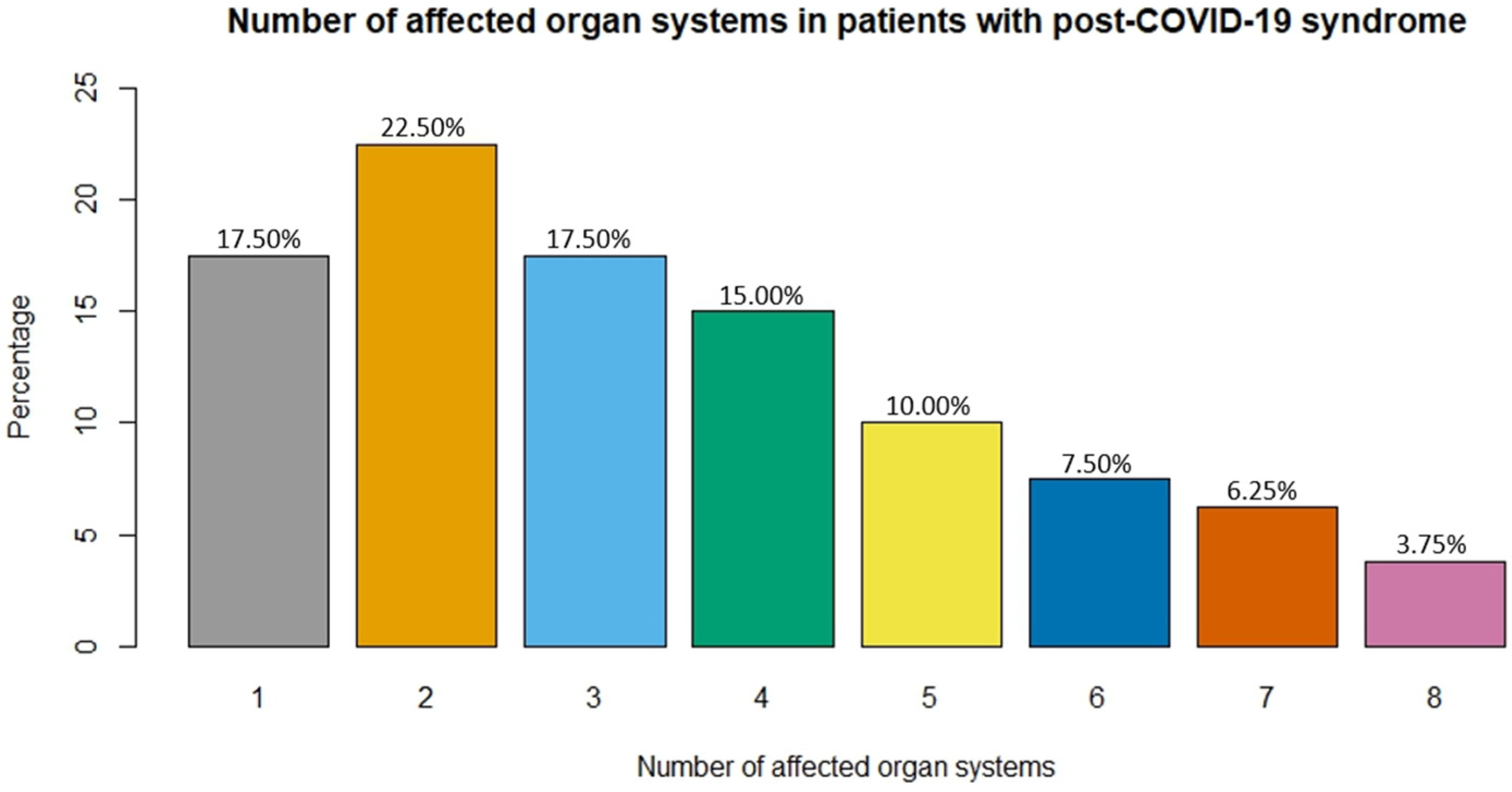
3.3. Post-COVID-19 Symptoms
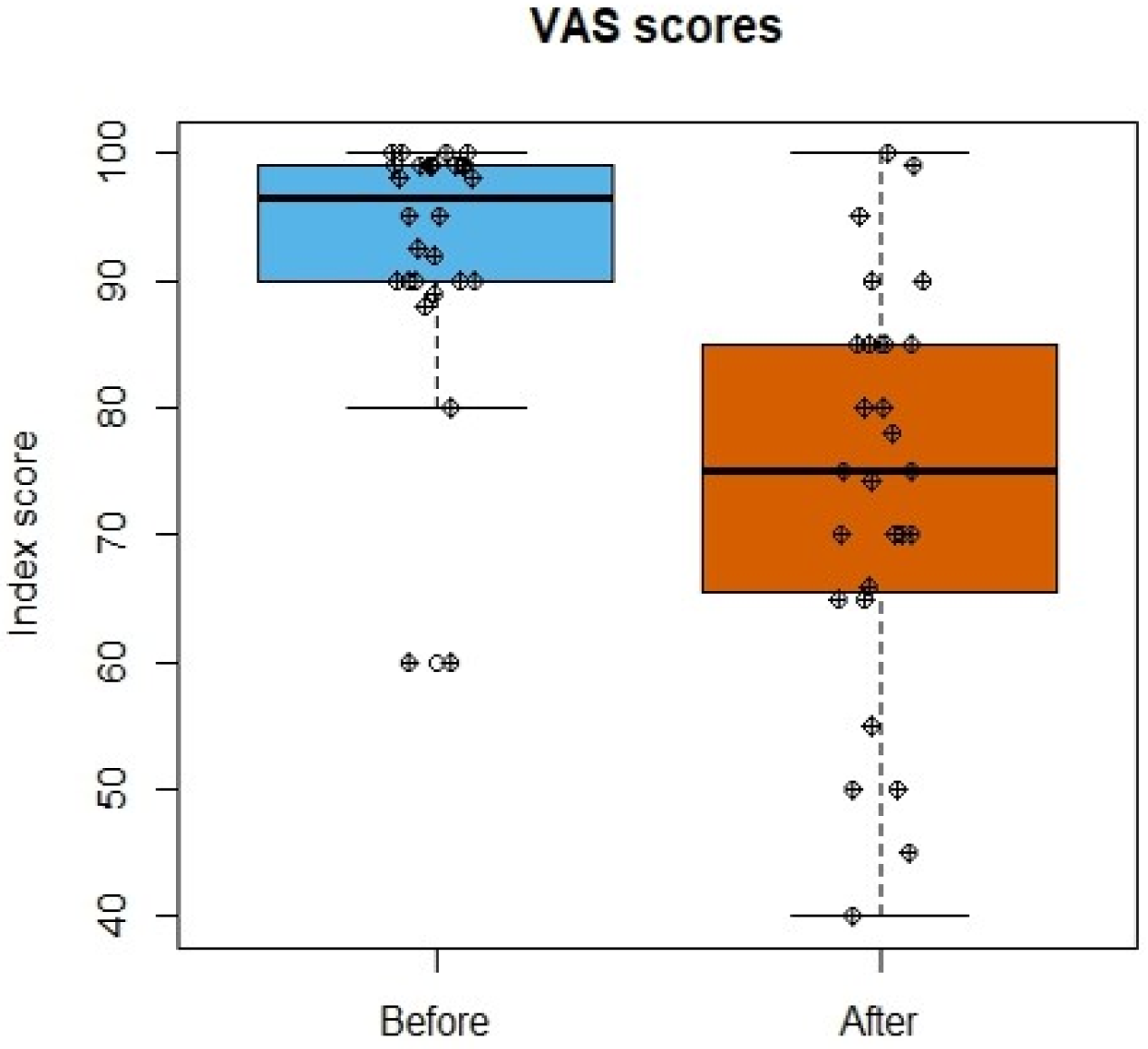
3.4. Life Quality Assessment
3.5. Smoking Habits
3.6. Vaccination Status
3.7. Clinical Severity Spectrum of COVID-19 versus Functional Lung Parameters in Post-COVID-19
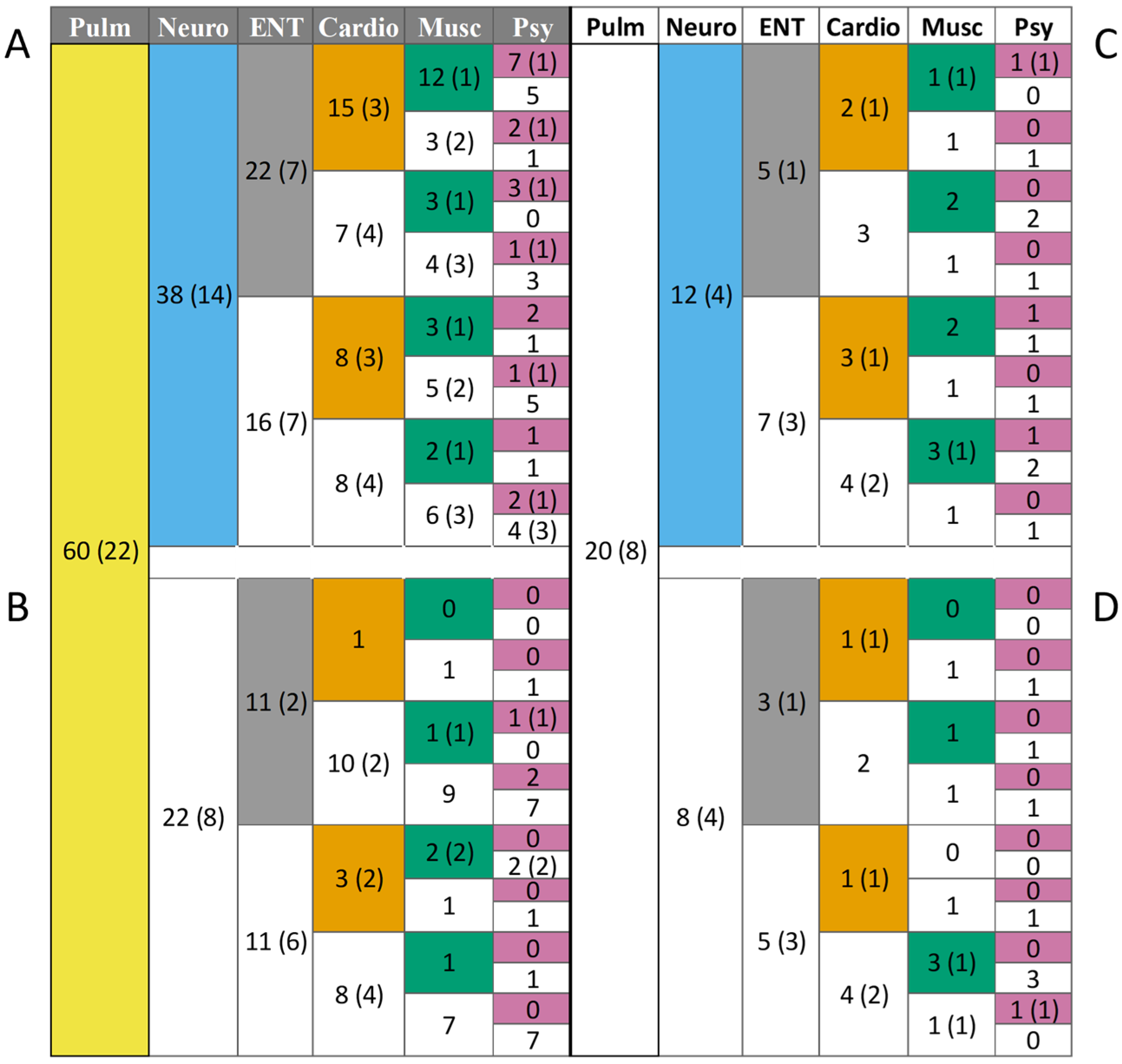
3.8. Co-Morbidities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- World Health Organisation. Post COVID-19 Condition (Long COVID). Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed on 22 December 2023).
- Wise, J. COVID-19: WHO urges action as 17 million long Covid cases are estimated in Europe. BMJ 2022, 378, o2232. [Google Scholar] [CrossRef]
- O’Mahoney, L.L.; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A.; et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. eClinicalMedicine 2023, 55, 101762. [Google Scholar] [CrossRef]
- Dryden, M.; Mudara, C.; Vika, C.; Blumberg, L.; Mayet, N.; Cohen, C.; Tempia, S.; Parker, A.; Nel, J.; Perumal, R.; et al. Post-COVID-19 condition 3 months after hospitalisation with SARS-CoV-2 in South Africa: A prospective cohort study. Lancet Glob. Health 2022, 10, e1247–e1256. [Google Scholar] [CrossRef]
- Dotan, A.; Shoenfeld, Y. Post-COVID syndrome: The aftershock of SARS-CoV-2. Int. J. Infect. Dis. 2022, 114, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.J.; Baldwin, M.M.; Daynes, E.; Evans, R.A.; Greening, N.J.; Jenkins, R.G.; Lone, N.I.; McAuley, H.; Mehta, P.; Newman, J.; et al. Respiratory sequelae of COVID-19: Pulmonary and extrapulmonary origins, and approaches to clinical care and rehabilitation. Lancet Respir. Med. 2023, 11, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Elrobaa, I.H.; New, K.J. COVID-19: Pulmonary and extra pulmonary manifestations. Front. Public Health 2021, 9, 711616. [Google Scholar] [CrossRef]
- Raman, B.; McCracken, C.; Cassar, M.P.; Moss, A.J.; Finnigan, L.; Samat, A.H.; Ogbole, G.; Tunnicliffe, E.M.; Alfaro-Almagro, F.; Menke, R.; et al. Multiorgan MRI findings after hospitalisation with COVID-19 in the UK (C-MORE): A prospective, multicentre, observational cohort study. Lancet Respir. Med. 2023, 11, 1003–1019. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Patel, K.; Pinto, C.; Jaiswal, R.; Tirupathi, R.; Pillai, S.; Patel, U. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)—A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Larson, C.; Hammond, T.C.; Oronsky, A.; Kesari, S.; Lybeck, M.; Reid, T.R. A review of persistent post-COVID syndrome (PPCS). Clin. Rev. Allergy Immunol. 2023, 64, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Porcher, R.; Pane, I.; Ravaud, P. Course of post COVID-19 disease symptoms over time in the ComPaRe long COVID prospective e-cohort. Nat. Commun. 2022, 13, 1812. [Google Scholar] [CrossRef]
- Wolf, S.; Zechmeister-Koss, I.; Erdös, J. Possible long COVID healthcare pathways: A scoping review. BMC Health Serv. Res. 2022, 22, 1076. [Google Scholar] [CrossRef]
- Montani, D.; Savale, L.; Beurnier, A.; Colle, R.; Noël, N.; Pham, T.; Monnet, X.; Humbert, M. Multidisciplinary approach for post-acute COVID-19 syndrome: Time to break down the walls. Eur. Respir. J. 2021, 58, 2101090. [Google Scholar] [CrossRef]
- Jones, P.W.; Tabberer, M.; Chen, W.H. Creating scenarios of the impact of COPD and their relationship to COPD Assessment Test (CAT™) scores. BMC Pulm. Med. 2011, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- GlaxoSmithKline. COPD Assessment Test (CAT). Available online: https://www.catestonline.org/patient-site-test-page-hungarian.html (accessed on 20 December 2023).
- Daynes, E.; Gerlis, C.; Briggs-Price, S.; Jones, P.; Singh, S.J. COPD assessment test for the evaluation of COVID-19 symptoms. Thorax 2020, 76, 185–187. [Google Scholar] [CrossRef] [PubMed]
- GlaxoSmithKline. COPD Assessment Test (CAT). Available online: https://www.catestonline.org/patient-site-test-page-english.html (accessed on 14 February 2024).
- Dolan, P.; Sutton, M. Mapping visual analogue scale health state valuations onto standard gamble and time trade-off values. Soc. Sci. Med. 1997, 44, 1519–1530. [Google Scholar] [CrossRef]
- Shmueli, A. The visual analog rating scale of health-related quality of life: An examination of end-digit preferences. Health Qual. Life Outcomes 2005, 3, 71. [Google Scholar] [CrossRef]
- National Institutes of Health. COVID-19 Treatment Guidelines: Clinical Spectrum of SARS-CoV-2 Infection. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 20 February 2024).
- Yang, X.; Hou, C.; Shen, Y.; Zhang, M.; Zhang, K.; Wang, F.; Liu, Y.; Ma, X.; Cheng, L.; Kang, J.; et al. Two-year health outcomes in hospitalized COVID-19 survivors in China. JAMA Netw. Open 2022, 5, e2231790. [Google Scholar] [CrossRef]
- Torres-Castro, R.; Vasconcello-Castillo, L.; Alsina-Restoy, X.; Solís-Navarro, L.; Burgos, F.; Puppo, H.; Vilaró, J. Respiratory function in patients post-infection by COVID-19: A systematic review and meta-analysis. Pulmonology 2021, 27, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.W.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; Borzakova, S.; et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604–1615. [Google Scholar] [CrossRef]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Abou Shaar, B.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef]
- Long, Q.; Li, J.; Hu, X.; Bai, Y.; Zheng, Y.; Gao, Z. Follow-ups on persistent symptoms and pulmonary function among post-acute COVID-19 patients: A systematic review and meta-analysis. Front. Med. 2021, 8, 702635. [Google Scholar] [CrossRef]
- Mocanu, A.; Lazureanu, V.E.; Laza, R.; Marinescu, A.R.; Cut, T.G.; Sincaru, S.V.; Marza, A.M.; Popescu, I.M.; Herlo, L.F.; Nelson-Twakor, A.; et al. Laboratory Findings and Clinical Outcomes of ICU-Admitted COVID-19 Patients: A Retrospective Assessment of Particularities Identified among Romanian Minorities. J. Pers. Med. 2023, 13, 195. [Google Scholar] [CrossRef]
- Mazzalai, E.; Giannini, D.; Tosti, M.E.; D’Angelo, F.; Declich, S.; Jaljaa, A.; Caminada, S.; Turatto, F.; De Marchi, C.; Gatta, A.; et al. Risk of COVID-19 Severe Outcomes and Mortality in Migrants and Ethnic Minorities Compared to the General Population in the European WHO Region: A Systematic Review. J. Int. Migr. Integr. 2023, 24, 1305–1335. [Google Scholar] [CrossRef]
- Scheibenbogen, C.; Bellmann-Strobl, J.T.; Heindrich, C.; Wittke, K.; Stein, E.; Franke, C.; Prüss, H.; Preßler, H.; Machule, M.L.; Audebert, H.; et al. Fighting Post-COVID and ME/CFS–development of curative therapies. Front. Med. 2023, 10, 1194754. [Google Scholar] [CrossRef]
- Mehandru, S.; Merad, M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022, 23, 194–202. [Google Scholar] [CrossRef]
- Haffke, M.; Freitag, H.; Rudolf, G.; Seifert, M.; Doehner, W.; Scherbakov, N.; Hanitsch, L.; Wittke, K.; Bauer, S.; Konietschke, F.; et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2022, 20, 138. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.A.; Maksoud, R.; Beeraka, N.M.; Madhunapantula, S.V.; Sinelnikov, M.; Nikolenko, V.N.; Neganova, M.E.; Klochkov, S.G.; Kamal, M.A.; Staines, D.R.; et al. Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome. J. Adv. Res. 2022, 40, 179–196. [Google Scholar] [CrossRef]
- Son, K.; Jamil, R.; Chowdhury, A.; Mukherjee, M.; Venegas, C.; Miyasaki, K.; Zhang, K.; Patel, Z.; Salter, B.; Yuen, A.C.; et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. Eur. Respir. J. 2023, 61, 2200970. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.A.; Pearmain, L.; Knight, S.B.; Brand, O.; Morgan, D.J.; Jagger, C.; Harbach, S.; Khan, S.; Shuwa, H.A.; Franklin, M.; et al. Monocyte migration profiles define disease severity in acute COVID-19 and unique features of long COVID. Eur. Respir. J. 2023, 61, 2202226. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.; Efstathiou, C.; Openshaw, P.J. Long COVID: Clues about causes. Eur. Respir. J. 2023, 61, 2300409. [Google Scholar] [CrossRef]
- Miklós, Z.; Horváth, I. The Role of Oxidative Stress and Antioxidants in Cardiovascular Comorbidities in COPD. Antioxidants 2023, 12, 1196. [Google Scholar] [CrossRef]
- Freund, O.; Breslavsky, A.; Fried, S.; Givoli-Vilensky, R.; Cohen-Rubin, S.; Zacks, N.; Kleinhendler, E.; Unterman, A.; Frydman, S.; Wand, O.; et al. Interactions and clinical implications of serological and respiratory variables 3 months after acute COVID-19. Clin. Exp. Med. 2023, 23, 3729–3736. [Google Scholar] [CrossRef]
- Freund, O.; Breslavsky, A.; Givoli-Vilensky, R.; Zacks, N.; Gershman, E.; Melloul, A.; Wand, O.; Bilenko, N.; Bar-Shai, A. Assessment of a close respiratory follow-up schedule at 3 and 6 months after acute COVID-19 and its related investigations. Respir. Med. 2023, 217, 107367. [Google Scholar] [CrossRef]
- de Jong, C.M.; Le, Y.J.; Boon, G.J.; Barco, S.; Klok, F.A.; Siegerink, B. Eight lessons from 2 years of use of the Post-COVID-19 Functional Status scale. Eur. Respir. J. 2023, 61, 2300416. [Google Scholar] [CrossRef]
- Trongtrakul, K.; Tajarernmuang, P.; Limsukon, A.; Theerakittikul, T.; Niyatiwatchanchai, N.; Surasit, K.; Glunriangsang, P.; Liwsrisakun, C.; Bumroongkit, C.; Pothirat, C.; et al. The National Early Warning Score 2 with Age and Body Mass Index (NEWS2 Plus) to Determine Patients with Severe COVID-19 Pneumonia. J. Clin. Med. 2024, 13, 298. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Seo, Y.B.; Seo, J.W.; Lee, J.; Nham, E.; Seong, H.; Yoon, J.G.; Noh, J.Y.; Cheong, H.J.; Kim, W.J.; et al. Effectiveness of Antiviral Therapy on Long COVID: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 7375. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, M.; Tiple, D.; Agostoni, P.; Armocida, B.; Biardi, L.; Bonfigli, A.R.; Campana, A.; Ciardi, M.; Di Marco, F.; Floridia, M.; et al. Italian good practice recommendations on management of persons with Long-COVID. Front. Public Health 2023, 11, 1122141. [Google Scholar] [CrossRef] [PubMed]
- McNarry, M.A.; Berg, R.M.; Shelley, J.; Hudson, J.; Saynor, Z.L.; Duckers, J.; Lewis, K.; Davies, G.A.; Mackintosh, K.A. Inspiratory muscle training enhances recovery post-COVID-19: A randomised controlled trial. Eur. Respir. J. 2022, 60, 2103101. [Google Scholar] [CrossRef] [PubMed]
- Chee, Y.J.; Fan, B.E.; Young, B.E.; Dalan, R.; Lye, D.C. Clinical trials on the pharmacological treatment of long COVID: A systematic review. J. Med. Virol. 2023, 95, e28289. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Leber, A.; Jawad, M.Y.; Yu, M.; Lui, L.M.; Subramaniapillai, M.; Di Vincenzo, J.D.; Gill, H.; Rodrigues, N.B.; Cao, B.; et al. Registered clinical trials investigating treatment of long COVID: A scoping review and recommendations for research. Infect. Dis. 2022, 54, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Notarte, K.I.; de Oliveira, M.H.; Peligro, P.J.; Velasco, J.V.; Macaranas, I.; Ver, A.T.; Pangilinan, F.C.; Pastrana, A.; Goldrich, N.; Kavteladze, D.; et al. Age, sex and previous comorbidities as risk factors not associated with SARS-CoV-2 infection for long COVID-19: A systematic review and meta-analysis. J. Clin. Med. 2022, 11, 7314. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Yon, D.K.; Lee, S.W.; Soysal, P.; Koyanagi, A.; Jacob, L.; Li, Y.; Park, J.M.; Kim, Y.W.; Shin, J.I.; et al. New-onset neurodegenerative diseases as long-term sequelae of SARS-CoV-2 infection: A systematic review and meta-analysis. J. Med. Virol. 2023, 95, e28909. [Google Scholar] [CrossRef] [PubMed]


| Factors | Values |
|---|---|
| Age (years; mean ± SD) | 55.16 ± 13.1 |
| Male | 110 (46) * |
| Female | 128 (54) * |
| Respiratory diseases | 47 (20) * |
| Asthma | 35 (15) * |
| COPD | 11 (5) * |
| Allergic rhinitis | 4 (2) * |
| Lung cancer | 1 (0.4) * |
| Pulmonary Hypertension | 2 (0.8) * |
| Psychiatric conditions | 8 (3) * |
| Cardiovascular diseases | 106 (44) * |
| Ischemic heart disease | 20 (8) * |
| Heart valve disease | 5 (2) * |
| Hypertension | 94 (39) * |
| Cardiac arrhythmia | 10 (4) * |
| Oncological diseases | 14 (6) * |
| Diabetes mellitus | 18 (8) * |
| Renal disease | 1 (0.4) * |
| Number of Patients | % of Patients | Lung Function as % of Predicted Value * | ||
|---|---|---|---|---|
| All | 191 | 100 | 95.00 (82.00–105.00) | |
| FEV1 | Low | 38 | 19.90 | 71.00 (62.25–75.00) |
| Normal | 153 | 80.10 | 98.00 (91–108) | |
| Tiffeneau-index | All | 187 | 100 | 78.95 (74.16–82.70) |
| Low | 110 | 58.82 | 75.06 (68.97–77.89) | |
| Normal | 77 | 41.18 | 84.06 (81.67–88.31) | |
| Dlco | All | 59 | 100 | 86.00 (73.00–96.50) |
| Low | 23 | 38.98 | 67.00 (61.00–75.00) | |
| Normal | 36 | 61.02 | 94.50 (86.75–101.25) | |
| Number of Patients | % | |
|---|---|---|
| Total Number of patients | 80 | 100 |
| General pain | 26 | 32.50 |
| Fatigue | 59 | 73.75 |
| Pulmonary symptoms | 60 | 75.00 |
| Shortness of breath, dyspnoe | 39 | 48.75 |
| Cough | 37 | 46.25 |
| Neurological symptoms | 50 | 62.50 |
| Sleep disorder | 29 | 36.25 |
| Cognitive disorder | 20 | 25.00 |
| Peripheral neuropathy | 12 | 15.00 |
| Headache | 22 | 27.50 |
| Dizziness | 21 | 26.25 |
| Delirium | 1 | 1.25 |
| Ear-Nose-Throat symptoms | 41 | 51.25 |
| Earache | 8 | 10.00 |
| Tinnitus | 16 | 20.00 |
| Pharyngalgia | 16 | 20.00 |
| Hearing loss | 11 | 13.75 |
| Loss of smell | 7 | 8.75 |
| Loss of taste | 5 | 6.25 |
| Cardiovascular symptoms | 34 | 42.50 |
| Chest pain | 31 | 38.75 |
| Palpitation | 5 | 6.25 |
| Musculoskeletal symptoms | 35 | 43.75 |
| Myalgia | 26 | 32.50 |
| Arthralgia | 25 | 31.25 |
| Psychiatric symptoms | 25 | 31.25 |
| Anxiety | 10 | 12.50 |
| Nightmares | 6 | 7.50 |
| Depression | 7 | 8.50 |
| Gastrointestinal symptoms | 21 | 26.25 |
| Diarrhea | 9 | 11.25 |
| Abdominal pain | 12 | 15.00 |
| Decreased appetite | 6 | 7.50 |
| Nausea | 6 | 7.50 |
| Dermatological symptoms | 6 | 7.50 |
| Dry skin | 1 | 1.25 |
| Itching | 1 | 1.25 |
| Hair loss | 3 | 3.75 |
| Skin rash | 3 | 3.75 |
| Other symptoms (vision impairment, tremors) | 3 | 3.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sárközi, A.T.; Tornyi, I.; Békési, E.; Horváth, I. Co-Morbidity Clusters in Post-COVID-19 Syndrome. J. Clin. Med. 2024, 13, 1457. https://doi.org/10.3390/jcm13051457
Sárközi AT, Tornyi I, Békési E, Horváth I. Co-Morbidity Clusters in Post-COVID-19 Syndrome. Journal of Clinical Medicine. 2024; 13(5):1457. https://doi.org/10.3390/jcm13051457
Chicago/Turabian StyleSárközi, Anna Teréz, Ilona Tornyi, Erik Békési, and Ildikó Horváth. 2024. "Co-Morbidity Clusters in Post-COVID-19 Syndrome" Journal of Clinical Medicine 13, no. 5: 1457. https://doi.org/10.3390/jcm13051457
APA StyleSárközi, A. T., Tornyi, I., Békési, E., & Horváth, I. (2024). Co-Morbidity Clusters in Post-COVID-19 Syndrome. Journal of Clinical Medicine, 13(5), 1457. https://doi.org/10.3390/jcm13051457







