Isolated Depo-Medrol Administration under Tenon’s Capsule for Post-COVID-19 Uveitis in a Child: A Case Report and Literature Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
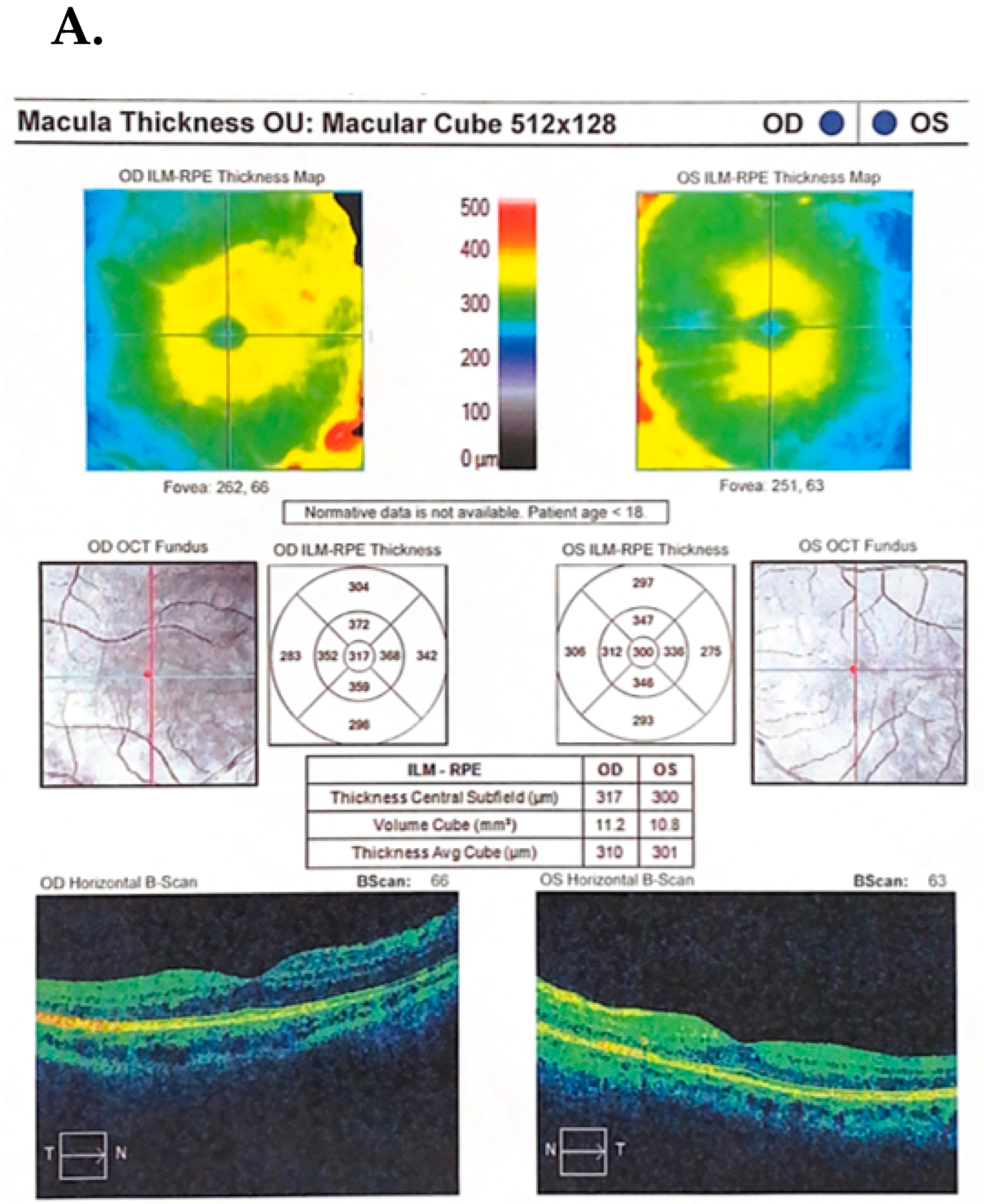
Case Description
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghosh, N.; Nandi, S.; Saha, I. A review on evolution of emerging SARS-CoV-2 variants based on spike glycoprotein. Int. Immunopharmacol. 2022, 105, 108565. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: The XBB.1.5 (‘Kraken’) Subvariant of Omicron SARS-CoV-2 and its Rapid Global Spread. Med. Sci. Monit. 2023, 29, e939580. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. Editorial: A Rapid Global Increase in COVID-19 is Due to the Emergence of the EG.5 (Eris) Subvariant of Omicron SARS-CoV-2. Med. Sci. Monit. 2023, 29, e942244. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, F.; Gutiérrez-Sacristán, A.; Makwana, S.; Li, X.; Rofeberg, V.N.; Cai, T.; Bourgeois, F.T.; Omenn, G.S.; Hanauer, D.A.; Sáez, C.; et al. Clinical phenotypes and outcomes in children with multisystem inflammatory syndrome across SARS-CoV-2 variant eras: A multinational study from the 4CE consortium. EClinicalMedicine 2023, 64, 102212. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, E.; Dubey, A.K.; Teodori, L.; Ramakrishna, S.; Kaushik, A. SARS-CoV-2 Omicron variant: A next phase of the COVID-19 pandemic and a call to arms for system sciences and precision medicine. MedComm 2022, 3, e119. [Google Scholar] [CrossRef]
- Alnahdi, M.A.; Alkharashi, M. Ocular manifestations of COVID-19 in the pediatric age group. Eur. J. Ophthalmol. 2023, 33, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Worldmeter. COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 22 September 2022).
- Akbari, M.; Dourandeesh, M. Update on overview of ocular manifestations of COVID-19. Front. Med. 2022, 9, 877023. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Abrishami, M.; Zamani, G.; Hemmati, A.; Momtahen, S.; Hassani, M.; Omidtabrizi, A. Acute Bilateral Neuroretinitis and Panuveitis in A Patient with Coronavirus Disease 2019: A Case Report. Ocul. Immunol. Inflamm. 2021, 29, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Braceros, K.K.; Asahi, M.G.; Gallemore, R.P. Visual Snow-Like Symptoms and Posterior Uveitis following COVID-19 Infection. Case Rep. Ophthalmol. Med. 2021, 2021, 6668552. [Google Scholar] [CrossRef]
- Ichhpujani, P.; Singh, R.B.; Dhillon, H.K.; Kumar, S. Ocular manifestations of COVID-19 in pediatric patients. Ther. Adv. Ophthalmol. 2023, 15, 25158414221149916. [Google Scholar] [CrossRef]
- Guo, C.X.; He, L.; Yin, J.Y.; Meng, X.G.; Tan, W.; Yang, G.P.; Bo, T.; Liu, J.P.; Lin, X.J.; Chen, X. Epidemiological and clinical features of pediatric COVID-19. BMC Med. 2020, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Valente, P.; Iarossi, G.; Federici, M.; Petroni, S.; Palma, P.; Cotugno, N.; De Ioris, M.A.; Campana, A.; Buzzonetti, L. Ocular manifestations and viral shedding in tears of pediatric patients with coronavirus disease 2019: A preliminary report. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2020, 24, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Kumari, E.; Roy, A.; Bandyopadhyay, M. Ocular manifestations and clinical profile of multisystemic inflammatory syndrome in children during COVID-19 pandemic. Int. J. Res. Med. Sci. 2021, 10, 173. [Google Scholar] [CrossRef]
- Madani, S. Acute and sub-acute ocular manifestations in pediatric patients with COVID-19: A systematic review. Med. Hypothesis Discov. Innov. Ophthalmol. 2022, 11, 11–18. [Google Scholar] [CrossRef]
- Singh, S.; Garcia, G., Jr.; Shah, R.; Kramerov, A.A.; Wright, R.E., III; Spektor, T.M.; Ljubimov, A.V.; Arumugaswami, V.; Kumar, A. SARS-CoV-2 and its beta variant of concern infect human conjunctival epithelial cells and induce differential antiviral innate immune response. Ocul. Surf. 2022, 23, 184–194. [Google Scholar] [CrossRef]
- Willcox, M.D.; Walsh, K.; Nichols, J.J.; Morgan, P.B.; Jones, L.W. The ocular surface, coronaviruses and COVID-19. Clin. Exp. Optom. 2020, 103, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Low, R.; Tong, L.; Gupta, V.; Veeraraghavan, A.; Agrawal, R. COVID-19 and the Ocular Surface: A Review of Transmission and Manifestations. Ocul. Immunol. Inflamm. 2020, 28, 726–734. [Google Scholar] [CrossRef]
- Hong, N.; Yu, W.; Xia, J.; Shen, Y.; Yap, M.; Han, W. Evaluation of ocular symptoms and tropism of SARS-CoV-2 in patients confirmed with COVID-19. Acta Ophthalmol. 2020, 98, e649–e655. [Google Scholar] [CrossRef]
- Xia, J.; Tong, J.; Liu, M.; Shen, Y.; Guo, D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020, 92, 589–594. [Google Scholar] [CrossRef]
- Kyrou, I.; Randeva, H.S.; Spandidos, D.A.; Karteris, E. Not only ACE2-the quest for additional host cell mediators of SARS-CoV-2 infection: Neuropilin-1 (NRP1) as a novel SARS-CoV-2 host cell entry mediator implicated in COVID-19. Signal Transduct. Target Ther. 2021, 6, 21. [Google Scholar] [CrossRef]
- Collin, J.; Queen, R.; Zerti, D.; Dorgau, B.; Georgiou, M.; Djidrovski, I.; Hussain, R.; Coxhead, J.M.; Joseph, A.; Rooney, P.; et al. Co-expression of SARS-CoV-2 entry genes in the superficial adult human conjunctival, limbal and corneal epithelium suggests an additional route of entry via the ocular surface. Ocul. Surf. 2021, 19, 190–200. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, K.; Zhu, Y.; Lyu, D.; Yu, Y.; Li, S.; Yao, K. Ocular manifestations in COVID-19 patients: A systematic review and meta-analysis. Travel. Med. Infect. Dis. 2021, 44, 102191. [Google Scholar] [CrossRef]
- Eissa, M.; Abdelrazek, N.A.; Saady, M. COVID-19 and its relation to the human eye: Transmission, infection, and ocular manifestations. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Patel, J.; Swiston, C.; Patel, B.C. Ophthalmic Manifestations of Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Soni, A.; Narayanan, R.; Tyagi, M.; Belenje, A.; Basu, S. Acute Retinal Necrosis as a presenting ophthalmic manifestation in COVID 19 recovered patients. Ocul. Immunol. Inflamm. 2021, 29, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Chimal, L.G.; Cuevas, G.G.; Di-Luciano, A.; Chamartín, P.; Amadeo, G.; Martínez-Castellanos, M.A. Ophthalmic manifestations associated with SARS-CoV-2 in newborn infants: A preliminary report. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2021, 25, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Diwakar, J.; Samaddar, A.; Konar, S.K.; Bhat, M.D.; Manuel, E.; Veenakumari, H.B.; Nandeesh, B.N.; Parveen, A.; Hajira, S.N.; Srinivas, D.; et al. First report of COVID-19-associated rhino-orbito-cerebral mucormycosis in pediatric patients with type 1 diabetes mellitus. J. Mycol. Med. 2021, 31, 101203. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.K.; Mohanan-Earatt, A. An analysis of the clinical profile of patients with uveitis following COVID-19 infection. Indian. J. Ophthalmol. 2022, 70, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Merticariu, C.I.; Merticariu, M.; Cobzariu, C.; Mihai, M.M.; Dragomir, M.S. Pediatric inflammatory multisystem syndrome induced Panuveitis associated with SARS-CoV-2 infection: What the Ophthalmologists need to know. Rom. J. Ophthalmol. 2022, 66, 198–208. [Google Scholar] [CrossRef]
- Yeo, S.; Kim, H.; Lee, J.; Yi, J.; Chung, Y.R. Retinal vascular occlusions in COVID-19 infection and vaccination: A literature review. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 1793–1808. [Google Scholar] [CrossRef] [PubMed]
- Shiroma, H.F.; Lima, L.H.; Shiroma, Y.B.; Kanadani, T.C.; Nobrega, M.J.; Andrade, G.; de Moraes Filho, M.N.; Penha, F.M. Retinal vascular occlusion in patients with the COVID-19 virus. Int. J. Retina Vitreous. 2022, 8, 45. [Google Scholar] [CrossRef]
- Seirafianpour, F.; Mozafarpoor, S.; Fattahi, N.; Sadeghzadeh-Bazargan, A.; Hanifiha, M.; Goodarzi, A. Treatment of COVID-19 with pentoxifylline: Could it be a potential adjuvant therapy? Dermatol. Ther. 2020, 33, e13733. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, M.T.; Cesarone, M.R.; Belcaro, G.; Incandela, L.; Steigerwalt, R.; Nicolaides, A.N.; Griffin, M.; Geroulakos, G. Treatment of retinal vein thrombosis with pentoxifylline: A controlled, randomized trial. Angiology 2002, 53 (Suppl. S1), S35–S38. [Google Scholar] [PubMed]
- Mostafa-Hedeab, G.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Jeandet, P.; Saad, H.M.; Batiha, G.E. A raising dawn of pentoxifylline in management of inflammatory disorders in COVID-19. Inflammopharmacology 2022, 30, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Griesel, M.; Mikolajewska, A.; Metzendorf, M.I.; Fischer, A.L.; Stegemann, M.; Spagl, M.; Nair, A.A.; Daniel, J.; Fichtner, F.; et al. Systemic corticosteroids for the treatment of COVID-19: Equity-related analyses and update on evidence. Cochrane Database Syst. Rev. 2022, 11, CD014963. [Google Scholar] [CrossRef] [PubMed]
- Tempest-Roe, S.; Joshi, L.; Dick, A.D.; Taylor, S.R. Local therapies for inflammatory eye disease in translation: Past, present and future. BMC Ophthalmol. 2013, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Shivpuri, A.; Turtsevich, I.; Solebo, A.L.; Compeyrot-Lacassagne, S. Pediatric uveitis: Role of the pediatrician. Front. Pediatr. 2022, 10, 874711. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, D.; Ali, Y.; Menezo, V.; Taylor, S.R.J. The Use of Sustained Release Intravitreal Steroid Implants in Non-Infectious Uveitis Affecting the Posterior Segment of the Eye. Ophthalmol. Ther. 2022, 11, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Su, G.; Yang, P. Risk factors, clinical features and treatment of Behçet’s disease uveitis. Prog. Retin. Eye Res. 2023, 97, 101216. [Google Scholar] [CrossRef]
- Capittini, C.; Rebuffi, C.; Lenti, M.V.; Di Sabatino, A.; Tinelli, C.; Martinetti, M.; De Silvestri, A. Global Meta-Analysis on the Association between Behcet Syndrome and Polymorphisms from the HLA Class I (A, B, and C) and Class II (DRB1, DQB1, and DPB1) Genes. Dis. Markers 2021, 2021, 9348697. [Google Scholar] [CrossRef]
- Kong, N.C.; Nasruruddin, B.A.; Murad, S.; Ong, K.J.; Sukumaran, K.D. HLA antigens in Malay patients with systemic lupus erythematosus. Lupus 1994, 3, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Rigby, R.J.; Dawkins, R.L.; Wetherall, J.D.; Hawkins, B.R. HLA in systemic lupus erythematosus: Influence on severity. Tissue Antigens 1978, 12, 25–31. [Google Scholar] [PubMed]
- Scharf, Y.; Zonis, S. Histocompatibility antigens (HLA) and uveitis. Surv. Ophthalmol. 1980, 24, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.J.; Vaughan, H.; Tait, B.D.; Mackay, I.R. Histocompatibility antigens (HLA): Associations with immunopathic diseases and with responses to microbial antigens. Aust. N. Z. J. Med. 1977, 7, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Bertrams, H.J.; Kuwert, E.K. Association of histocompatibility haplotype HLA-A3-B7 with multiple sclerosis. J. Immunol. 1976, 117 Pt 2, 1906–1912. [Google Scholar] [CrossRef]
- Matsumoto, K.; Fukunari, K.; Ikeda, Y.; Miyazono, M.; Kishi, T.; Matsumoto, R.; Fukuda, M.; Uchiumi, S.; Yoshizaki, M.; Nonaka, Y.; et al. A report of an adult case of tubulointerstitial nephritis and uveitis (TINU) syndrome, with a review of 102 Japanese cases. Am. J. Case Rep. 2015, 28, 119–123. [Google Scholar] [CrossRef]
- Matyushkina, D.; Shokina, V.; Tikhonova, P.; Manuvera, V.; Shirokov, D.; Kharlampieva, D.; Lazarev, V.; Varizhuk, A.; Vedekhina, T.; Pavlenko, A.; et al. Autoimmune Effect of Antibodies against the SARS-CoV-2 Nucleoprotein. Viruses 2022, 14, 1141. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modrzejewska, M.; Cyrankiewicz, J.; Zdanowska, O.; Bosy-Gąsior, W. Isolated Depo-Medrol Administration under Tenon’s Capsule for Post-COVID-19 Uveitis in a Child: A Case Report and Literature Review. J. Clin. Med. 2024, 13, 1341. https://doi.org/10.3390/jcm13051341
Modrzejewska M, Cyrankiewicz J, Zdanowska O, Bosy-Gąsior W. Isolated Depo-Medrol Administration under Tenon’s Capsule for Post-COVID-19 Uveitis in a Child: A Case Report and Literature Review. Journal of Clinical Medicine. 2024; 13(5):1341. https://doi.org/10.3390/jcm13051341
Chicago/Turabian StyleModrzejewska, Monika, Joanna Cyrankiewicz, Oliwia Zdanowska, and Wiktoria Bosy-Gąsior. 2024. "Isolated Depo-Medrol Administration under Tenon’s Capsule for Post-COVID-19 Uveitis in a Child: A Case Report and Literature Review" Journal of Clinical Medicine 13, no. 5: 1341. https://doi.org/10.3390/jcm13051341
APA StyleModrzejewska, M., Cyrankiewicz, J., Zdanowska, O., & Bosy-Gąsior, W. (2024). Isolated Depo-Medrol Administration under Tenon’s Capsule for Post-COVID-19 Uveitis in a Child: A Case Report and Literature Review. Journal of Clinical Medicine, 13(5), 1341. https://doi.org/10.3390/jcm13051341





