Therapist versus Machine—Immediate Effects of Manual versus Mechanical Lymphatic Drainage in Patients with Secondary Lymphedema
Abstract
1. Introduction
2. Materials and Methods
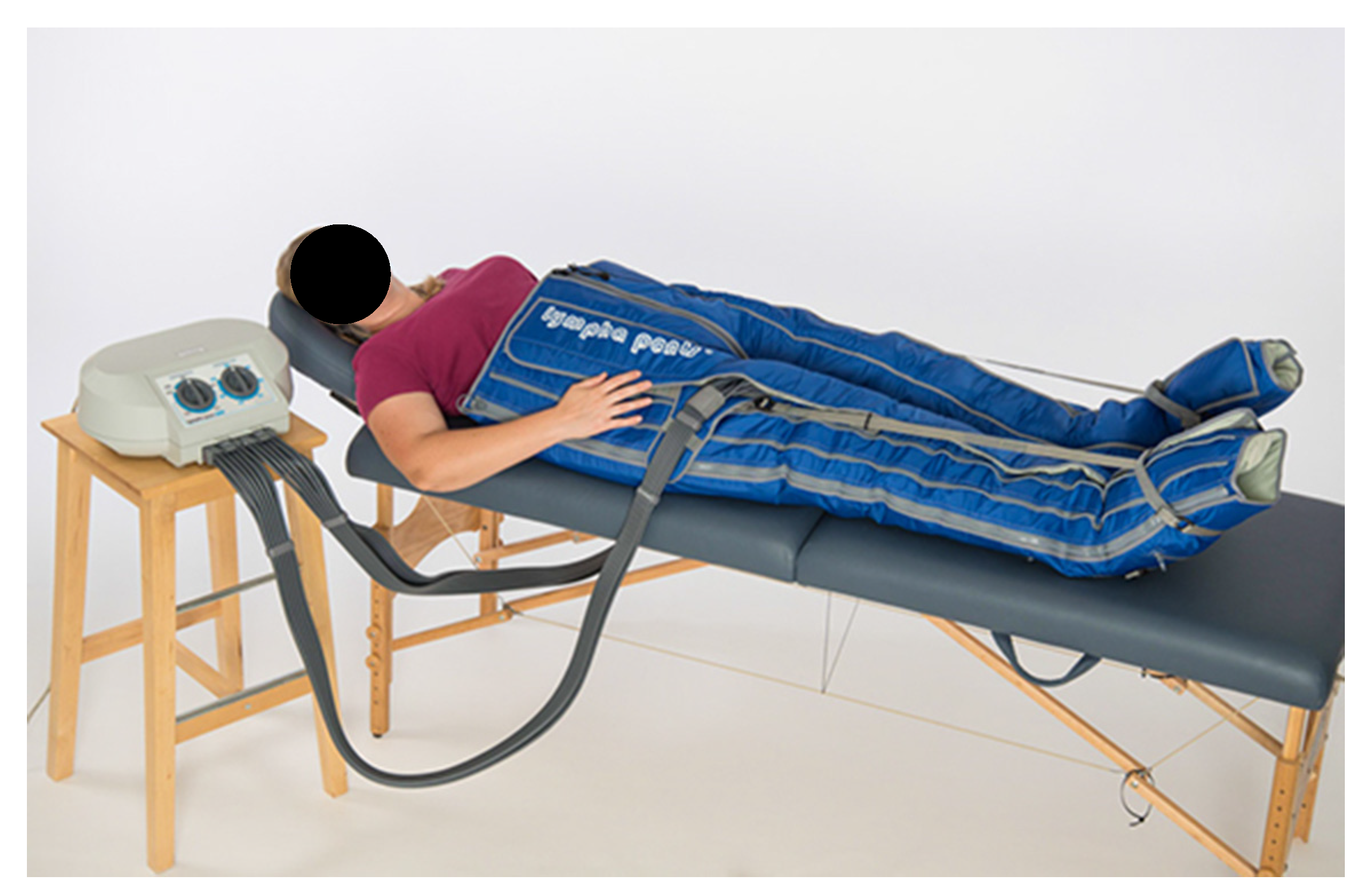
2.1. Objective Measurements (3D Volumetry)
2.2. Subjective Measurements
2.2.1. Questionnaire 1
2.2.2. Questionnaire 2
2.3. Statistics
2.4. Patient Recruitment
3. Results
3.1. Objective Measurements (3D Volumetry)
3.1.1. Immediate Effect
3.1.2. Effect after Two Days
3.2. Subjective Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Executive Committee of the International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Leak, L.V.; Liotta, L.A.; Krutzsch, H.; Jones, M.; Fusaroa, V.A.; Ross, S.J.; Zhao, Y.; Petricoin, E.F., III. Proteomic analysis of lymph. Proteomics 2004, 4, 753–765. [Google Scholar] [CrossRef]
- Liu, N.F.; Zhang, L.R. Changes of tissue fluid hyaluronan (hyaluronic acid) in peripheral lymphedema. Lymphology 1998, 31, 173–179. [Google Scholar]
- Koller, M.B.R.; Döller, W.; Földi, E.; Wilting, J.; Ure, C.; Brauer, W.; Földi, M.; Albert, U. S2k Guideline ‘Diagnostics and Therapy of Lymphoedema’; AWMF online; AWMF: Duesseldorf, Germany, 2017. [Google Scholar]
- Moffatt, C.; Franks, P.; Doherty, D.; Williams, A.; Badger, C.; Jeffs, E.; Bosanquet, N.; Mortimer, P. Lymphoedema: An underestimated health problem. QJM 2003, 96, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Jäger, G.; Döller, W.; Roth, R. Quality-of-life and body image impairments in patients with lymphedema. Lymphology 2006, 39, 193–200. [Google Scholar] [PubMed]
- McWayne, J.; Heiney, S.P. Psychologic and social sequelae of secondary lymphedema: A review. Cancer 2005, 104, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.F.; Moffatt, C.J.; Franks, P.J. A phenomenological study of the lived experiences of people with lymphoedema. Int. J. Palliat. Nurs. 2004, 10, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.A.; Phillips, T.J. Lymphedema: Pathophysiology and clinical manifestations. J. Am. Acad. Dermatol. 2017, 7, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Tzani, I.; Tsichlaki, M.; Zerva, E.; Papathanasiou, G.; Dimakakos, E. Physiotherapeutic rehabilitation of lymphedema: State-of-the-art. Lymphology 2018, 51, 1–12. [Google Scholar] [PubMed]
- Bergmann, A.; Baiocchi, J.M.T.; de Andrade, M.F.C. Conservative treatment of lymphedema: The state of the art. J. Vasc. Bras. 2021, 20, e20200091. [Google Scholar] [CrossRef] [PubMed]
- Lasinski, B.B.; Thrift, K.M.; Squire, D.; Austin, M.K.; Smith, K.M.; Wanchai, A.; Green, J.M.; Stewart, B.R.; Cormier, J.N.; Armer, J.M. A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PMR 2012, 4, 580–601. [Google Scholar] [CrossRef]
- Huang, T.W.; Tseng, S.H.; Lin, C.C.; Bai, C.H.; Chen, C.S.; Hung, C.S.; Wu, C.H.; Tam, K.W. Effects of manual lymphatic drainage on breast cancer-related lymphedema: A systematic review and me-ta-analysis of randomized controlled trials. World J. Surg. Oncol. 2013, 11, 15. [Google Scholar] [CrossRef]
- Mayrovitz, H.N.; Davey, S.; Shapiro, E. Localized tissue water changes accompanying one manual lymphatic drainage (MLD) therapy session assessed by changes in tissue dielectric constant inpatients with lower extremity lymphedema. Lymphology 2008, 41, 87–92. [Google Scholar] [PubMed]
- Konschake, W.; Riebe, H.; Vollmer, M.; Jünger, M. Optimisation of intermittent pneumatic compression in patients with lymphoedema of the legs. Eur. J. Dermatol. 2022, 32, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Otero, V.P.; Delgado, E.G.; Cortijo, C.M.; Ramos, M.L.R.; Iriarte, E.D.C.; Gil García, A.; Romay-Barrero, H.; Avendaño-Coy, J. Intensive complex physical therapy combined with intermittent pneumatic compression versus Kinesio taping for treating breast cancer-related lymphedema of the upper limb: A randomised cross-over clinical trial. Eur. J. Cancer Care 2022, 31, e13625. [Google Scholar] [CrossRef]
- Zaleska, M.; Olszewski, W.L.; Cakala, M.; Cwikla, J.; Budlewski, T. Intermittent Pneumatic Compression Enhances Formation of Edema Tissue Fluid Channels in Lymphedema of Lower Limbs. Lymphat. Res. Biol. 2015, 13, 146–153. [Google Scholar] [CrossRef]
- Zaleska, M.T.; Olszewski, W.L. The Effectiveness of Intermittent Pneumatic Compression in Therapy of Lymphedema of Lower Limbs: Methods of Evaluation and Results. Lymphat. Res. Biol. 2019, 17, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Zaleska, M.; Olszewski, W.L.; Durlik, M. The effectiveness of intermittent pneumatic compression in long-term therapy of lymphedema of lower limbs. Lymphat. Res. Biol. 2014, 12, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, S.; Lim, J.Y.; Hwang, C.M.; Ko, M.-H.; Hwang, J.H. Home-Based Intermittent Pneumatic Compression Therapy: The Impact in Chronic Leg Lymphedema in Patients Treated for Gynecologic Cancer. Healthcare 2022, 10, 638. [Google Scholar] [CrossRef] [PubMed]
- Finnane, A.; Janda, M.; Hayes, S.C. Review of the evidence of lymphedema treatment effect. Am. J. Phys. Med. Rehabil. 2015, 94, 483–498. [Google Scholar] [CrossRef]
- Williams, A. Manual lymphatic drainage: Exploring the history and evidence base. Br. J. Community Nurs. 2010, 15, S18–S24. [Google Scholar] [CrossRef] [PubMed]
- Schwahn-Schreiber, C.; Breu, F.X.; Rabe, E.; Buschmann, I.; Döller, W.; Lulay, G.R.; Miller, A.; Valesky, E.; Reich-Schupke, S. S1 guideline on intermittent pneumatic compression (IPC). Hautarzt 2018, 69, 662–673. [Google Scholar] [CrossRef]
- Koban, K.C.; Titze, V.; Etzel, L.; Frank, K.; Schenck, T.; Giunta, R. Quantitative volumetric analysis of the lower extremity: Validation against established tape measurement and water displacement. Handchir. Mikrochir. Plast. Chir. 2018, 50, 393–399. [Google Scholar] [PubMed]
- Peschke, D. Appropriateness of physiotherapy care in Germany: A scoping review. Z. Evidenz Fortbild. Qual. Gesundheitswesen 2019, 141–142, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Neuhüttler, S.B.E. Beitrag zur Epidemiologie des Lymphödems. Phlebologie 2006, 35, 181–187. [Google Scholar] [CrossRef]
- Greene, A.K.; Grant, F.D.; Slavin, S.A. Lower-extremity lymphedema and elevated body-mass index. N. Engl. J. Med. 2012, 366, 2136–2137. [Google Scholar] [CrossRef]
- Johansson, K.; Lie, E.; Ekdahl, C.; Lindfeldt, J. A randomized study comparing manual lymph drainage with sequential pneumatic compression for treatment of postoperative arm lymphedema. Lymphology 1998, 31, 56–64. [Google Scholar]
- Olszewski, W.L.; Jain, P.; Ambujam, G.; Zaleska, M.; Cakala, M.; Gradalski, T. Tissue fluid pressure and flow during pneumatic compression in lymphedema of lower limbs. Lymphat. Res. Biol. 2011, 9, 77–83. [Google Scholar] [CrossRef]
- Ridner, S.H.; McMahon, E.; Dietrich, M.S.; Hoy, S. Home-based lymphedema treatment in patients with cancer-related lymphedema or noncancer-related lymphedema. Oncol. Nurs. Forum 2008, 35, 671–680. [Google Scholar] [CrossRef]
- Uzkeser, H.; Karatay, S.; Erdemci, B.; Koc, M.; Senel, K. Efficacy of manual lymphatic drainage and intermittent pneumatic compression pump use in the treatment of lymphedema after mastectomy: A randomized controlled trial. Breast Cancer 2013, 22, 300–307. [Google Scholar] [CrossRef]
- Szolnoky, G.; Lakatos, B.; Keskeny, T.; Varga, E.; Varga, M.; Dobozy, A.; Kemény, L. Intermittent pneumatic compression acts synergistically with manual lymphatic drainage in complex de-congestive physiotherapy for breast cancer treatment-related lymphedema. Lymphology 2009, 42, 188–194. [Google Scholar]
- Szuba, A.; Achalu, R.; Rockson, S.G. Decongestive lymphatic therapy for patients with breast carcinoma-associated lymphedema. A randomized, prospective study of a role for adjunctive intermittent pneumatic compression. Cancer 2002, 95, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.J.; Gordon, S.J. Intermittent Pneumatic Compression Dosage for Adults and Children with Lymphedema: A Systematic Review. Lymphat. Res. Biol. 2019, 17, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Besley, J.; Kayes, N.M.; McPherson, K.M. Assessing Therapeutic Relationships in Physiotherapy: Literature Review. N. Z. J. Physiother. 2011, 39, 81–91. [Google Scholar]
- Mendoza, E.; Amsler, F. Effectiveness of manual lymphatic drainage and intermittent pneumatic compression in lymphedema maintenance therapy. Vasa 2023, 52, 423–431. [Google Scholar] [CrossRef]


| Therapy | ||||||||||
| MLD | IPCT | |||||||||
| m | 95% CI | m | 95% CI | Mean Difference | 95% CI | p | ||||
| immediate effect | −57.01 | −65.93 | −48.09 | −58.62 | −67.59 | −49.65 | 1.61 | −9.99 | 13.22 | 0.780 |
| two-day effect | −16.33 | −35.86 | 3.19 | −12.3 | −34.24 | 9.64 | −4.04 | −37.91 | 29.84 | 0.811 |
| BMI categories | ||||||||||
| Obesity | non−obesity | |||||||||
| immediate effect | −51.96 | −60.74 | −43.19 | −63.67 | −75.37 | −51.97 | 11.70 | −3.88 | 27.29 | 0.137 |
| two-day effect | −11.35 | −26.66 | 3.97 | −17.28 | −37.68 | 3.11 | 5.94 | −21.17 | 33.04 | 0.660 |
| lymphedema stage | ||||||||||
| I | II | |||||||||
| immediate effect | −53.99 | −63.56 | −44.41 | −61.64 | −70.96 | −52.33 | 7.66 | −5.45 | 20.76 | 0.245 |
| two-day effect | −6.10 | −22.79 | 10.6 | −22.53 | −38.77 | −6.30 | 16.44 | −6.33 | 39.20 | 0.152 |
| treatment order | ||||||||||
| MLD | IPCT | |||||||||
| immediate effect | −53.13 | −62.71 | −43.55 | −62.5 | −72.14 | −52.87 | 9.37 | −4.20 | 22.95 | 0.171 |
| two-day effect | −12.27 | −28.97 | 4.44 | −16.36 | −33.16 | 0.44 | 4.10 | −19.49 | 27.68 | 0.727 |
| Subjective Effectiveness | IPCT | Total | |||
|---|---|---|---|---|---|
| Moderate | Strong | Very Strong | |||
| MLD | moderate | 2 | 5 | 0 | 7 |
| strong | 6 | 9 | 5 | 20 | |
| very strong | 1 | 8 | 4 | 13 | |
| Total | 9 | 22 | 9 | 40 | |
| Subjective Long-Term Effectiveness in Hours | IPCT | Total | |||||
|---|---|---|---|---|---|---|---|
| <12 | 12–24 | 24–36 | 36–48 | >48 | |||
| MLD | <12 | 4 | 1 | 0 | 1 | 0 | 6 |
| 12–24 | 3 | 9 | 5 | 2 | 0 | 19 | |
| 24–36 | 0 | 5 | 3 | 4 | 1 | 13 | |
| 36–48 | 0 | 0 | 1 | 0 | 0 | 1 | |
| >48 | 0 | 0 | 0 | 0 | 1 | 1 | |
| Total | 7 | 15 | 9 | 7 | 2 | 40 | |
| Paresthesia | ICPT | Total | |||
|---|---|---|---|---|---|
| None | Mild | Moderate | |||
| MLD | none | 32 | 5 | 0 | 37 |
| mild | 1 | 1 | 0 | 2 | |
| moderate | 0 | 1 | 0 | 1 | |
| Total | 33 | 7 | 0 | 40 | |
| Pleasant/Unpleasant | IPCT | Total | |||||
|---|---|---|---|---|---|---|---|
| Very Unpleasant | Slightly Unpleasant | Neither | Slightly Pleasant | Very Pleasant | |||
| MLD | very unpleasant | 0 | 0 | 0 | 0 | 1 | 1 |
| slightly unpleasant | 0 | 0 | 0 | 1 | 0 | 1 | |
| neither | 0 | 0 | 0 | 0 | 0 | 0 | |
| slightly pleasant | 0 | 1 | 3 | 3 | 4 | 11 | |
| very pleasant | 1 | 3 | 4 | 7 | 12 | 27 | |
| Total | 1 | 4 | 7 | 11 | 17 | 40 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiltz, D.; Eibl, D.; Mueller, K.; Biermann, N.; Prantl, L.; Taeger, C.D. Therapist versus Machine—Immediate Effects of Manual versus Mechanical Lymphatic Drainage in Patients with Secondary Lymphedema. J. Clin. Med. 2024, 13, 1277. https://doi.org/10.3390/jcm13051277
Schiltz D, Eibl D, Mueller K, Biermann N, Prantl L, Taeger CD. Therapist versus Machine—Immediate Effects of Manual versus Mechanical Lymphatic Drainage in Patients with Secondary Lymphedema. Journal of Clinical Medicine. 2024; 13(5):1277. https://doi.org/10.3390/jcm13051277
Chicago/Turabian StyleSchiltz, Daniel, Dominik Eibl, Karolina Mueller, Niklas Biermann, Lukas Prantl, and Christian Dirk Taeger. 2024. "Therapist versus Machine—Immediate Effects of Manual versus Mechanical Lymphatic Drainage in Patients with Secondary Lymphedema" Journal of Clinical Medicine 13, no. 5: 1277. https://doi.org/10.3390/jcm13051277
APA StyleSchiltz, D., Eibl, D., Mueller, K., Biermann, N., Prantl, L., & Taeger, C. D. (2024). Therapist versus Machine—Immediate Effects of Manual versus Mechanical Lymphatic Drainage in Patients with Secondary Lymphedema. Journal of Clinical Medicine, 13(5), 1277. https://doi.org/10.3390/jcm13051277







