Entero-Cutaneous and Entero-Atmospheric Fistulas: Insights into Management Using Negative Pressure Wound Therapy
Abstract
1. Introduction
2. Definition of Fistula
3. Etiology of ECF
4. EAF and OA
5. Classification and Evolution
6. Physiopathology
7. Outcomes
8. Treatment
- (a)
- Recognition and management of sepsis: organ dysfunction or progressive organ failure should be promptly managed;
- (b)
- Source control is crucial for the resolution of sepsis;
- (c)
- Antibiotic regimen based on culture results;
- (d)
- Reducing fistula output: nil per os, a nasogastric tube as well as an attempt to reduce secretions of the gastrointestinal tract by administering proton pump inhibitors and to reduce enteric and pancreatic secretions using somatostatin or octreotide [106];
- (e)
- Nutritional support: In the presence of OA, the patient is in a hypercatabolic state. Hypercatabolism is further worsened by the presence of an EAF. The main parameters on which nutritional support should be based are the following: (a) increased caloric requirements, usually calculated by 30–35 kcal/kg/day; (b) increased protein depriving, calculated by adding 1.5 g protein/kg/day and 2 g protein losses for each liter of fluid collected from the raw surface of the OA; and (c) deficiencies of vitamins and trace elements. Adequate nutritional support based on the patient’s nutritional status, a positive nitrogen balance, adequate trace minerals, and vitamin replacement, along with glycemic control, may allow the surgeon to proceed to surgical treatment of the fistula. Additionally, several known parameters, such as weight, prealbumin, albumin, and transferrin, are correlated with postoperative mortality and morbidity and spontaneous fistula closure rates [92,107,108].
9. NPWT Assisted Closure
| Author | Number of Patients/ | EAFs | Fistula Closure | Mortality |
|---|---|---|---|---|
| Woodfield et al. [148] | 3 | 0 | 100% | 0 |
| Goverman et al. [124] | 10 | 5 | 60% | 40% |
| Wainstein et al. [88] | 91 | 179 | 80.2% | 16.5% |
| Verhaalen et al. [149] | 8 | 16 | 90% | 10% |
| D’Hondt et al. [150] | 9 | 17 | 100% | 11.1% |
| Wang et al. [125] | 11 | 11 | 100% | 0 |
| Ozer et al. [151] | 1 | 1 | 100% | 0 |
| Yetisir et al. [152] | 1 | 1 | 0 | 0 |
| Tavusbay et al. [84] | 18 | Not reported | 55.6% | 44.4% |
| Pepe et al. [1] | 8 | 4 | 62.5% | 0 |
| Heineman et al. [153] | 4 | 4 | 0 | 0 |
| Jaguscik et al. [154] | 1 | 1 | 0 | 0 |
| Bobkiewicz et al. [144] | 16 | 31 | 61.3% | 9.7% |
| Miranda et al. [126] | 2 | 2 | Not reported | 0 |
| Ortiz et al. [155] | 31 | 5 | 74% | 6% |
| Wirth et al. [156] | 3 | 3 | 100% | 0 |
| Sun et al. [123] | 83 | Not reported | 71.1% | 0 |
| Wainstein et al. [30] | 77 | 77 | 81.8% | 9% |
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pepe, G.; Magalini, S.; Callari, C.; Persiani, R.; Lodoli, C.; Gui, D. Vacuum Assisted Closure (VAC) therapyTM as a swiss knife multi-tool for enteric fistula closure: Tips and tricks: A pilot study. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2527–2532. [Google Scholar] [PubMed]
- Di Saverio, S.; Tarasconi, A.; Inaba, K.; Navsaria, P.; Coccolini, F.; Costa Navarro, D.; Mandrioli, M.; Vassiliu, P.; Jovine, E.; Catena, F.; et al. Open abdomen with concomitant enteroatmospheric fistula: Attempt to rationalize the approach to a surgical nightmare and proposal of a clinical algorithm. J. Am. Coll. Surg. 2015, 220, e23–e33. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Andrade, D.F.; Campos, G.M.; Matias, J.E.; Coelho, J.C. A multivariate model to determine prognostic factors in gastrointestinal fistulas. J. Am. Coll. Surg. 1999, 188, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Galie, K.L.; Whitlow, C.B. Postoperative enterocutaneous fistula: When to reoperate and how to succeed. Clin. Colon. Rectal Surg. 2006, 19, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.E.; Fabian, T.C.; Magnotti, L.J.; Schroeppel, T.J.; Bee, T.K.; Maish, G.O., 3rd; Savage, S.A.; Laing, A.E.; Barker, A.B.; Croce, M.A. A ten-year review of enterocutaneous fistulas after laparotomy for trauma. J. Trauma. 2009, 67, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Bruhin, A.; Ferreira, F.; Chariker, M.; Smith, J.; Runkel, N. Systematic review and evidence based recommendations for the use of negative pressure wound therapy in the open abdomen. Int. J. Surg. 2014, 12, 1105–1114. [Google Scholar] [CrossRef]
- Brisinda, G.; Chiarello, M.M.; Crocco, A.; Adams, N.J.; Fransvea, P.; Vanella, S. Postoperative mortality and morbidity after D2 lymphadenectomy for gastric cancer: A retrospective cohort study. World J. Gastroenterol. 2022, 28, 381–398. [Google Scholar] [CrossRef]
- Navsaria, P.; Nicol, A.; Hudson, D.; Cockwill, J.; Smith, J. Negative pressure wound therapy management of the “open abdomen” following trauma: A prospective study and systematic review. World J. Emerg. Surg. 2013, 8, 4. [Google Scholar] [CrossRef]
- Fitzgerald, C.A.; Broecker, J.; Park, C.; Dumas, R.P. Primary Repair Versus Resection for AAST Grade I and II Colon Injuries: Does the Type of Repair Matter? J. Surg. Res. 2023, 295, 370–375. [Google Scholar] [CrossRef]
- Vengail, S.; Chandrakar, D.; Naik, A.K.; Nayak, A.K.; Mahajan, A.; Dutta, P. Assessment of Risk Factors for Enteric Fistula and Intra-Abdominal Sepsis in Patients with Open Abdomen in Trauma: An Original Research. J. Pharm. Bioallied Sci. 2023, 15, S273–S276. [Google Scholar] [CrossRef]
- Fico, V.; Altieri, G.; Di Grezia, M.; Bianchi, V.; Chiarello, M.M.; Pepe, G.; Tropeano, G.; Brisinda, G. Surgical complications of oncological treatments: A narrative review. World J. Gastrointest. Surg. 2023, 15, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.J.; Leppaniemi, A.; Tolonen, M.; Mentula, P.; Bjorck, M.; Kirkpatrick, A.W.; Sugrue, M.; Pereira, B.M.; Petersson, U.; Coccolini, F.; et al. The open abdomen in trauma, acute care, and vascular and endovascular surgery: Comprehensive, expert, narrative review. BJS Open 2023, 7, zrad084. [Google Scholar] [CrossRef] [PubMed]
- Dubose, J.J.; Lundy, J.B. Enterocutaneous fistulas in the setting of trauma and critical illness. Clin. Colon. Rectal Surg. 2010, 23, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.F., Jr.; Ivatury, R.R. Enterocutaneous fistulas: An overview. Eur. J. Trauma. Emerg. Surg. 2011, 37, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Latifi, R.; Joseph, B.; Kulvatunyou, N.; Wynne, J.L.; O’Keeffe, T.; Tang, A.; Friese, R.; Rhee, P.M. Enterocutaneous fistulas and a hostile abdomen: Reoperative surgical approaches. World J. Surg. 2012, 36, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Polk, T.M.; Schwab, C.W. Metabolic and nutritional support of the enterocutaneous fistula patient: A three-phase approach. World J. Surg. 2012, 36, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.J.; Dubose, J.J.; Scalea, T.M.; Holcomb, J.B.; Shrestha, B.; Okoye, O.; Inaba, K.; Bee, T.K.; Fabian, T.C.; Whelan, J.F.; et al. Independent predictors of enteric fistula and abdominal sepsis after damage control laparotomy: Results from the prospective AAST Open Abdomen registry. JAMA Surg. 2013, 148, 947–954. [Google Scholar] [CrossRef]
- Wainstein, D.E.; Sisco, P.; Deforel, M.L.; Irigoyen, M.; Devoto, J.; Zarate, J.M. Systematic and Specific Treatment of Patients with Enteroatmospheric Fistulas: From Initial Conservative Treatment to Definitive Surgery. Surg. Technol. Int. 2016, 28, 73–81. [Google Scholar]
- Gribovskaja-Rupp, I.; Melton, G.B. Enterocutaneous Fistula: Proven Strategies and Updates. Clin. Colon. Rectal Surg. 2016, 29, 130–137. [Google Scholar] [CrossRef]
- Haffejee, A.A. Surgical management of high output enterocutaneous fistulae: A 24-year experience. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 309–316. [Google Scholar] [CrossRef]
- Alvarez, C.; McFadden, D.W.; Reber, H.A. Complicated enterocutaneous fistulas: Failure of octreotide to improve healing. World J. Surg. 2000, 24, 533–537; discussion 538. [Google Scholar] [CrossRef]
- Erdmann, D.; Drye, C.; Heller, L.; Wong, M.S.; Levin, S.L. Abdominal wall defect and enterocutaneous fistula treatment with the Vacuum-Assisted Closure (V.A.C.) system. Plast. Reconstr. Surg. 2001, 108, 2066–2068. [Google Scholar] [CrossRef]
- Gunn, L.A.; Follmar, K.E.; Wong, M.S.; Lettieri, S.C.; Levin, L.S.; Erdmann, D. Management of enterocutaneous fistulas using negative-pressure dressings. Ann. Plast. Surg. 2006, 57, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.G.; Johnson, E.K. Controversies in the care of the enterocutaneous fistula. Surg. Clin. N. Am. 2013, 93, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Abu-Zidan, F.M.; Ansaloni, L.; Bala, M.; Beltran, M.A.; Biffl, W.L.; Catena, F.; Chiara, O.; Coccolini, F.; Coimbra, R.; et al. The role of the open abdomen procedure in managing severe abdominal sepsis: WSES position paper. World J. Emerg. Surg. 2015, 10, 35. [Google Scholar] [CrossRef]
- Coccolini, F.; Biffl, W.; Catena, F.; Ceresoli, M.; Chiara, O.; Cimbanassi, S.; Fattori, L.; Leppaniemi, A.; Manfredi, R.; Montori, G.; et al. The open abdomen, indications, management and definitive closure. World J. Emerg. Surg. 2015, 10, 32. [Google Scholar] [CrossRef]
- Chiara, O.; Cimbanassi, S.; Biffl, W.; Leppaniemi, A.; Henry, S.; Scalea, T.M.; Catena, F.; Ansaloni, L.; Chieregato, A.; de Blasio, E.; et al. International consensus conference on open abdomen in trauma. J. Trauma. Acute Care Surg. 2016, 80, 173–183. [Google Scholar] [CrossRef]
- Pereira, B.; Duchesne, J.; Concon-Filho, A.; Leppaniemi, A. Entero-atmospheric fistula migration: A new management alternative for complex septic open abdomen. Anaesthesiol. Intensive Ther. 2020, 52, 56–62. [Google Scholar] [CrossRef]
- Di Saverio, S.; Tarasconi, A.; Walczak, D.A.; Cirocchi, R.; Mandrioli, M.; Birindelli, A.; Tugnoli, G. Classification, prevention and management of entero-atmospheric fistula: A state-of-the-art review. Langenbecks Arch. Surg. 2016, 401, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wainstein, D.E.; Calvi, R.J.; Rezzonico, F.; Deforel, M.L.; Perrone, N.; Sisco, P. Management of enteroatmospheric fistula: A ten-year experience following fifteen years of learning. Surgery 2023, 173, 1079–1085. [Google Scholar] [CrossRef]
- Li, J.; Ren, J.; Zhu, W.; Yin, L.; Han, J. Management of enterocutaneous fistulas: 30-year clinical experience. Chin. Med. J. 2003, 116, 171–175. [Google Scholar] [PubMed]
- Visschers, R.G.; Olde Damink, S.W.; Winkens, B.; Soeters, P.B.; van Gemert, W.G. Treatment strategies in 135 consecutive patients with enterocutaneous fistulas. World J. Surg. 2008, 32, 445–453. [Google Scholar] [CrossRef]
- Denicu, M.M.; Cartu, D.; Ciorbagiu, M.; Nemes, R.N.; Surlin, V.; Ramboiu, S.; Chiutu, L.C. Therapeutic Options in Postoperative Enterocutaneous Fistula-A Retrospective Case Series. Medicina 2022, 58, 880. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.; Kearney, S.; Zhou, K.; Woo, K. Topical Management of Enterocutaneous and Enteroatmospheric Fistulas: A Systematic Review. Wound Manag. Prev. 2020, 66, 26–37. [Google Scholar] [CrossRef]
- Terzi, C.; Egeli, T.; Canda, A.E.; Arslan, N.C. Management of enteroatmospheric fistulae. Int. Wound J. 2014, 11 (Suppl. S1), 17–21. [Google Scholar] [CrossRef]
- Chiarello, M.M.; Fransvea, P.; Cariati, M.; Adams, N.J.; Bianchi, V.; Brisinda, G. Anastomotic leakage in colorectal cancer surgery. Surg. Oncol. 2022, 40, 101708. [Google Scholar] [CrossRef]
- Brisinda, G.; Chiarello, M.M.; Pepe, G.; Cariati, M.; Fico, V.; Mirco, P.; Bianchi, V. Anastomotic leakage in rectal cancer surgery: Retrospective analysis of risk factors. World J. Clin. Cases 2022, 10, 13321–13336. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, M.M.; Pepe, G.; Fico, V.; Bianchi, V.; Tropeano, G.; Altieri, G.; Brisinda, G. Therapeutic strategies in Crohn’s disease in an emergency surgical setting. World J. Gastroenterol. 2022, 28, 1902–1921. [Google Scholar] [CrossRef]
- Andreyev, H.J.; Davidson, S.E.; Gillespie, C.; Allum, W.H.; Swarbrick, E. Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut 2012, 61, 179–192. [Google Scholar] [CrossRef]
- Birch, J.C.; Khatri, G.; Watumull, L.M.; Arriaga, Y.E.; Leyendecker, J.R. Unintended Consequences of Systemic and Ablative Oncologic Therapy in the Abdomen and Pelvis. Radiographics 2018, 38, 1158–1179. [Google Scholar] [CrossRef]
- Andreyev, H.J.; Vlavianos, P.; Blake, P.; Dearnaley, D.; Norman, A.R.; Tait, D. Gastrointestinal symptoms after pelvic radiotherapy: Role for the gastroenterologist? Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1464–1471. [Google Scholar] [CrossRef]
- Andreyev, J. Gastrointestinal complications of pelvic radiotherapy: Are they of any importance? Gut 2005, 54, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gao, W.; Spratt, D.E.; Sun, Y.; Xing, L. Management of gastrointestinal perforation related to radiation. Int. J. Clin. Oncol. 2020, 25, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Anker, C.J.; Grossmann, K.F.; Atkins, M.B.; Suneja, G.; Tarhini, A.A.; Kirkwood, J.M. Avoiding Severe Toxicity From Combined BRAF Inhibitor and Radiation Treatment: Consensus Guidelines from the Eastern Cooperative Oncology Group (ECOG). Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 632–646. [Google Scholar] [CrossRef]
- Inoue, T.; Kinoshita, H.; Komai, Y.; Kawabata, T.; Kawa, G.; Uemura, Y.; Matsuda, T. Two cases of gastrointestinal perforation after radiotherapy in patients receiving tyrosine kinase inhibitor for advanced renal cell carcinoma. World J. Surg. Oncol. 2012, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, T.; Tsukuda, K.; Toyooka, S.; Kagawa, S.; Naomoto, Y.; Takemoto, M.; Katsui, K.; Kanazawa, S.; Maki, Y.; Masuda, H.; et al. Ileal perforation induced by acute radiation injury under gefitinib treatment. Int. J. Clin. Oncol. 2011, 16, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Reginelli, A.; Sangiovanni, A.; Vacca, G.; Belfiore, M.P.; Pignatiello, M.; Viscardi, G.; Clemente, A.; Urraro, F.; Cappabianca, S. Chemotherapy-induced bowel ischemia: Diagnostic imaging overview. Abdom. Radiol. 2022, 47, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Katabathina, V.S.; Restrepo, C.S.; Betancourt Cuellar, S.L.; Riascos, R.F.; Menias, C.O. Imaging of oncologic emergencies: What every radiologist should know. Radiographics 2013, 33, 1533–1553. [Google Scholar] [CrossRef]
- Sussman, J.J. Surgical emergencies in the cancer patient. In Surgery: Basic Science and Clinical Evidence; Norton, J.A., Ed.; Springer-Verlag: New York, NY, USA, 2007; p. 2117. [Google Scholar]
- Heimann, D.M.; Schwartzentruber, D.J. Gastrointestinal perforations associated with interleukin-2 administration. J. Immunother. 2004, 27, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Cronin, C.G.; O’Connor, M.; Lohan, D.G.; Keane, M.; Roche, C.; Bruzzi, J.F.; Murphy, J.M. Imaging of the gastrointestinal complications of systemic chemotherapy. Clin. Radiol. 2009, 64, 724–733. [Google Scholar] [CrossRef]
- Liaw, C.C.; Huang, J.S.; Wang, H.M.; Wang, C.H. Spontaneous gastroduodenal perforation in patients with cancer receiving chemotherapy and steroids. Report of four cases combining 5-fluorouracil infusion and cisplatin with antiemetics dexamethasone. Cancer 1993, 72, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.G.; Piver, M.S. Intestinal perforation secondary to paclitaxel. Gynecol. Oncol. 1995, 57, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Hapani, S.; Chu, D.; Wu, S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: A meta-analysis. Lancet Oncol. 2009, 10, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Garant, A.; Des Groseilliers, S.; Martin, L.; Ferland, E.; Vuong, T. Late anastomotic dehiscence during bevacizumab therapy for patients with colorectal cancer. Clin. Oncol. 2011, 23, 497–498. [Google Scholar] [CrossRef] [PubMed]
- Kabbinavar, F.F.; Flynn, P.J.; Kozloff, M.; Ashby, M.A.; Sing, A.; Barr, C.E.; Grothey, A. Gastrointestinal perforation associated with bevacizumab use in metastatic colorectal cancer: Results from a large treatment observational cohort study. Eur. J. Cancer 2012, 48, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Badgwell, B.D.; Camp, E.R.; Feig, B.; Wolff, R.A.; Eng, C.; Ellis, L.M.; Cormier, J.N. Management of bevacizumab-associated bowel perforation: A case series and review of the literature. Ann. Oncol. 2008, 19, 577–582. [Google Scholar] [CrossRef]
- Bonifazi, M.; Rossi, M.; Moja, L.; Scigliano, V.D.; Franchi, M.; La Vecchia, C.; Zocchetti, C.; Negri, E. Bevacizumab in clinical practice: Prescribing appropriateness relative to national indications and safety. Oncologist 2012, 17, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Yoshikawa, K.; Higashijima, J.; Miyatani, T.; Tokunaga, T.; Nishi, M.; Takasu, C.; Kashihara, H.; Takehara, Y.; Shimada, M. Bevacizumab-associated intestinal perforation and perioperative complications in patients receiving bevacizumab. Ann. Gastroenterol. Surg. 2020, 4, 151–155. [Google Scholar] [CrossRef]
- Rutkowski, P.; Ruka, W. Emergency surgery in the era of molecular treatment of solid tumours. Lancet Oncol. 2009, 10, 157–163. [Google Scholar] [CrossRef]
- Sliesoraitis, S.; Tawfik, B. Bevacizumab-induced bowel perforation. J. Am. Osteopath. Assoc. 2011, 111, 437–441. [Google Scholar]
- Ouchi, R.; Okada, K.; Usui, K.; Kurata, N.; Suzuki, S.; Nagao, M.; Watanabe, Y.; Koyama, K. Intestinal Perforation in a Patient with Colon Cancer during Treatment with Regorafenib: A Case Report and Review of the Literature. Tohoku J. Exp. Med. 2021, 254, 207–211. [Google Scholar] [CrossRef]
- Cheon, Y.H.; Kim, M.J.; Kang, M.G.; Kim, H.J.; Lee, S.S.; Kim, C.Y.; Jeon, D.H.; Kim, Y.E.; Lee, G.W. Bowel perforation after erlotinib treatment in a patient with non-small cell lung cancer. Yonsei Med. J. 2011, 52, 695–698. [Google Scholar] [CrossRef][Green Version]
- Walraven, M.; Witteveen, P.O.; Lolkema, M.P.; van Hillegersberg, R.; Voest, E.E.; Verheul, H.M. Antiangiogenic tyrosine kinase inhibition related gastrointestinal perforations: A case report and literature review. Angiogenesis 2011, 14, 135–141. [Google Scholar] [CrossRef]
- Okamoto, I.; Miyazaki, M.; Morinaga, R.; Kaneda, H.; Ueda, S.; Hasegawa, Y.; Satoh, T.; Kawada, A.; Fukuoka, M.; Fukino, K.; et al. Phase I clinical and pharmacokinetic study of sorafenib in combination with carboplatin and paclitaxel in patients with advanced non-small cell lung cancer. Investig. New Drugs 2010, 28, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Eng, F.C.; Easson, A.M.; Szentgyorgyi, E.; Knox, J.J. Sorafenib and surgical complications: A case report of adverse reaction to sorafenib during treatment for renal cell carcinoma. Eur. J. Surg. Oncol. 2009, 35, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Frieling, T.; Heise, J.; Wassilew, S.W. Multiple colon ulcerations, perforation and death during treatment of malignant melanoma with sorafenib. Dtsch. Med. Wochenschr. 2009, 134, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Yetisir, F.; Sarer, A.E. Operative Management of Enteroatmospheric Fistula in Bjorck 4 Open Abdomen Patients by the Help of Laparoscopic Lateral Approach. Indian. J. Surg. 2017, 79, 173–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Serrano Concha, K.; Morales Mayorga, H.; Acosta Farina, D.; Mendoza Saldarreaga, L.; Polit Guerrero, V.; Oliveros Rivero, J.; Acosta Bowen, D. Negative pressure device used in pediatric patients with Hostile abdomen. Case series. Cir. Pediatr. 2024, 37, 37–41. [Google Scholar] [CrossRef]
- Theodorou, A.; Jedig, A.; Manekeller, S.; Willms, A.; Pantelis, D.; Matthaei, H.; Schafer, N.; Kalff, J.C.; von Websky, M.W. Long Term Outcome After Open Abdomen Treatment: Function and Quality of Life. Front. Surg. 2021, 8, 590245. [Google Scholar] [CrossRef]
- Bleier, J.I.; Hedrick, T. Metabolic support of the enterocutaneous fistula patient. Clin. Colon. Rectal Surg. 2010, 23, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Barie, P.S. Serious intra-abdominal infections. Curr. Opin. Crit. Care 2001, 7, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Waibel, B.H.; Rotondo, M.F. Damage control in trauma and abdominal sepsis. Crit. Care Med. 2010, 38, S421–S430. [Google Scholar] [CrossRef] [PubMed]
- Bjorck, M.; Kirkpatrick, A.W.; Cheatham, M.; Kaplan, M.; Leppaniemi, A.; De Waele, J.J. Amended Classification of the Open Abdomen. Scand. J. Surg. 2016, 105, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, E.J.; Bugaev, N.; Appelbaum, R.; Goldenberg-Sandau, A.; Baltazar, G.A.; Posluszny, J.; Dultz, L.; Kartiko, S.; Kasotakis, G.; Como, J.; et al. Management of the open abdomen: A systematic review with meta-analysis and practice management guideline from the Eastern Association for the Surgery of Trauma. J. Trauma. Acute Care Surg. 2022, 93, e110–e118. [Google Scholar] [CrossRef]
- Coccolini, F.; Roberts, D.; Ansaloni, L.; Ivatury, R.; Gamberini, E.; Kluger, Y.; Moore, E.E.; Coimbra, R.; Kirkpatrick, A.W.; Pereira, B.M.; et al. The open abdomen in trauma and non-trauma patients: WSES guidelines. World J. Emerg. Surg. 2018, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, A.W.; Roberts, D.J.; Jaeschke, R.; De Waele, J.J.; De Keulenaer, B.L.; Duchesne, J.; Bjorck, M.; Leppaniemi, A.; Ejike, J.C.; Sugrue, M.; et al. Methodological background and strategy for the 2012-2013 updated consensus definitions and clinical practice guidelines from the abdominal compartment society. Anaesthesiol. Intensive Ther. 2015, 47, s63–s77. [Google Scholar] [CrossRef]
- Sermoneta, D.; Di Mugno, M.; Spada, P.L.; Lodoli, C.; Carvelli, M.E.; Magalini, S.C.; Cavicchioni, C.; Bocci, M.G.; Martorelli, F.; Brizi, M.G.; et al. Intra-abdominal vacuum-assisted closure (VAC) after necrosectomy for acute necrotising pancreatitis: Preliminary experience. Int. Wound J. 2010, 7, 525–530. [Google Scholar] [CrossRef]
- Brooke, J.; El-Ghaname, A.; Napier, K.; Sommerey, L. Nurses Specialized in Wound, Ostomy and Continence Canada (NSWOCC) Management of Enterocutaneous Fistula and Enteroatmospheric Fistula: Development of Best Practice Recommendations. J. Wound Ostomy Cont. Nurs. 2019, 46, 346–347. [Google Scholar] [CrossRef]
- Coccolini, F.; Ceresoli, M.; Kluger, Y.; Kirkpatrick, A.; Montori, G.; Salvetti, F.; Fugazzola, P.; Tomasoni, M.; Sartelli, M.; Ansaloni, L.; et al. Open abdomen and entero-atmospheric fistulae: An interim analysis from the International Register of Open Abdomen (IROA). Injury 2019, 50, 160–166. [Google Scholar] [CrossRef]
- Di Saverio, S.; Villani, S.; Biscardi, A.; Giorgini, E.; Tugnoli, G. Open abdomen with concomitant enteroatmospheric fistula: Validation, refinements, and adjuncts to a novel approach. J. Trauma. 2011, 71, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Gross, D.J.; Smith, M.C.; Zangbar-Sabegh, B.; Chao, K.; Chang, E.; Boudourakis, L.; Muthusamy, M.; Roudnitsky, V.; Schwartz, T. Challenge of uncontrolled enteroatmospheric fistulas. Trauma. Surg. Acute Care Open 2019, 4, e000381. [Google Scholar] [CrossRef] [PubMed]
- Tavusbay, C.; Genc, H.; Cin, N.; Kar, H.; Kamer, E.; Atahan, K.; Haciyanli, M. Use of a vacuum-assisted closure system for the management of enteroatmospheric fistulae. Surg. Today 2015, 45, 1102–1111. [Google Scholar] [CrossRef]
- Diaz, J.J., Jr.; Mauer, A.; May, A.K.; Miller, R.; Guy, J.S.; Morris, J.A., Jr. Bedside laparotomy for trauma: Are there risks? Surg. Infect. 2004, 5, 15–20. [Google Scholar] [CrossRef]
- Anastasiu, M.; Surlin, V.; Beuran, M. The Management of the Open Abdomen—A Literature Review. Chirurgia 2021, 116, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Schecter, W.P. Management of enterocutaneous fistulas. Surg. Clin. North Am. 2011, 91, 481–491. [Google Scholar] [CrossRef]
- Wainstein, D.E.; Fernandez, E.; Gonzalez, D.; Chara, O.; Berkowski, D. Treatment of high-output enterocutaneous fistulas with a vacuum-compaction device. A ten-year experience. World J. Surg. 2008, 32, 430–435. [Google Scholar] [CrossRef]
- Martinez, J.L.; Luque-de-Leon, E.; Mier, J.; Blanco-Benavides, R.; Robledo, F. Systematic management of postoperative enterocutaneous fistulas: Factors related to outcomes. World J. Surg. 2008, 32, 436–443; discussion 444. [Google Scholar] [CrossRef]
- Williams, L.J.; Zolfaghari, S.; Boushey, R.P. Complications of enterocutaneous fistulas and their management. Clin. Colon. Rectal Surg. 2010, 23, 209–220. [Google Scholar] [CrossRef]
- Dudrick, S.J.; Panait, L. Metabolic consequences of patients with gastrointestinal fistulas. Eur. J. Trauma. Emerg. Surg. 2011, 37, 215–225. [Google Scholar] [CrossRef]
- Pironi, L.; Arends, J.; Baxter, J.; Bozzetti, F.; Pelaez, R.B.; Cuerda, C.; Forbes, A.; Gabe, S.; Gillanders, L.; Holst, M.; et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin. Nutr. 2015, 34, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Klek, S.; Forbes, A.; Gabe, S.; Holst, M.; Wanten, G.; Irtun, O.; Damink, S.O.; Panisic-Sekeljic, M.; Pelaez, R.B.; Pironi, L.; et al. Management of acute intestinal failure: A position paper from the European Society for Clinical Nutrition and Metabolism (ESPEN) Special Interest Group. Clin. Nutr. 2016, 35, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Frileux, P.; Cugnenc, P.H.; Honiger, J.; Ollivier, J.M.; Parc, R. High-output external fistulae of the small bowel: Management with continuous enteral nutrition. Br. J. Surg. 1989, 76, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Hollington, P.; Mawdsley, J.; Lim, W.; Gabe, S.M.; Forbes, A.; Windsor, A.J. An 11-year experience of enterocutaneous fistula. Br. J. Surg. 2004, 91, 1646–1651. [Google Scholar] [CrossRef]
- Quinn, M.; Falconer, S.; McKee, R.F. Management of Enterocutaneous Fistula: Outcomes in 276 Patients. World J. Surg. 2017, 41, 2502–2511. [Google Scholar] [CrossRef]
- Boolaky, K.N.; Tariq, A.H.; Hardcastle, T.C. Open abdomen in the trauma ICU patient: Who? when? why? and what are the outcome results? Eur. J. Trauma. Emerg. Surg. 2022, 48, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Rohith, V.N.; Arya, S.V.; Rani, A.; Chejara, R.K.; Sharma, A.; Arora, J.K.; Kalwaniya, D.S.; Tolat, A.; Pawan, G.; Singh, A.; et al. Preoperative Serum Albumin Level as a Predictor of Abdominal Wound-Related Complications After Emergency Exploratory Laparotomy. Cureus 2022, 14, e31980. [Google Scholar] [CrossRef]
- Marinis, A.; Gkiokas, G.; Argyra, E.; Fragulidis, G.; Polymeneas, G.; Voros, D. “Enteroatmospheric fistulae”—Gastrointestinal openings in the open abdomen: A review and recent proposal of a surgical technique. Scand. J. Surg. 2013, 102, 61–68. [Google Scholar] [CrossRef]
- Becker, H.P.; Willms, A.; Schwab, R. Small bowel fistulas and the open abdomen. Scand. J. Surg. 2007, 96, 263–271. [Google Scholar] [CrossRef]
- Bjorck, M.; Bruhin, A.; Cheatham, M.; Hinck, D.; Kaplan, M.; Manca, G.; Wild, T.; Windsor, A. Classification—Important step to improve management of patients with an open abdomen. World J. Surg. 2009, 33, 1154–1157. [Google Scholar] [CrossRef]
- Kaushal, M.; Carlson, G.L. Management of enterocutaneous fistulas. Clin. Colon. Rectal Surg. 2004, 17, 79–88. [Google Scholar] [CrossRef]
- Samad, S.; Anele, C.; Akhtar, M.; Doughan, S. Implementing a Pro-forma for Multidisciplinary Management of an Enterocutaneous Fistula: A Case Study. Ostomy Wound Manag. 2015, 61, 46–52. [Google Scholar]
- Tang, Q.Q.; Hong, Z.W.; Ren, H.J.; Wu, L.; Wang, G.F.; Gu, G.S.; Chen, J.; Zheng, T.; Wu, X.W.; Ren, J.A.; et al. Nutritional Management of Patients With Enterocutaneous Fistulas: Practice and Progression. Front. Nutr. 2020, 7, 564379. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, L.; Veverkova, L.; Zak, J.; Reska, M. Nutrition in open abdomen. Rozhl. Chir. 2021, 100, 83–87. [Google Scholar]
- Assenza, M.; Rossi, D.; De Gruttola, I.; Ballanti, C. Enterocutaneous fistula treatment: Case report and review of the literature. G. Chir. 2018, 39, 143–151. [Google Scholar]
- Meguid, M.M.; Muscaritoli, M. Current uses of total parenteral nutrition. Am. Fam. Physician 1993, 47, 383–394. [Google Scholar] [PubMed]
- Lloyd, D.A.; Gabe, S.M.; Windsor, A.C. Nutrition and management of enterocutaneous fistula. Br. J. Surg. 2006, 93, 1045–1055. [Google Scholar] [CrossRef]
- Connolly, P.T.; Teubner, A.; Lees, N.P.; Anderson, I.D.; Scott, N.A.; Carlson, G.L. Outcome of reconstructive surgery for intestinal fistula in the open abdomen. Ann. Surg. 2008, 247, 440–444. [Google Scholar] [CrossRef]
- Fazio, V.W.; Coutsoftides, T.; Steiger, E. Factors influencing the outcome of treatment of small bowel cutaneous fistula. World J. Surg. 1983, 7, 481–488. [Google Scholar] [CrossRef]
- Rahbour, G.; Siddiqui, M.R.; Ullah, M.R.; Gabe, S.M.; Warusavitarne, J.; Vaizey, C.J. A meta-analysis of outcomes following use of somatostatin and its analogues for the management of enterocutaneous fistulas. Ann. Surg. 2012, 256, 946–954. [Google Scholar] [CrossRef]
- Lynch, A.C.; Delaney, C.P.; Senagore, A.J.; Connor, J.T.; Remzi, F.H.; Fazio, V.W. Clinical outcome and factors predictive of recurrence after enterocutaneous fistula surgery. Ann. Surg. 2004, 240, 825–831. [Google Scholar] [CrossRef]
- Rahbour, G.; Gabe, S.M.; Ullah, M.R.; Thomas, G.P.; Al-Hassi, H.O.; Yassin, N.A.; Tozer, P.J.; Warusavitarne, J.; Vaizey, C.J. Seven-year experience of enterocutaneous fistula with univariate and multivariate analysis of factors associated with healing: Development of a validated scoring system. Colorectal Dis. 2013, 15, 1162–1170. [Google Scholar] [CrossRef]
- Runstrom, B.; Hallbook, O.; Nystrom, P.O.; Sjodahl, R.; Olaison, G. Outcome of 132 consecutive reconstructive operations for intestinal fistula—Staged operation without primary anastomosis improved outcome in retrospective analysis. Scand. J. Surg. 2013, 102, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Sriussadaporn, S.; Sriussadaporn, S.; Kritayakirana, K.; Pak-art, R. Operative management of small bowel fistulae associated with open abdomen. Asian J. Surg. 2006, 29, 1–7. [Google Scholar] [CrossRef]
- Singh, B.; Haffejee, A.A.; Allopi, L.; Moodley, J. Surgery for high-output small bowel enterocutaneous fistula: A 30-year experience. Int. Surg. 2009, 94, 262–268. [Google Scholar]
- Owen, R.M.; Love, T.P.; Perez, S.D.; Srinivasan, J.K.; Sharma, J.; Pollock, J.D.; Haack, C.I.; Sweeney, J.F.; Galloway, J.R. Definitive surgical treatment of enterocutaneous fistula: Outcomes of a 23-year experience. JAMA Surg. 2013, 148, 118–126. [Google Scholar] [CrossRef]
- Demetriades, D. Total management of the open abdomen. Int. Wound J. 2012, 9 (Suppl. S1), 17–24. [Google Scholar] [CrossRef]
- Rabel, T.; Bonnot, P.E.; Hadeedi, O.; Kepenekian, V.; Bernard, L.; Friggeri, A.; Glehen, O.; Passot, G. Negative-Pressure Wound Therapy for Open Abdomen in Surgical Reintervention after Curative Surgery of Peritoneal Malignancy Increases the Risk of Recurrence. Adv. Skin. Wound Care 2023, 36, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, M.; Agresta, F.; Attina, G.M.; Amabile, D.; Marchi, D.; “Complex Abdominal Wall Study” Italian Collaborative Group. “Complex abdominal wall” management: Evidence-based guidelines of the Italian Consensus Conference. Updates Surg. 2019, 71, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Gefen, R.; Garoufalia, Z.; Zhou, P.; Watson, K.; Emile, S.H.; Wexner, S.D. Treatment of enterocutaneous fistula: A systematic review and meta-analysis. Tech. Coloproctol. 2022, 26, 863–874. [Google Scholar] [CrossRef]
- Suter, K.J.L.; Fairweather, L.; Al-Habbal, Y.; Houli, N.; Jacobs, R.; Bui, H.T. How to isolate a high output enteroatmospheric fistula in the open abdomen with negative pressure therapy: An institution’s step by step guide to the VAC donut. ANZ J. Surg. 2023, 93, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Xu, X.; Luo, S.; Zhao, R.; Tian, W.; Huang, M.; Yao, Z. An alternative negative pressure treatment for enteroatmospheric fistula resulting from small intestinal leakage caused by incision dehiscence. Heliyon 2023, 9, e22045. [Google Scholar] [CrossRef] [PubMed]
- Goverman, J.; Yelon, J.A.; Platz, J.J.; Singson, R.C.; Turcinovic, M. The “Fistula VAC”, a technique for management of enterocutaneous fistulae arising within the open abdomen: Report of 5 cases. J. Trauma. 2006, 60, 428–431; discussion 431. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ren, J.; Liu, S.; Wu, X.; Gu, G.; Li, J. “Fistula patch”: Making the treatment of enteroatmospheric fistulae in the open abdomen easier. J. Trauma. Acute Care Surg. 2013, 74, 1175–1177. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.E.; Miranda, A.C. Enteroatmospheric fistula management by endoscopic gastrostomy PEG tube. Int. Wound J. 2017, 14, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Morykwas, M.J.; Argenta, L.C.; Shelton-Brown, E.I.; McGuirt, W. Vacuum-assisted closure: A new method for wound control and treatment: Animal studies and basic foundation. Ann. Plast. Surg. 1997, 38, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Trevelyan, S.L.; Carlson, G.L. Is TNP in the open abdomen safe and effective? J. Wound Care 2009, 18, 24–25. [Google Scholar] [CrossRef]
- Bee, T.K.; Croce, M.A.; Magnotti, L.J.; Zarzaur, B.L.; Maish, G.O., 3rd; Minard, G.; Schroeppel, T.J.; Fabian, T.C. Temporary abdominal closure techniques: A prospective randomized trial comparing polyglactin 910 mesh and vacuum-assisted closure. J. Trauma. 2008, 65, 337–342; discussion 342–334. [Google Scholar] [CrossRef]
- Fischer, J.E. A cautionary note: The use of vacuum-assisted closure systems in the treatment of gastrointestinal cutaneous fistula may be associated with higher mortality from subsequent fistula development. Am. J. Surg. 2008, 196, 1–2. [Google Scholar] [CrossRef]
- Caro, A.; Olona, C.; Jimenez, A.; Vadillo, J.; Feliu, F.; Vicente, V. Treatment of the open abdomen with topical negative pressure therapy: A retrospective study of 46 cases. Int. Wound J. 2011, 8, 274–279. [Google Scholar] [CrossRef]
- Fleischmann, W.; Strecker, W.; Bombelli, M.; Kinzl, L. Vacuum sealing as treatment of soft tissue damage in open fractures. Unfallchirurg 1993, 96, 488–492. [Google Scholar] [PubMed]
- Fleischmann, W.; Becker, U.; Bischoff, M.; Hoekstra, H. Vacuum sealing: Indication, technique, and results. Eur. J. Orthop. Surg. Traumatol. 1995, 5, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, W.; Russ, M.K.; Moch, D. Surgical wound treatment. Chirurg 1998, 69, W222–W232. [Google Scholar] [CrossRef] [PubMed]
- Muller, G. Vacuum dressing in septic wound treatment. Langenbecks Arch. Chir. Suppl. Kongressbd 1997, 114, 537–541. [Google Scholar] [PubMed]
- Smith, L.A.; Barker, D.E.; Chase, C.W.; Somberg, L.B.; Brock, W.B.; Burns, R.P. Vacuum pack technique of temporary abdominal closure: A four-year experience. Am. Surg. 1997, 63, 1102–1107; discussion 1107–1108. [Google Scholar] [PubMed]
- Plikaitis, C.M.; Molnar, J.A. Subatmospheric pressure wound therapy and the vacuum-assisted closure device: Basic science and current clinical successes. Expert. Rev. Med. Devices 2006, 3, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, M.L.; Demetriades, D.; Fabian, T.C.; Kaplan, M.J.; Miles, W.S.; Schreiber, M.A.; Holcomb, J.B.; Bochicchio, G.; Sarani, B.; Rotondo, M.F. Prospective study examining clinical outcomes associated with a negative pressure wound therapy system and Barker’s vacuum packing technique. World J. Surg. 2013, 37, 2018–2030. [Google Scholar] [CrossRef] [PubMed]
- Carlson, G.L.; Patrick, H.; Amin, A.I.; McPherson, G.; MacLennan, G.; Afolabi, E.; Mowatt, G.; Campbell, B. Management of the open abdomen: A national study of clinical outcome and safety of negative pressure wound therapy. Ann. Surg. 2013, 257, 1154–1159. [Google Scholar] [CrossRef]
- Perez Dominguez, L.; Pardellas Rivera, H.; Caceres Alvarado, N.; Lopez Saco, A.; Rivo Vazquez, A.; Casal Nunez, E. Vacuum assisted closure in open abdomen and deferred closure: Experience in 23 patients. Cir. Esp. 2012, 90, 506–512. [Google Scholar] [CrossRef]
- Reinisch, A.; Liese, J.; Woeste, G.; Bechstein, W.; Habbe, N. A Retrospective, Observational Study of Enteral Nutrition in Patients with Enteroatmospheric Fistulas. Ostomy Wound Manag. 2016, 62, 36–47. [Google Scholar]
- Porziella, V.; Nachira, D.; Boskoski, I.; Trivisonno, A.; Costamagna, G.; Margaritora, S. Emulsified stromal vascular fraction tissue grafting: A new frontier in the treatment of esophageal fistulas. Gastrointest. Endosc. 2020, 92, 1262–1263. [Google Scholar] [CrossRef]
- Nachira, D.; Trivisonno, A.; Costamagna, G.; Toietta, G.; Margaritora, S.; Pontecorvi, V.; Punzo, G.; Porziella, V.; Boskoski, I. Successful Therapy of Esophageal Fistulas by Endoscopic Injection of Emulsified Adipose Tissue Stromal Vascular Fraction. Gastroenterology 2021, 160, 1026–1028. [Google Scholar] [CrossRef] [PubMed]
- Bobkiewicz, A.; Walczak, D.; Smolinski, S.; Kasprzyk, T.; Studniarek, A.; Borejsza-Wysocki, M.; Ratajczak, A.; Marciniak, R.; Drews, M.; Banasiewicz, T. Management of enteroatmospheric fistula with negative pressure wound therapy in open abdomen treatment: A multicentre observational study. Int. Wound J. 2017, 14, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Willms, A.; Gusgen, C.; Schaaf, S.; Bieler, D.; von Websky, M.; Schwab, R. Management of the open abdomen using vacuum-assisted wound closure and mesh-mediated fascial traction. Langenbecks Arch. Surg. 2015, 400, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, M.H.; Liscum, K.R.; Hirshberg, A. The floating stoma: A new technique for controlling exposed fistulae in abdominal trauma. J. Trauma. 2002, 53, 386–388. [Google Scholar] [CrossRef]
- Lenz, S.; Doll, D.; Harder, K.; Lieber, A.; Muller, U.; Dusel, W.; Siewert, J.R. Procedures of temporary wall closure in abdominal trauma and sepsis. Chirurg 2006, 77, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, J.C.; Parry, B.R.; Bissett, I.P.; McKee, M. Experience with the use of vacuum dressings in the management of acute enterocutaneous fistulas. ANZ J. Surg. 2006, 76, 1085–1087. [Google Scholar] [CrossRef]
- Verhaalen, A.; Watkins, B.; Brasel, K. Techniques and cost effectiveness of enteroatmospheric fistula isolation. Wounds 2010, 22, 212–217. [Google Scholar] [PubMed]
- D’Hondt, M.; Devriendt, D.; Van Rooy, F.; Vansteenkiste, F.; D’Hoore, A.; Penninckx, F.; Miserez, M. Treatment of small-bowel fistulae in the open abdomen with topical negative-pressure therapy. Am. J. Surg. 2011, 202, e20–e24. [Google Scholar] [CrossRef]
- Ozer, M.T.; Sinan, H.; Zeybek, N.; Peker, Y. A simple novel technique for enteroatmospheric fistulae: Silicone fistula plug. Int. Wound J. 2014, 11 (Suppl. S1), 22–24. [Google Scholar] [CrossRef]
- Yetisir, F.; Sarer, A.E.; Acar, H.Z. Management of Necrotizing Fasciitis and Fecal Peritonitis following Ostomy Necrosis and Detachment by Using NPT and Flexi-Seal. Case Rep. Surg. 2015, 2015, 231450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heineman, J.T.; Garcia, L.J.; Obst, M.A.; Chong, H.S.; Langin, J.G.; Humpal, R.; Pezzella, P.A.; Dries, D.J. Collapsible Enteroatmospheric Fistula Isolation Device: A Novel, Simple Solution to a Complex Problem. J. Am. Coll. Surg. 2015, 221, e7–e14. [Google Scholar] [CrossRef] [PubMed]
- Jaguscik, R.; Walczak, D.A.; Porzezynska, J.; Trzeciak, P.W. The Use Of Negative Pressure Wound Therapy (NPWT) in The Management Of Enteroatmospheric Fistula—Case Report And Literature Review. Pol. J. Surg. 2015, 87, 522–527. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ortiz, L.A.; Zhang, B.; McCarthy, M.W.; Kaafarani, H.M.A.; Fagenholz, P.; King, D.R.; De Moya, M.; Velmahos, G.; Yeh, D.D. Treatment of Enterocutaneous Fistulas, Then and Now. Nutr. Clin. Pract. 2017, 32, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Wirth, U.; Renz, B.W.; Andrade, D.; Schiergens, T.S.; Arbogast, H.; Andrassy, J.; Werner, J. Successful treatment of enteroatmospheric fistulas in combination with negative pressure wound therapy: Experience on 3 cases and literature review. Int. Wound J. 2018, 15, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.E.; Kaufman, H.J.; Smith, L.A.; Ciraulo, D.L.; Richart, C.L.; Burns, R.P. Vacuum pack technique of temporary abdominal closure: A 7-year experience with 112 patients. J. Trauma. 2000, 48, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, M.C.; Riggle, A.; Beilman, G.; Chipman, J. A novel technique to skin graft abdominal wall wounds surrounding enterocutaneous fistulas. Surg. Infect. 2010, 11, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Al-Khoury, G.; Kaufman, D.; Hirshberg, A. Improved control of exposed fistula in the open abdomen. J. Am. Coll. Surg. 2008, 206, 397–398. [Google Scholar] [CrossRef]
- Layton, B.; Dubose, J.; Nichols, S.; Connaughton, J.; Jones, T.; Pratt, J. Pacifying the open abdomen with concomitant intestinal fistula: A novel approach. Am. J. Surg. 2010, 199, e48–e50. [Google Scholar] [CrossRef]
- Trevino, C.M.; Verhaalen, A.; Bruce, M.L.; Webb, T. Conversion of an enterocutaneous fistula associated with an open abdominal wound into a drain- controlled enterocutaneous fistula. Wounds 2014, 26, 43–46. [Google Scholar]
- Ramsay, P.T.; Mejia, V.A. Management of enteroatmospheric fistulae in the open abdomen. Am. Surg. 2010, 76, 637–639. [Google Scholar] [CrossRef] [PubMed]
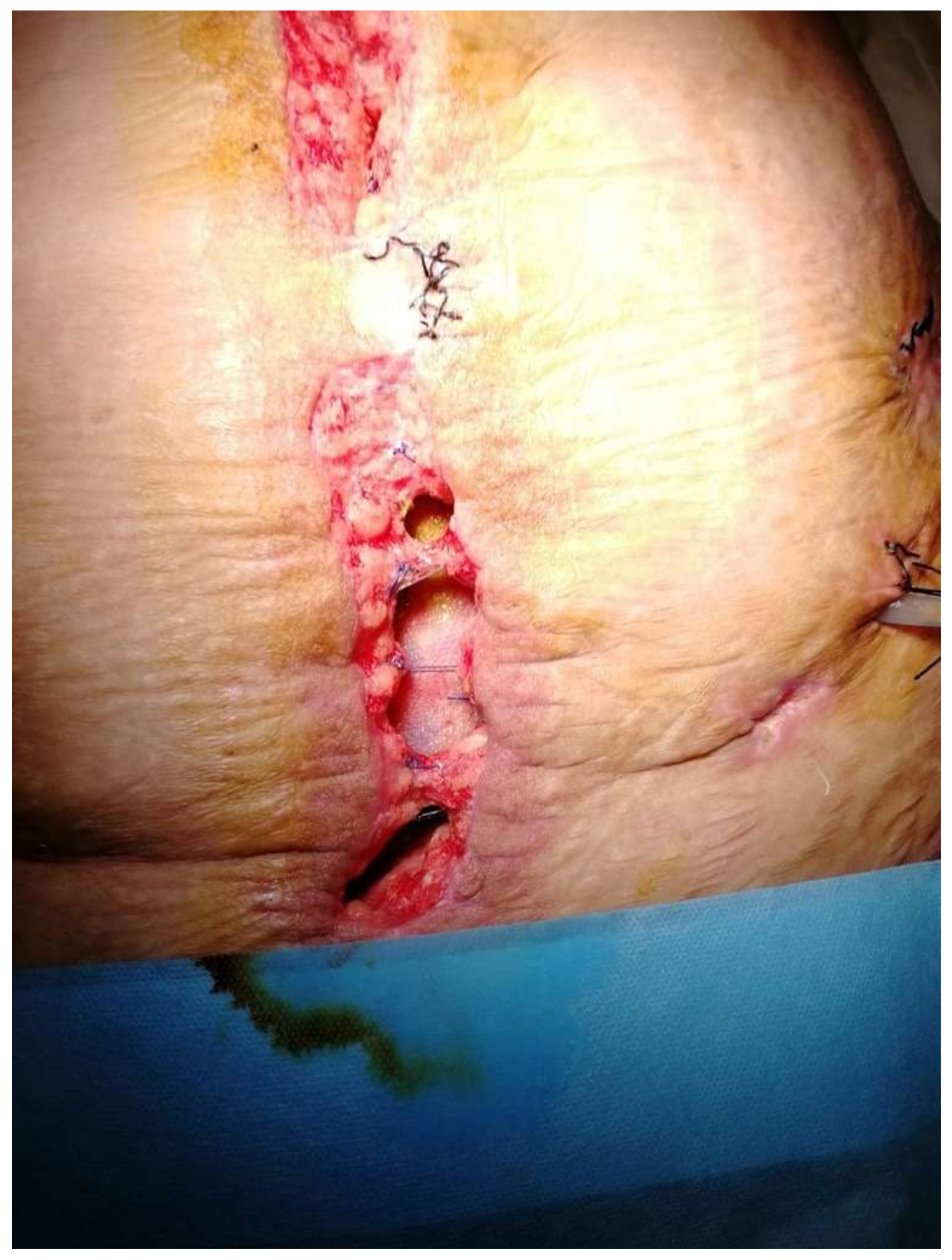
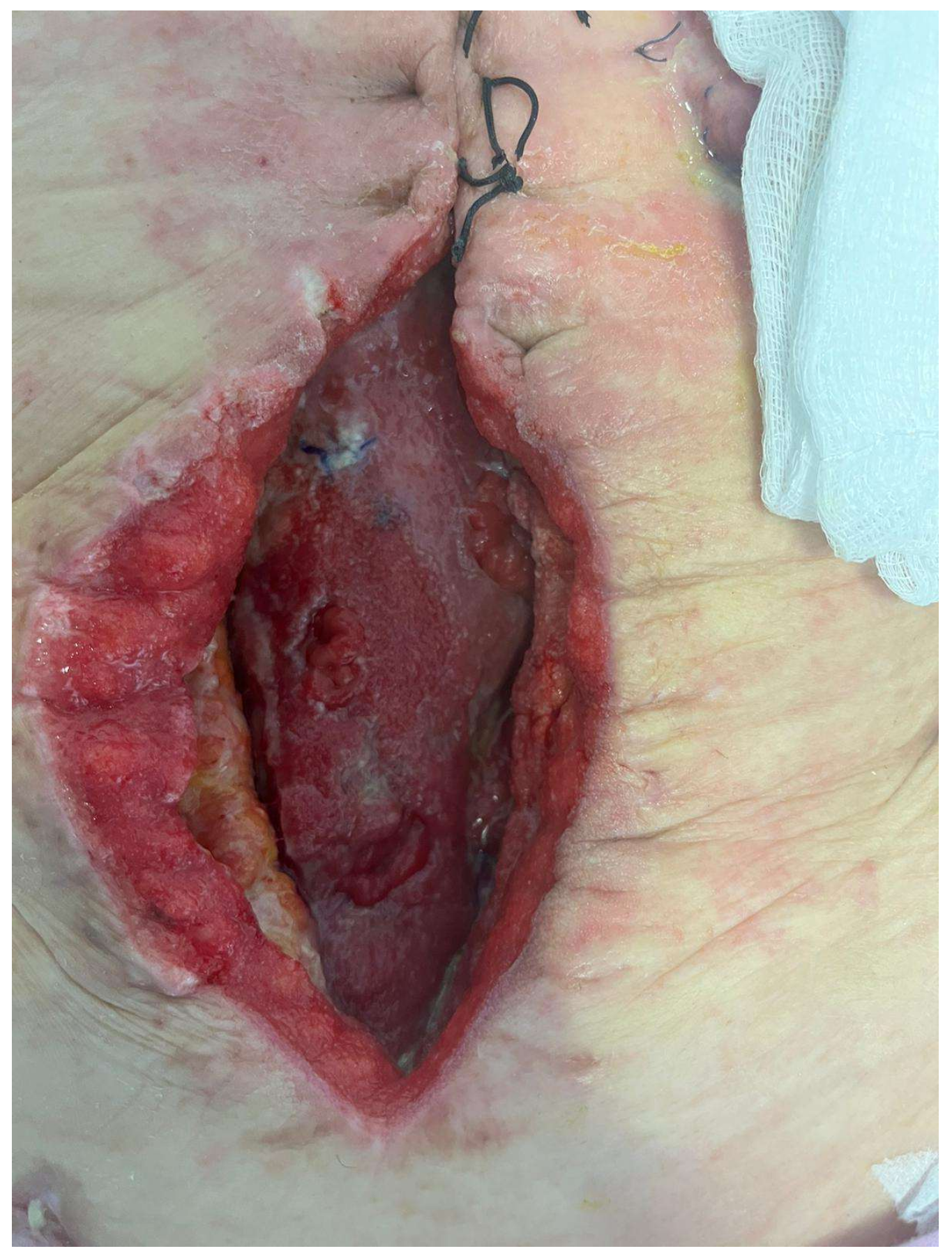
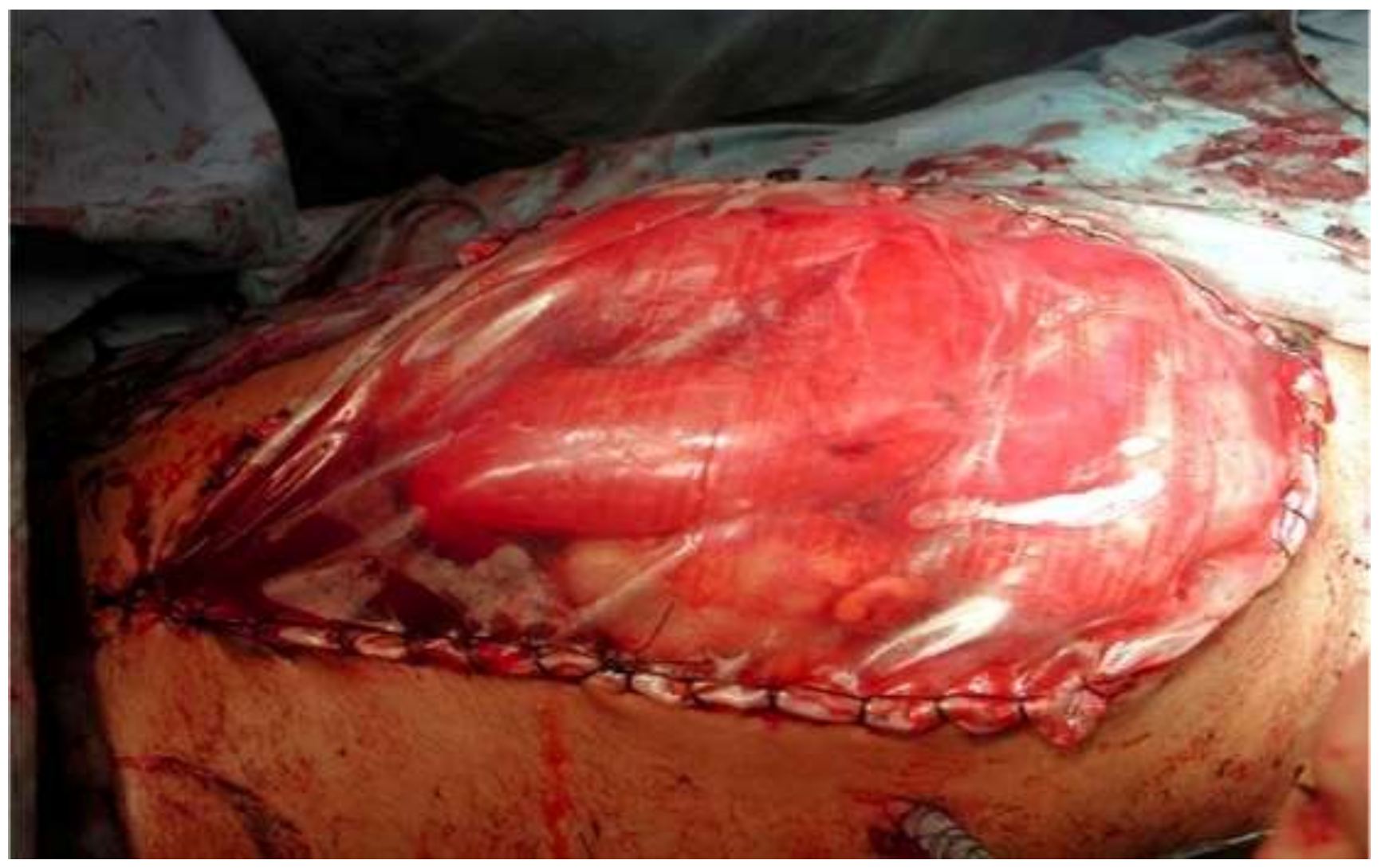
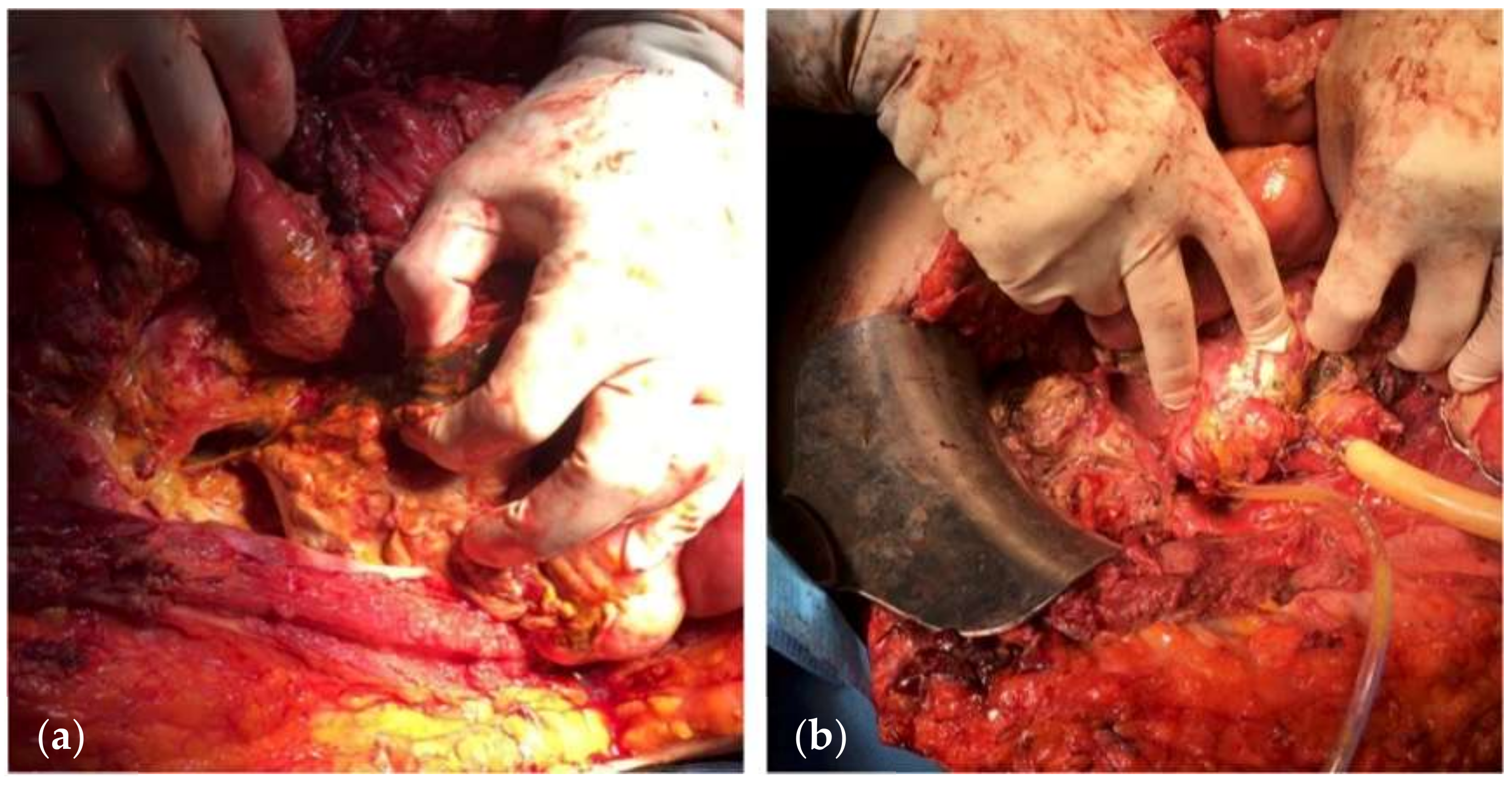
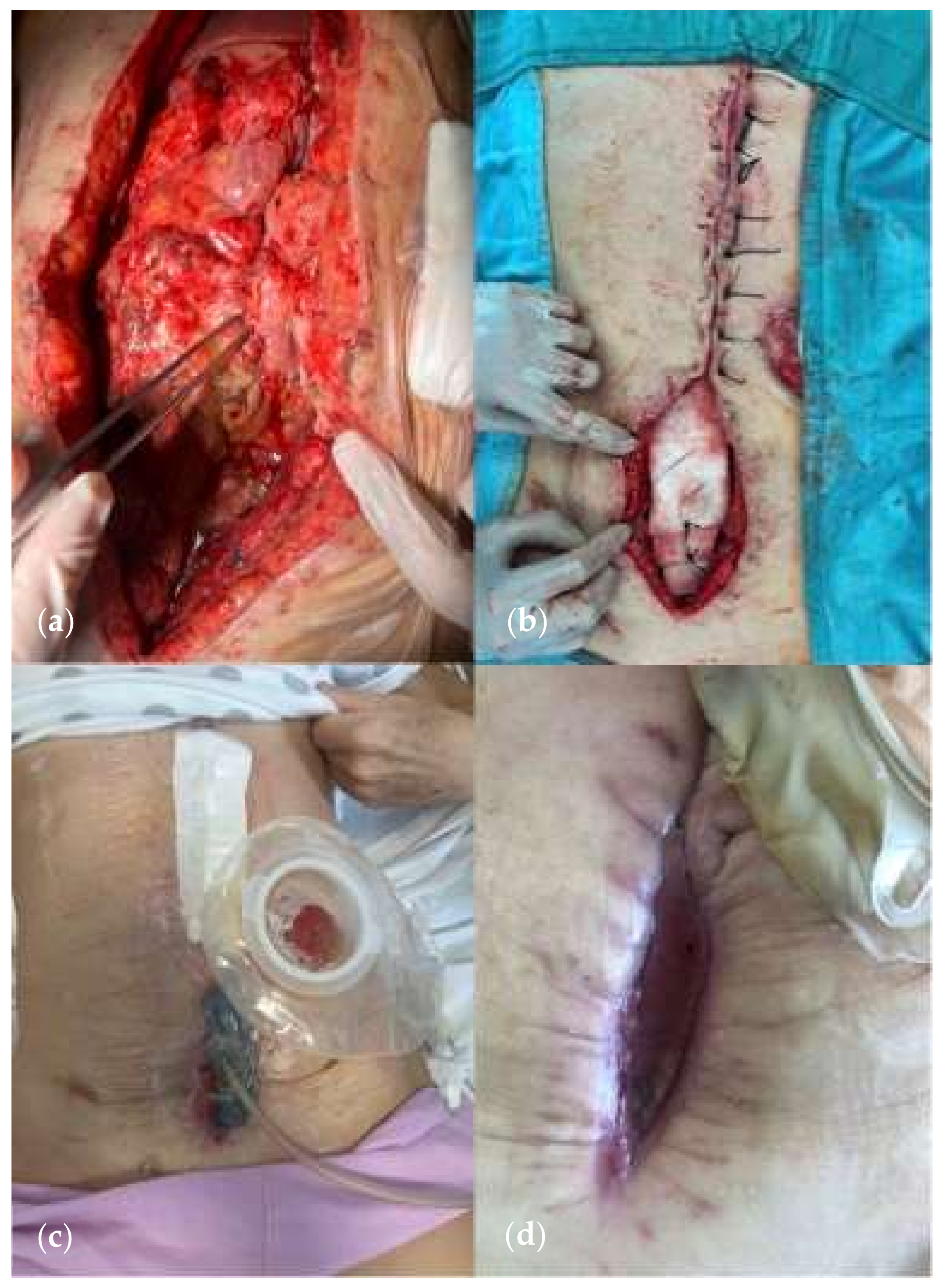
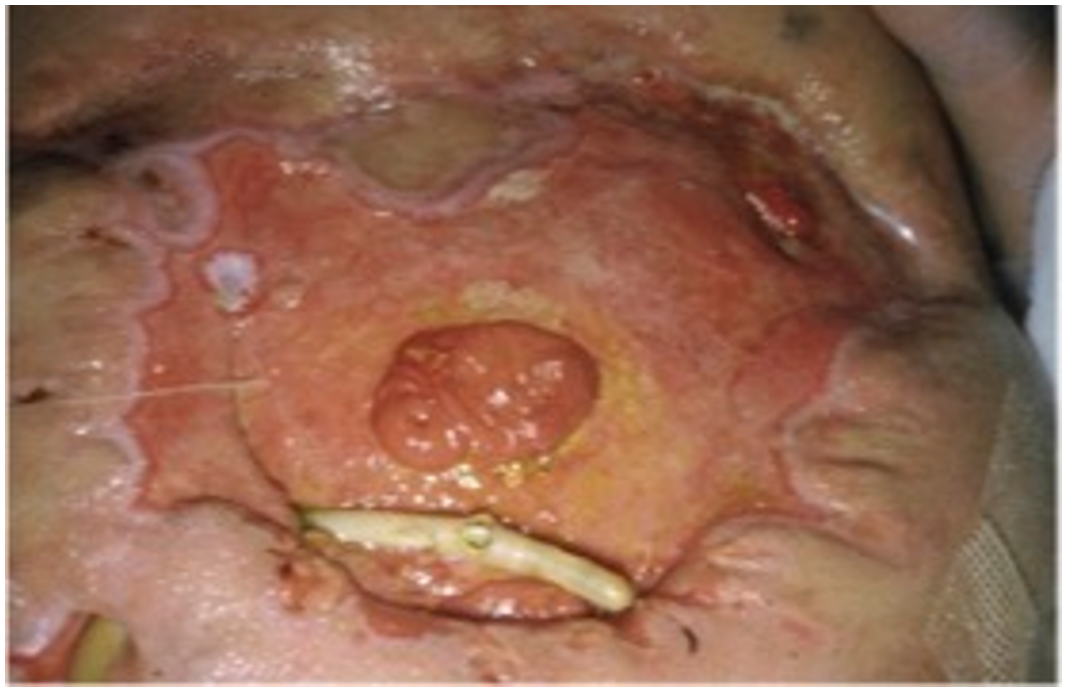
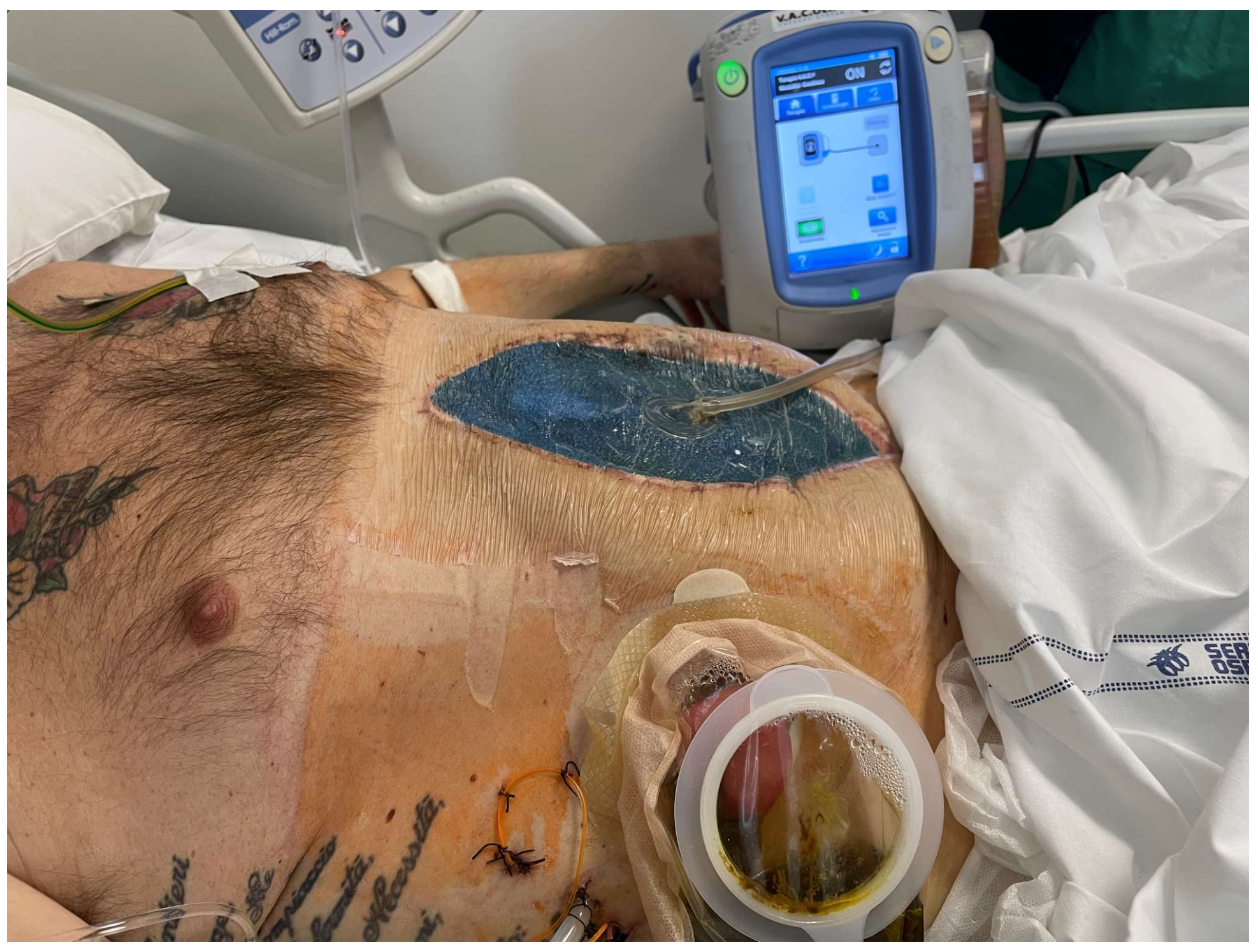

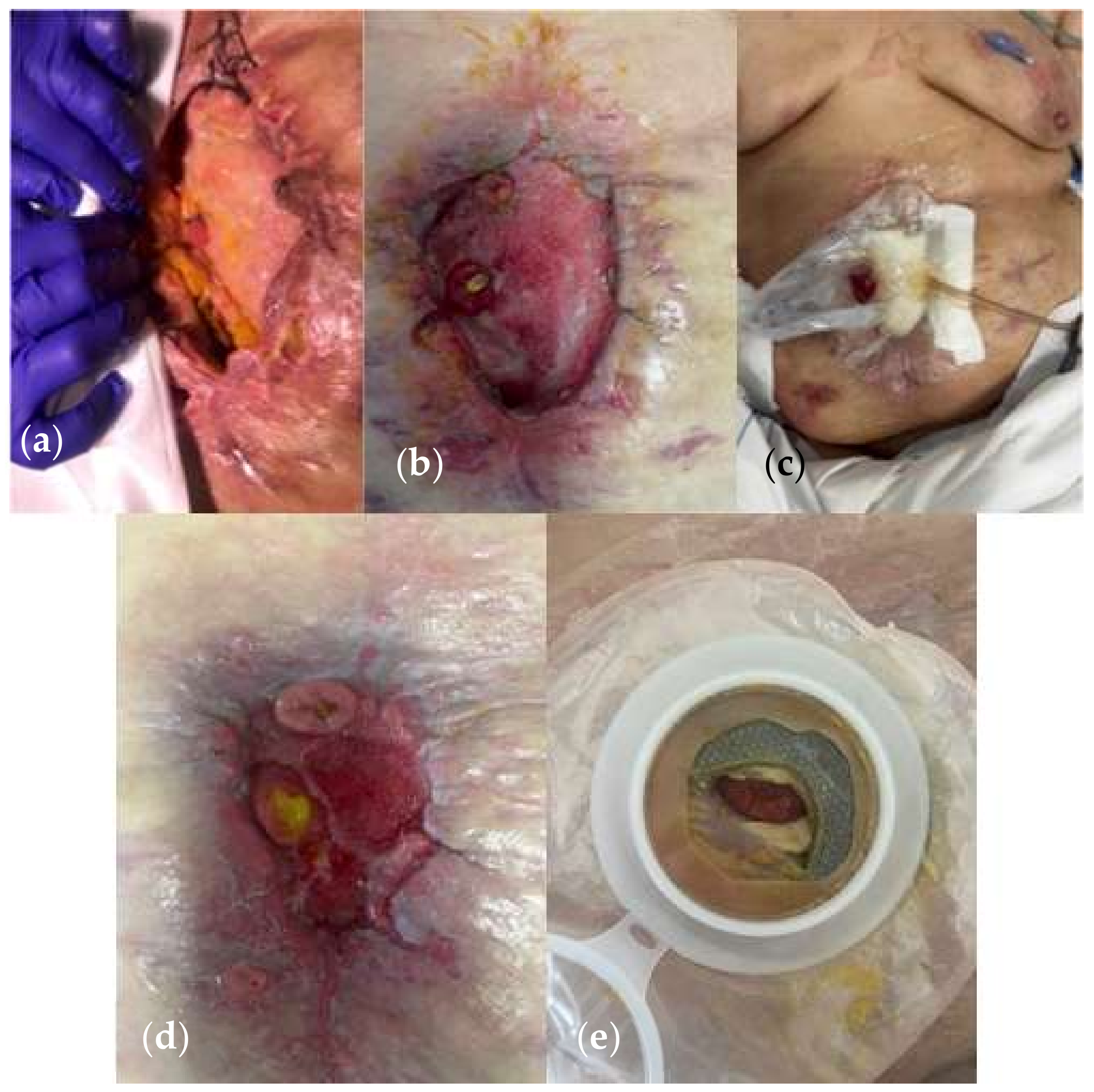
| Variables | Favorable | Unfavorable | |
|---|---|---|---|
| Site of origin | Esophagus | Stomach | |
| Duodenal stump | Duodenum | ||
| Pancreas | Proximal jejunum | ||
| Biliary tree | Ileum | ||
| Colon | |||
| Characteristics and environment | Enteric defect | <1 cm | >1 cm |
| Fistula tract | >3 cm | <3 cm | |
| Budding mucosa | Absent | Present | |
| Intestinal continuity | Intact | Disrupted | |
| Distal obstruction | Absent | Present | |
| Adjacent abscess | Absent | Present | |
| Bowel disease | Absent | Present | |
| Foreign body | Absent | Present | |
| Previous chemoradiation | No | Yes | |
| Physiology | Fistula output | <500 mL/day | >500 mL/day |
| Nutrition status | Well nourished | Malnourished | |
| Transferrin | >200 mg/dL | <200 mg/dL | |
| Sepsis | Absent/infrequent | Present/frequent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepe, G.; Chiarello, M.M.; Bianchi, V.; Fico, V.; Altieri, G.; Tedesco, S.; Tropeano, G.; Molica, P.; Di Grezia, M.; Brisinda, G. Entero-Cutaneous and Entero-Atmospheric Fistulas: Insights into Management Using Negative Pressure Wound Therapy. J. Clin. Med. 2024, 13, 1279. https://doi.org/10.3390/jcm13051279
Pepe G, Chiarello MM, Bianchi V, Fico V, Altieri G, Tedesco S, Tropeano G, Molica P, Di Grezia M, Brisinda G. Entero-Cutaneous and Entero-Atmospheric Fistulas: Insights into Management Using Negative Pressure Wound Therapy. Journal of Clinical Medicine. 2024; 13(5):1279. https://doi.org/10.3390/jcm13051279
Chicago/Turabian StylePepe, Gilda, Maria Michela Chiarello, Valentina Bianchi, Valeria Fico, Gaia Altieri, Silvia Tedesco, Giuseppe Tropeano, Perla Molica, Marta Di Grezia, and Giuseppe Brisinda. 2024. "Entero-Cutaneous and Entero-Atmospheric Fistulas: Insights into Management Using Negative Pressure Wound Therapy" Journal of Clinical Medicine 13, no. 5: 1279. https://doi.org/10.3390/jcm13051279
APA StylePepe, G., Chiarello, M. M., Bianchi, V., Fico, V., Altieri, G., Tedesco, S., Tropeano, G., Molica, P., Di Grezia, M., & Brisinda, G. (2024). Entero-Cutaneous and Entero-Atmospheric Fistulas: Insights into Management Using Negative Pressure Wound Therapy. Journal of Clinical Medicine, 13(5), 1279. https://doi.org/10.3390/jcm13051279







