Systemic Immune-Inflammation Index and Systemic Inflammatory Response Index as Predictors of Mortality in ST-Elevation Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
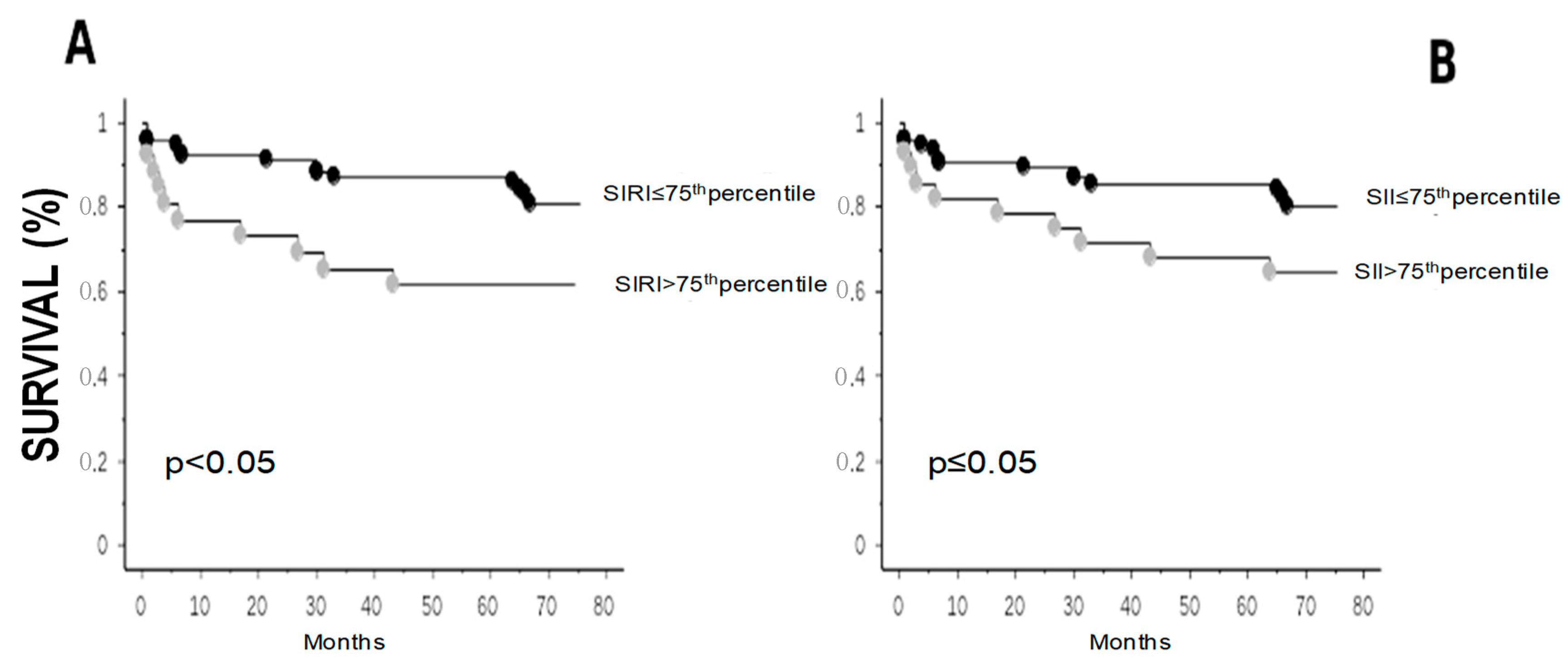
4. Results
4.1. SII Determinants in the STEMI Population
4.2. SIRI Determinants in the STEMI Population
4.3. Follow-Up
5. Discussion
Limitations and Strengths of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Ma, X.; Shao, Q.; Yang, Z.; Wang, Y.; Gao, F.; Zhou, Y.; Yang, L.; Wang, Z. Prognostic Impact of Multiple Lymphocyte-Based Inflammatory Indices in Acute Coronary Syndrome Patients. Front. Cardiovasc. Med. 2022, 9, 811790. [Google Scholar] [CrossRef]
- Fan, W.; Wei, C.; Liu, Y.; Sun, Q.; Tian, Y.; Wang, X.; Liu, J.; Zhang, Y.; Sun, L. The Prognostic Value of Hematologic Inflammatory Markers in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221146183. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.M.; Qiu, S.J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Meng, L.; Yang, Y.; Hu, X.; Zhang, R.; Li, X. Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: A systematic review and meta-analysis. J. Transl. Med. 2023, 21, 79. [Google Scholar] [CrossRef]
- Yang, Y.L.; Wu, C.H.; Hsu, P.F.; Chen, S.C.; Huang, S.S.; Chan, W.L.; Lin, S.J.; Chou, C.Y.; Chen, J.W.; Pan, J.P.; et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur. J. Clin. Investig. 2020, 50, e13230. [Google Scholar] [CrossRef] [PubMed]
- Mangalesh, S.; Dudani, S.; Mahesh, N.K. Development of a Novel Inflammatory Index to Predict Coronary Artery Disease Severity in Patients with Acute Coronary Syndrome. Angiology 2024, 75, 231–239. [Google Scholar] [CrossRef]
- Han, K.; Shi, D.; Yang, L.; Wang, Z.; Li, Y.; Gao, F.; Liu, Y.; Ma, X.; Zhou, Y. Prognostic value of systemic inflammatory response index in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Ann. Med. 2022, 54, 1667–1677. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H. A nonlinear relationship between systemic inflammation response index and short-term mortality in patients with acute myocardial infarction: A retrospective study from MIMIC-IV. Front. Cardiovasc. Med. 2023, 10, 1208171. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, K.; Han, Y.; Xu, Q.; Zhao, X. An Easy-to-Use Nomogram Based on SII and SIRI to Predict in-Hospital Mortality Risk in Elderly Patients with Acute Myocardial Infarction. J. Inflamm. Res. 2023, 16, 4061–4071. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Q.; Wang, R.; Ji, H.; Chen, Y.; Quan, X.; Zhang, C. Systemic Immune-Inflammatory Index Predicts Clinical Outcomes for Elderly Patients with Acute Myocardial Infarction Receiving Percutaneous Coronary Intervention. Med. Sci. Monit. 2019, 25, 9690–9701. [Google Scholar] [CrossRef]
- Su, G.; Zhang, Y.; Xiao, R.; Zhang, T.; Gong, B. Systemic immune-inflammation index as a promising predictor of mortality in patients with acute coronary syndrome: A real-world study. J. Int. Med. Res. 2021, 49, 3000605211016274. [Google Scholar] [CrossRef]
- Tosu, A.R.; Kalyoncuoglu, M.; Biter, H.İ.; Cakal, S.; Selcuk, M.; Çinar, T.; Belen, E.; Can, M.M. Prognostic Value of Systemic Immune-Inflammation Index for Major Adverse Cardiac Events and Mortality in Severe Aortic Stenosis Patients after TAVI. Medicina 2021, 7, 588. [Google Scholar] [CrossRef]
- Gur, D.O.; Efe, M.M.; Alpsoy, S.; Akyüz, A.; Uslu, N.; Çelikkol, A.; Gur, O. Systemic Immune-Inflammatory Index as a Determinant of Atherosclerotic Burden and High-Risk Patients with Acute Coronary Syndromes. Arq. Bras. Cardiol. 2022, 119, 382–390. [Google Scholar]
- Yildiz, C.; Yuksel, Y.; Efe, S.C.; Altintas, M.S.; Katkat, F.; Ayca, B.; Karabulut, D.; Çağlar, F.N.T.; Köse, S. Value of systemic inflammation-response index in predicting contrast-induced nephropathy in patients with ST-elevation myocardial infarction. Acta Cardiol. 2023, 78, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Kelesoglu, S.; Yilmaz, Y.; Elcık, D.; Çetınkaya, Z.; Inanc, M.T.; Dogan, A.; Oguzhan, A.; Kalay, N. Systemic Immune Inflammation Index: A Novel Predictor of Contrast-Induced Nephropathy in Patients with Non-ST Segment Elevation Myocardial Infarction. Angiology 2021, 72, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, D.; Xu, T.; Chen, Z.; Shan, Y.; Zhao, L.; Fu, G.; Luan, Y.; Xia, S.; Zhang, W. Systemic Immune-Inflammation Index Predicts Contrast-Induced Acute Kidney Injury in Patients Undergoing Coronary Angiography: A Cross-Sectional Study. Front. Med. 2022, 9, 841601. [Google Scholar] [CrossRef]
- Zhu, Y.; Qiu, H.; Wang, Z.; Shen, G.; Li, W. Predictive value of systemic immune-inflammatory index combined with CHA2DS2-VASC score for contrast-induced acute kidney injury in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Int. Urol. Nephrol. 2023, 55, 2897–2903. [Google Scholar] [CrossRef]
- Guo, W.; Song, Y.; Sun, Y.; Du, H.; Cai, Y.; You, Q.; Fu, H.; Shao, L. Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: Evidence from NHANES 2011–2018. Front. Endocrinol. 2022, 13, 1071465. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Liu, M.; Zhou, H.; Xu, H. Association between neutrophil-to-lymphocyte ratio and diabetic kidney disease in type 2 diabetes mellitus patients: A cross-sectional study. Front. Endocrinol. 2024, 14, 1285509. [Google Scholar] [CrossRef]
- Zheng, H.; Yin, Z.; Luo, X.; Zhou, Y.; Zhang, F.; Guo, Z. Associations between systemic immunity-inflammation index and heart failure: Evidence from the NHANES 1999–2018. Int. J. Cardiol. 2024, 395, 131400. [Google Scholar] [CrossRef] [PubMed]
- Balci, K.; Erbay, İ.; Demirkan, B.; Balci, M.M.; Temizhan, A. The association of hemodynamic markers of right ventricular dysfunction with SII index and clinical outcomes in reduced ejection fraction heart failure. Medicine 2023, 102, e34809. [Google Scholar] [CrossRef] [PubMed]
- Chi, R.; Shan, X.; Guan, C.; Yang, H.; Wang, X.; Li, B.; Zhang, Q. Association between systemic inflammatory response index and left ventricular remodeling and systolic dysfunction in atrial fibrillation patients. BMC Cardiovasc. Disord. 2023, 23, 37. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction. Circulation 2018, 138, e618–e651. [Google Scholar]
- Michelucci, E.; Rocchiccioli, S.; Gaggini, M.; Ndreu, R.; Berti, S.; Vassalle, C. Ceramides and Cardiovascular Risk Factors, Inflammatory Parameters and Left Ventricular Function in AMI Patients. Biomedicines 2022, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Paradossi, U.; Taglieri, N.; Massarelli, G.; Palmieri, C.; De Caterina, A.R.; Bruno, A.G.; Taddei, A.; Nardi, E.; Ghetti, G.; Palmerini, T.; et al. Female gender and mortality in ST-segment-elevation myocardial infarction treated with primary PCI. J. Cardiovasc. Med. 2022, 23, 234–241. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Adatia, K.; Farag, M.F.; Gue, Y.X.; Srinivasan, M.; Gorog, D.A. Relationship of Platelet Reactivity and Inflammatory Markers to Recurrent Adverse Events in Patients with ST-Elevation Myocardial Infarction. Thromb. Haemost. 2019, 119, 1785–1794. [Google Scholar] [CrossRef]
- Mehu, M.; Narasimhulu, C.A.; Singla, D.K. Inflammatory Cells in Atherosclerosis. Antioxidants 2022, 11, 233. [Google Scholar] [CrossRef]
- Doran, A.C.; Lipinski, M.J.; Oldham, S.N.; Garmey, J.C.; Campbell, K.A.; Skaflen, M.D.; Cutchins, A.; Lee, D.J.; Glover, D.K.; Kelly, K.A.; et al. B-cell aortic homing and atheroprotection depend on Id3. Circ. Res. 2012, 110, e1–e12. [Google Scholar] [CrossRef]
- Biswas, M.; Suvarna, R.; Krishnan, S.V.; Devasia, T.; Shenoy Belle, V.; Prabhu, K. The mechanistic role of neutrophil lymphocyte ratio perturbations in the leading non communicable lifestyle diseases. F1000Res 2022, 11, 960. [Google Scholar] [CrossRef]
- Arı, E.; Köseoğlu, H.; Eroğlu, T. Predictive value of SIRI and SII for metastases in RCC: A prospective clinical study. BMC Urol. 2024, 24, 14. [Google Scholar] [CrossRef]
- Wenpei, G.; Yuan, L.; Liangbo, L.; Jingjun, M.; Bo, W.; Zhiqiang, N.; Yijie, N.; Lixin, L. Predictive value of preoperative inflammatory indexes for postoperative early recurrence of hepatitis B-related hepatocellular carcinoma. Front. Oncol. 2023, 13, 1142168. [Google Scholar] [CrossRef]
- Atasever Akkas, E.; Erdis, E.; Yucel, B. Prognostic value of the systemic immune-inflammation index, systemic inflammation response index, and prognostic nutritional index in head and neck cancer. Eur. Arch. Otorhinolaryngol. 2023, 280, 3821–3830. [Google Scholar] [CrossRef]
- Xu, M.; Chen, R.; Liu, L.; Liu, X.; Hou, J.; Liao, J.; Zhang, P.; Huang, J.; Lu, L.; Chen, L.; et al. Systemic immune-inflammation index and incident cardiovascular diseases among middle-aged and elderly Chinese adults: The Dongfeng-Tongji cohort study. Atherosclerosis 2021, 323, 20–29. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, C.; Wu, L.; Li, Z.; Li, H.; Zhang, J. Systemic Immune Inflammation Index (SII), System Inflammation Response Index (SIRI) and Risk of All-Cause Mortality and Cardiovascular Mortality: A 20-Year Follow-Up Cohort Study of 42,875 US Adults. J. Clin. Med. 2023, 12, 1128. [Google Scholar] [CrossRef]
- Jin, Z.; Wu, Q.; Chen, S.; Gao, J.; Li, X.; Zhang, X.; Zhou, Y.; He, D.; Cheng, Z.; Zhu, Y.; et al. The Associations of Two Novel Inflammation Indexes, SII and SIRI with the Risks for Cardiovascular Diseases and All-Cause Mortality: A Ten-Year Follow-Up Study in 85,154 Individuals. J. Inflamm. Res. 2021, 14, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Mo, C.; Li, Y.; Gui, C. Systemic immune-inflammation index associated with contrast-induced nephropathy after elective percutaneous coronary intervention in a case-control study. Coron. Artery Dis. 2023, 34, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Almeida, I.; Chin, J.; Santos, H.; Miranda, H.; Santos, M.; Sá, C.; Almeida, S.; Sousa, C.; Almeida, L. National Cardiology Data Collection Center, Portuguese Society of Cardiology, Coimbra, Portugal, National Register of Acute Coronary Syndromes Investigators. Prognostic value of brain natriuretic peptide in ST-elevation myocardial infarction patients: A Portuguese registry. Rev. Port. Cardiol. 2022, 41, 87–95. [Google Scholar] [PubMed]
- Zuo, H.; Xie, X.; Peng, J.; Wang, L.; Zhu, R. Predictive Value of Novel Inflammation-Based Biomarkers for Pulmonary Hypertension in the Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Anal. Cell. Pathol. 2019, 2019, 5189165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; He, H.; Qiu, H.; Shen, G.; Wang, Z.; Li, W. Prognostic Value of Systemic Immune-Inflammation Index and NT-proBNP in Patients with Acute ST-Elevation Myocardial Infarction. Clin. Interv. Aging 2023, 18, 397–407. [Google Scholar] [CrossRef]
- Ząbczyk, M.; Ariëns, R.A.S.; Undas, A. Fibrin clot properties in cardiovascular disease: From basic mechanisms to clinical practice. Cardiovasc. Res. 2023, 119, 94–111. [Google Scholar] [CrossRef] [PubMed]

| Variable | Value |
|---|---|
| Number | 105 |
| Age (years) | 70 ± 11 |
| Males/Females | 74 (70)/31 (30) |
| Diabetes | 20 (19) |
| Hypertension | 63 (60) |
| Dyslipidemia | 52 (49) |
| BMI (Kg/m2) | 27 ± 5 |
| Smoking history (current or former smoking habit) | 44 (43) |
| LVEF (%) | 48 ± 9 |
| BNP (ng/L) | 128 (51–245) |
| Creatinine (mg/dL)) | 1 ± 0.3 |
| Neutrophils (103/µL) | 6 (4.7–8.1) |
| Lymphocytes (103/µL) | 1.8 (1.4–2.1) |
| Monocytes (103/µL) | 0.8 (0.6–1) |
| Platelets (103/µL) | 234 ± 76 |
| Variable | Standard Coefficient | t-Value | p Value |
|---|---|---|---|
| Creatinine | 0.17 | 1.6 | ns |
| BNP | 0.26 | 2.5 | <0.05 |
| Variable | Standard Coefficient | t-Value | p Value |
|---|---|---|---|
| Creatinine | 0.28 | 2.7 | <0.01 |
| BNP | 0.15 | 1.3 | ns |
| Age | 0.1 | 0.8 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchi, F.; Pylypiv, N.; Parlanti, A.; Storti, S.; Gaggini, M.; Paradossi, U.; Berti, S.; Vassalle, C. Systemic Immune-Inflammation Index and Systemic Inflammatory Response Index as Predictors of Mortality in ST-Elevation Myocardial Infarction. J. Clin. Med. 2024, 13, 1256. https://doi.org/10.3390/jcm13051256
Marchi F, Pylypiv N, Parlanti A, Storti S, Gaggini M, Paradossi U, Berti S, Vassalle C. Systemic Immune-Inflammation Index and Systemic Inflammatory Response Index as Predictors of Mortality in ST-Elevation Myocardial Infarction. Journal of Clinical Medicine. 2024; 13(5):1256. https://doi.org/10.3390/jcm13051256
Chicago/Turabian StyleMarchi, Federica, Nataliya Pylypiv, Alessandra Parlanti, Simona Storti, Melania Gaggini, Umberto Paradossi, Sergio Berti, and Cristina Vassalle. 2024. "Systemic Immune-Inflammation Index and Systemic Inflammatory Response Index as Predictors of Mortality in ST-Elevation Myocardial Infarction" Journal of Clinical Medicine 13, no. 5: 1256. https://doi.org/10.3390/jcm13051256
APA StyleMarchi, F., Pylypiv, N., Parlanti, A., Storti, S., Gaggini, M., Paradossi, U., Berti, S., & Vassalle, C. (2024). Systemic Immune-Inflammation Index and Systemic Inflammatory Response Index as Predictors of Mortality in ST-Elevation Myocardial Infarction. Journal of Clinical Medicine, 13(5), 1256. https://doi.org/10.3390/jcm13051256









