Lung Cancer in Women—Sociodemographic, Clinical and Psychological Characteristics with Comparison to Men
Abstract
1. Background
2. Methods
Statistical Analysis
3. Results
3.1. Study Group Description
3.2. Comparison of Clinical Data Related to Lung Cancer
3.3. Psychological Assessment
4. Discussion
5. Conclusions
6. Clinical Practice Points
- The role of collecting sociodemographic information among lung cancer patients continues to grow.
- Appropriate campaigns of screening programs aimed at different education levels in both sexes are needed.
- We should be more active in finding out the willingness to consult a psychologist or psychiatrist among women with lung cancer.
- The diagnosis of COPD should be considered more often among women due to the lack of differences in the reported incidence of COPD between men and women, despite a clear contrast in the number of pack-years.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIS | Acceptance of Illness Scale |
| ALK | anaplastic lymphoma kinase |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 |
| COPD | chronic obstructive pulmonary disease |
| EGFR | epidermal growth factor receptor |
| NOS | non-otherwise specified |
| PSS-10 | Perceived Stress Scale |
| ROS1 | ROS proto-oncogene 1, receptor tyrosine kinase |
| TNM | Tumor, Node, Metastasis |
References
- Jacobsen, M.M.; Silverstein, S.C.; Quinn, M.; Waterston, L.B.; Thomas, C.A.; Benneyan, J.C.; Han, P.K.J. Timeliness of access to lung cancer diagnosis and treatment: A scoping literature review. Lung Cancer 2017, 112, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A.; Fedewa, S.A.; Henley, S.J.; Pollack, L.A.; Jemal, A. Proportion of Never Smokers Among Men and Women with Lung Cancer in 7 US States. JAMA Oncol. 2021, 7, 302–304. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Report on Trends in Prevalence of Tobacco Use 2000–2025, 4th ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Oliver, A.L. Lung Cancer: Epidemiology and Screening. Surg. Clin. North Am. 2022, 102, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Domagala-Kulawik, J.; Trojnar, A. Lung cancer in women in 21st century. J. Thorac. Dis. 2020, 12, 4398–4410. [Google Scholar] [CrossRef]
- Pesch, B.; Kendzia, B.; Gustavsson, P. Cigarette smoking and lung cancer-relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int. J. Cancer 2012, 131, 1210–1219. [Google Scholar] [CrossRef]
- Subramanian, J.; Govindan, R. Lung cancer in never smokers: A review. J. Clin. Oncol. 2007, 25, 561–570. [Google Scholar] [CrossRef]
- Wakelee, H.A.; Chang, E.T.; Gomez, S.L.; Keegan, T.H.; Feskanich, D.; Clarke, C.A.; Holmberg, L.; Yong, L.C.; Kolonel, L.N.; Gould, M.K.; et al. Lung cancer incidence in never smokers. J. Clin. Oncol. 2007, 25, 472–478. [Google Scholar] [CrossRef]
- Kligerman, S.; White, C. Epidemiology of lung cancer in women: Risk factors, survival, and screening. AJR Am. J. Roentgenol. 2011, 196, 287–295. [Google Scholar] [CrossRef]
- Orzołek, I.; Sobieraj, J.; Domagała-Kulawik, J. Estrogens, Cancer and Immunity. Cancers 2022, 14, 2265. [Google Scholar] [CrossRef]
- Redondo-Sánchez, D.; Petrova, D.; Rodríguez-Barranco, M.; Fernández-Navarro, P.; Jiménez-Moleón, J.J.; Sánchez, M.J. Socio-Economic Inequalities in Lung Cancer Outcomes: An Overview of Systematic Reviews. Cancers 2022, 14, 398. [Google Scholar] [CrossRef]
- Zou, X.; Wang, R.; Yang, Z.; Wang, Q.; Fu, W.; Huo, Z.; Ge, F.; Zhong, R.; Jiang, Y.; Li, J.; et al. Family Socioeconomic Position and Lung Cancer Risk: A Meta-Analysis and a Mendelian Randomization Study. Front. Public Health 2022, 10, 780538. [Google Scholar] [CrossRef]
- Sullivan, D.R.; Forsberg, C.W.; Ganzini, L.; Au, D.H.; Gould, M.K.; Provenzale, D.; Slatore, C.G. Longitudinal Changes in Depression Symptoms and Survival Among Patients with Lung Cancer: A National Cohort Assessment. J. Clin. Oncol. 2016, 34, 3984–3991. [Google Scholar] [CrossRef]
- Andersen, B.L.; Valentine, T.R.; Lo, S.B.; Carbone, D.P.; Presley, C.J.; Shields, P.G. Newly diagnosed patients with advanced non-small cell lung cancer: A clinical description of those with moderate to severe depressive symptoms. Lung Cancer 2020, 145, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Polański, J.; Chabowski, M.; Chudiak, A.; Uchmanowicz, B.; Janczak, D.; Rosińczuk, J.; Mazur, G. Intensity of Anxiety and Depression in Patients with Lung Cancer in Relation to Quality of Life. Adv. Exp. Med. Biol. 2018, 1023, 29–36. [Google Scholar] [CrossRef]
- Trojnar, A.; Domagała-Kulawik, J.; Sienkiewicz-Ulita, A.; Zbytniewski, M.; Gryszko, G.M.; Cackowski, M.M.; Dziedzic, M.; Woźnica, K.; Orłowski, T.M.; Dziedzic, D.A.; et al. Sex differences in clinico-pathologic characteristics and long-term survival among 17,192 surgically treated NSCLC patients: Nationwide population-based propensity score-matching study. Surg. Oncol. 2022, 45, 101873. [Google Scholar] [CrossRef] [PubMed]
- Trojnar, A.; Domagała-Kulawik, J.; Sienkiewicz-Ulita, A.; Zbytniewski, M.; Gryszko, G.M.; Cackowski, M.M.; Dziedzic, M.; Woźnica, K.; Orłowski, T.M.; Dziedzic, D.A. The clinico-pathological characteristics of surgically treated young women with NSCLC. Transl. Lung Cancer Res. 2022, 11, 2382–2394. [Google Scholar] [CrossRef]
- Lim, W.; Ridge, C.A.; Nicholson, A.G.; Mirsadraee, S. The 8th lung cancer TNM classification and clinical staging system: Review of the changes and clinical implications. Quant. Imaging Med. Surg. 2018, 8, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef]
- Felton, B.J.; Revenson, T.A. Coping with chronic illness: A study of illness controllability and the influence of coping strategies on psychological adjustment. J. Consult. Clin. Psychol. 1984, 52, 343–353. [Google Scholar] [CrossRef]
- Jurczyński, Z. Measurement Tools in the Promotion and Psychology of Health; Pracownia Testów Psychologicznych: Warszawa, Poland, 2009. [Google Scholar]
- Mazurek, J.; Lurbiecki, J. Skala Akceptacji Choroby i jej znaczenie w praktyce klinicznej [Acceptance of illness scale and its clinical impact]. Pol. Merkur. Lek. 2014, 36, 106–108. [Google Scholar]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Melosky, B.; Kambartel, K.; Häntschel, M.; Bennetts, M.; Nickens, D.J.; Brinkmann, J.; Kayser, A.; Moran, M.; Cappuzzo, F. Worldwide Prevalence of Epidermal Growth Factor Receptor Mutations in Non-Small Cell Lung Cancer: A Meta-Analysis. Mol. Diagn. Ther. 2022, 26, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Giorlando, M.A.; Gabay, C.; Mendoza, L.A.; Mari, E.; Escorial, C.; Schnetter, L.; Ortega, E.; Andreasyan, A. Gender Differences in Non-Small Cell Lung Cancer: A Comparative Analysis of European and Asian Patients. J. Thorac. Oncol. 2023, 18, 123. [Google Scholar] [CrossRef]
- Ganti, A.K.; Klein, A.B.; Cotarla, I.; Seal, B.; Chou, E. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients with Non-Small Cell Lung Cancer in the US. JAMA Oncol. 2021, 7, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Aisner, D.L.; Marshall, C.B. Molecular pathology of non-small cell lung cancer: A practical guide. Am. J. Clin. Pathol. 2012, 138, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Stabellini, N.; Bruno, D.S.; Dmukauskas, M.; Barda, A.J.; Cao, L.; Shanahan, J.; Waite, K.; Montero, A.J.; Barnholtz-Sloan, J.S. Sex Differences in Lung Cancer Treatment and Outcomes at a Large Hybrid Academic-Community Practice. JTO Clin. Res. Rep. 2022, 3, 100307. [Google Scholar] [CrossRef]
- Sezer, A.; Kilickap, S.; Gümüş, M.; Bondarenko, I.; Özgüroğlu, M.; Gogishvili, M.; Turk, H.M.; Çiçin, İ.; Bentsion, D.; Gladkov, O.; et al. EMPOWER-Lung 1: Phase 3 First-line (1L) Cemiplimab Monotherapy vs Platinum-Doublet Chemotherapy (Chemo) in Advanced Non-Small Cell Lung Cancer (NSCLC) with Programmed Cell Death-Ligand 1 (PD-L1) ≥50%. Ann. Oncol. 2020, 31, 1182–1183. [Google Scholar] [CrossRef]
- Garassino, M.C.; Gadgeel, S.; Speranza, G.; Felip, E.; Esteban, E.; Dómine, M.; Hochmair, M.J.; Powell, S.F.; Bischoff, H.G.; Peled, N.; et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J. Clin. Oncol. 2023, 41, 1992–1998. [Google Scholar] [CrossRef]
- Novello, S.; Kowalski, D.M.; Luft, A.; Gümüş, M.; Vicente, D.; Mazières, J.; Rodríguez-Cid, J.; Tafreshi, A.; Cheng, Y.; Lee, K.H.; et al. Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J. Clin. Oncol. 2023, 41, 1999–2006. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Ruano-Ravina, A.; Provencio, M.; Calvo de Juan, V.; Carcereny, E.; Estival, A.; Rodríguez-Abreu, D.; Benítez, G.; López-Castro, R.; Belver, M.; Guirado-Risueño, M.; et al. Are there differences by sex in lung cancer characteristics at diagnosis? -a nationwide study. Transl. Lung Cancer Res. 2021, 10, 3902–3911. [Google Scholar] [CrossRef]
- Kreuzer, M.; Boffetta, P.; Whitley, E.; Ahrens, W.; Gaborieau, V.; Heinrich, J.; Jöckel, K.H.; Kreienbrock, L.; Mallone, S.; Merletti, F.; et al. Gender differences in lung cancer risk by smoking: A multicentre case-control study in Germany and Italy. Br. J. Cancer 2000, 82, 227–233. [Google Scholar] [CrossRef]
- Daniel, M.; Keefe, F.J.; Lyna, P.; Peterson, B.; Garst, J.; Kelley, M.; Bepler, G.; Bastian, L.A. Persistent smoking after a diagnosis of lung cancer is associated with higher reported pain levels. J. Pain. 2009, 10, 323–328. [Google Scholar] [CrossRef]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar] [CrossRef]
- Yoshida, K.; Takizawa, Y.; Nishino, Y.; Takahashi, S.; Kanemura, S.; Omori, J.; Kurosawa, H.; Maemondo, M.; Minami, Y. Association between Family History of Cancer and Lung Cancer Risk among Japanese Men and Women. Tohoku J. Exp. Med. 2019, 247, 99–110. [Google Scholar] [CrossRef]
- Young, R.P.; Hopkins, R. The potential impact of chronic obstructive pulmonary disease in lung cancer screening: Implications for the screening clinic. Expert Rev. Respir. Med. 2019, 13, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, R.S.; Stover, D.E.; Shi, W.; Venkatraman, E. Prevalence of COPD in women compared to men around the time of diagnosis of primary lung cancer. Chest 2006, 129, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dou, S.; Dong, W.; Xie, M.; Cui, L.; Zheng, C.; Xiao, W. Impact of COPD on prognosis of lung cancer: From a perspective on disease heterogeneity. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 3767–3776. [Google Scholar] [CrossRef] [PubMed]
- Dobek, R.; Farnik, M.; Franczuk, M.; Konieczny, Z.; Mierzwa, W.; Nowacka-Apiyo, J.; Obojski, A.; Przybyłowska, K.; Rasławska, K.; Przybyłowska, K.; et al. Ścieżka chorego na POChP w Polsce: Stan obecny i pożądany kierunek zmian. Perspektywa specjalistów pulmonologów. Pneumonol. Pol. 2022, 3, 23–32. [Google Scholar]
- Undrunas, A.; Kasprzyk, P.; Rajca, A.; Kuziemski, K.; Rzyman, W.; Zdrojewski, T. Prevalence, symptom burden and under-diagnosis of chronic obstructive pulmonary disease in Polish lung cancer screening population: A cohort observational study. BMJ Open 2022, 12, e055007. [Google Scholar] [CrossRef]
- Hovanec, J.; Siemiatycki, J.; Conway, D.I.; Olsson, A.; Stücker, I.; Guida, F.; Jöckel, K.H.; Pohlabeln, H.; Ahrens, W.; Brüske, I.; et al. Lung cancer and socioeconomic status in a pooled analysis of case-control studies. PLoS ONE 2018, 13, e0192999. [Google Scholar] [CrossRef]
- Mitra, D.; Shaw, A.; Tjepkema, M.; Peters, P. Social determinants of lung cancer incidence in Canada: A 13-year prospective study. Health Rep. 2015, 26, 12–20. [Google Scholar]
- Zhou, H.; Zhang, Y.; Liu, J.; Yang, Y.; Fang, W.; Hong, S.; Chen, G.; Zhao, S.; Zhang, Z.; Shen, J.; et al. Education and lung cancer: A Mendelian randomization study. Int. J. Epidemiol. 2019, 48, 743–750. [Google Scholar] [CrossRef]
- Herndon, J.E., 2nd; Kornblith, A.B.; Holland, J.C.; Paskett, E.D. Patient education level as a predictor of survival in lung cancer clinical trials. J. Clin. Oncol. 2008, 26, 4116–4123. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Jin, Y.; Tang, L.; Pi, Y.P.; Chen, W.Q.; Jiménez-Herrera, M.F. Predicting the Risk of Psychological Distress among Lung Cancer Patients: Development and Validation of a Predictive Algorithm Based on Sociodemographic and Clinical Factors. Asia Pac. J. Oncol. Nurs. 2021, 8, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Brintzenhofe-Szoc, K.M.; Levin, T.T.; Li, Y.; Kissane, D.W.; Zabora, J.R. Mixed anxiety/depression symptoms in a large cancer cohort: Prevalence by cancer type. Psychosomatics 2009, 50, 383–391. [Google Scholar] [CrossRef]
- Linden, W.; Vodermaier, A.; Mackenzie, R.; Greig, D. Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. J. Affect. Disord. 2012, 141, 343–351. [Google Scholar] [CrossRef]
- Grassi, L.; Caruso, R.; Riba, M.B.; Lloyd-Williams, M.; Kissane, D.; Rodin, G.; McFarland, D.; Campos-Ródenas, R.; Zachariae, R.; Santini, D.; et al. Anxiety and depression in adult cancer patients: ESMO Clinical Practice Guideline. ESMO Open 2023, 8, 101155. [Google Scholar] [CrossRef]
- Kadan-Lottick, N.S.; Vanderwerker, L.C.; Block, S.D.; Zhang, B.; Prigerson, H.G. Psychiatric disorders and mental health service use in patients with advanced cancer: A report from the coping with cancer study. Cancer 2005, 104, 2872–2881. [Google Scholar] [CrossRef] [PubMed]
- Mosher, C.E.; Winger, J.G.; Hanna, N.; Jalal, S.I.; Fakiris, A.J.; Einhorn, L.H.; Birdas, T.J.; Kesler, K.A.; Champion, V.L. Barriers to mental health service use and preferences for addressing emotional concerns among lung cancer patients. Psychooncology 2014, 23, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Chabowski, M.; Polański, J.; Jankowska-Polanska, B.; Lomper, K.; Janczak, D.; Rosinczuk, J. The acceptance of illness, the intensity of pain and the quality of life in patients with lung cancer. J. Thorac. Dis. 2017, 9, 2952–2958. [Google Scholar] [CrossRef] [PubMed]
- Karczmarek-Borowska, B.; Tobiasz, M.; Bukała, A. Akceptacja choroby przez chorych na nowotwór płuca [Acceptance of the disease in patients with lung cancer]. Pol. Merkur. Lek. 2016, 40, 89–93. [Google Scholar] [PubMed]
- Tian, X.; Jin, Y.; Chen, H.; Tang, L.; Jiménez-Herrera, M.F. Relationships among Social Support, Coping Style, Perceived Stress, and Psychological Distress in Chinese Lung Cancer Patients. Asia Pac. J. Oncol. Nurs. 2021, 8, 172–179. [Google Scholar] [CrossRef]
- European Lung Foundation. Available online: https://europeanlung.org/solace/about/ (accessed on 18 April 2023).
| Females (N = 50) | Males (N = 50) | p-Value | |
|---|---|---|---|
| Age (years) | 66 (59.5–71) | 65 (61–71.8) | 0.939 |
| Pack-years of smoking | 22.5 (6.2–30) | 45 (26.2–50) | <0.05 |
| Current smokers (N, %) | 7 (14%) | 12 (24%) | <0.05 |
| Ever smokers | 42 (84%) | 49 (98%) | |
| Non-smoker status (N, %) | 8 (16%) | 1 (2%) | |
| Second-hand smoke exposure (N, %) | 34 (68%) | 29 (58%) | 0.407 |
| COPD (N, %) | 13 (26%) | 11 (22%) | 0.815 |
| Primary symptoms | 0.745 | ||
| Cough (N, %) | 32 (64%) | 28 (56%) | |
| Dyspnoea (N, %) | 16 (32%) | 14 (28%) | |
| Weight loss (N, %) | 11 (22%) | 12 (24%) | |
| Chest pain (N, %) | 8 (16%) | 8 (16%) | |
| Hemoptysis (N, %) | 3 (6%) | 3 (6%) | |
| Hoarseness (N, %) | 6 (12%) | 11 (22%) | |
| Symptoms of pneumonia (N, %) | 6 (12%) | 5 (10%) | |
| Time from onset of symptoms to diagnosis (months) | 5 (2–6) | 3 (2–6) | 0.416 |
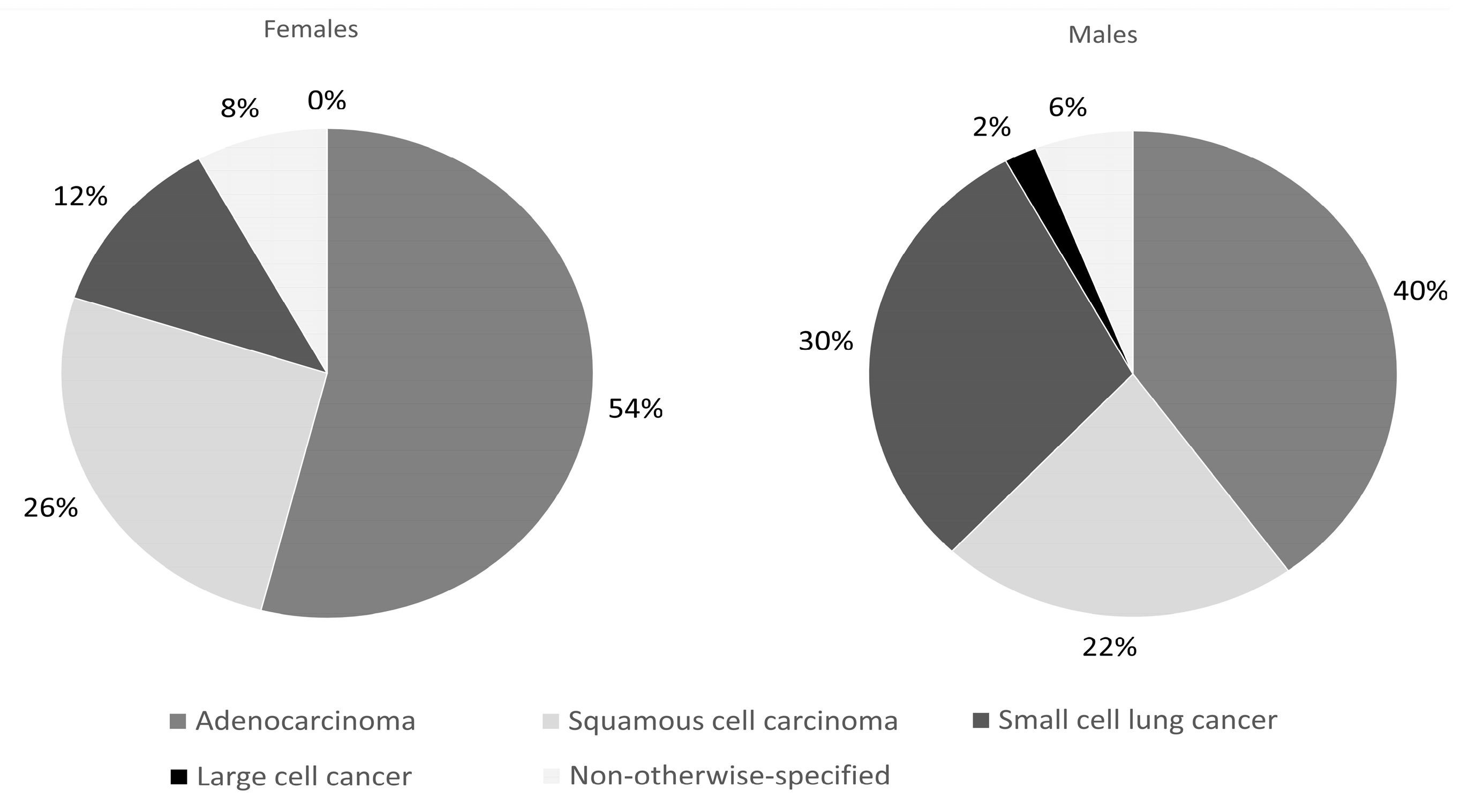
| Histopatology diagnosis | <0.05 | ||
| Adenocarcinoma (N, %) | 27 (54%) | 20 (40%) | |
| Squamous cell carcinoma (N, %) | 13 (26%) | 11 (22%) | |
| Small cell lung cancer (N, %) | 6 (12%) | 15 (30%) | |
| Large cell cancer (N, %) | 4 (8%) | 1 (2%) | |
| Not otherwise specified | 0 (0%) | 3 (6%) | |
| EGFR mutations (N, %) | 8 (29.6%) | 1 (4.3%) | <0.05 |
| ALK rearrangements (N, %) | 2 (7.4%) | 1 (4.3%) | 0.649 |
| ROS1 rearrangements (N, %) | 1 (3.7%) | 0 (0%) | 0.351 |
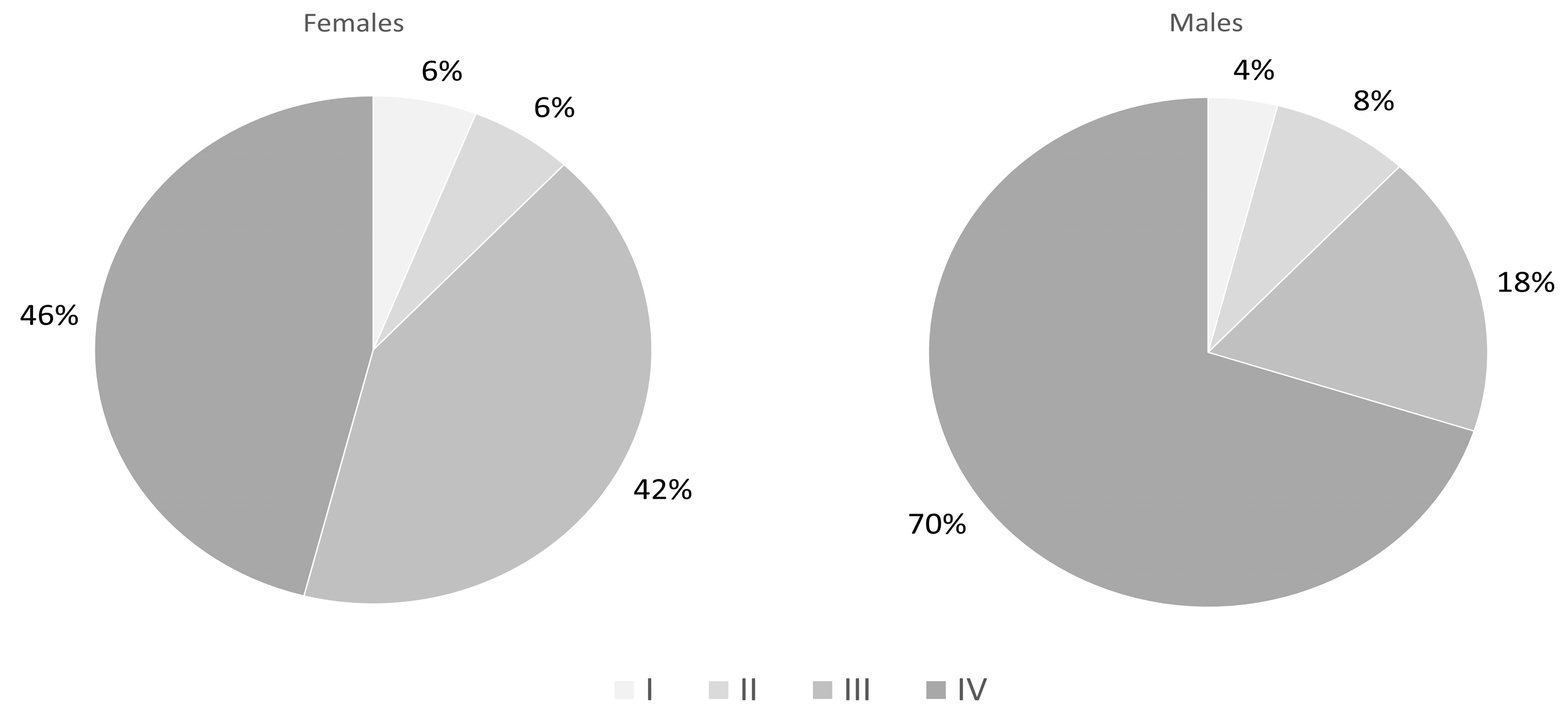
| Clinical stage | <0.05 | ||
| I (N, %) | 3 (6%) | 2 (4%) | |
| II (N, %) | 3 (6%) | 4 (8%) | |
| III (N, %) | 21 (42%) | 9 (18%) | |
| IV (N, %) | 23 (46%) | 35 (70%) | |
| Treatment | |||
| Surgery (N, %) | 10 (20%) | 7 (14%) | 0.594 |
| Chemiotherapy (N, %) | 35 (70%) | 40 (80%) | 0.421 |
| Radiotherapy (N, %) | 20 (40%) | 25 (50%) | 0.356 |
| Immunotherapy (N, %) | 13 (26%) | 25 (50%) | <0.05 |
| Targeted therapy (N, %) | 9 (18%) | 4 (8%) | 0.25 |
| Females (N = 50) | Males (N = 50) | p-Value | |
|---|---|---|---|
| Education level | 0.053 | ||
| Lower (N, %) | 6 (12%) | 8 (16%) | |
| Secondary (N, %) | 21 (44%) | 31 (62%) | |
| Higher (N, %) | 22 (44%) | 11 (22%) | |
| Residence | 0.165 | ||
| Village (N, %) | 10 (20%) | 13 (26%) | |
| City to 50,000 (N, %) | 10 (20%) | 10 (20%) | |
| City 50–100,000 (N, %) | 2 (4%) | 9 (18%) | |
| City 100–500,000 (N, %) | 3 (6%) | 3 (6%) | |
| City > 500,000 (N, %) | 23 (46%) | 15 (30%) | |
| Family history of lung cancer | 8 (16%) | 8 (16%) | 1 |
| Treatment Method | Odds Ratio with 95% Confidence Interval | p-Value |
|---|---|---|
| Surgery | 1.05 (0.27–4.05) | 0.94 |
| Chemotherapy | 0.58 (0.21–1.54) | 0.27 |
| Radiotherapy | 0.63 (0.27–1.45) | 0.28 |
| Immunotherapy | 0.38 (0.16–0.90) | 0.03 |
| Targeted therapy | 2.7 (0.81–10.6) | 0.12 |
| Females (N = 50) | Males (N = 50) | p-Value | |
|---|---|---|---|
| Need of consultation with psychologist/psychiatrist in terms of lung cancer | 12 (24%) | 3 (6%) | <0.05 |
| History of using a psychologist’s/psychiatrist’s consultation in terms of lung cancer | 6 (12%) | 3 (6%) | 0.485 |
| PSS-10 | 18.9 (12.7–25.1) | 16.3 (10.1–22.5) | <0.05 |
| AIS | 27 (22–32) | 27 (20.2–31) | 0.648 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trojnar, A.; Knetki-Wróblewska, M.; Sobieraj, P.; Domagała-Kulawik, J. Lung Cancer in Women—Sociodemographic, Clinical and Psychological Characteristics with Comparison to Men. J. Clin. Med. 2024, 13, 1450. https://doi.org/10.3390/jcm13051450
Trojnar A, Knetki-Wróblewska M, Sobieraj P, Domagała-Kulawik J. Lung Cancer in Women—Sociodemographic, Clinical and Psychological Characteristics with Comparison to Men. Journal of Clinical Medicine. 2024; 13(5):1450. https://doi.org/10.3390/jcm13051450
Chicago/Turabian StyleTrojnar, Anna, Magdalena Knetki-Wróblewska, Piotr Sobieraj, and Joanna Domagała-Kulawik. 2024. "Lung Cancer in Women—Sociodemographic, Clinical and Psychological Characteristics with Comparison to Men" Journal of Clinical Medicine 13, no. 5: 1450. https://doi.org/10.3390/jcm13051450
APA StyleTrojnar, A., Knetki-Wróblewska, M., Sobieraj, P., & Domagała-Kulawik, J. (2024). Lung Cancer in Women—Sociodemographic, Clinical and Psychological Characteristics with Comparison to Men. Journal of Clinical Medicine, 13(5), 1450. https://doi.org/10.3390/jcm13051450






