Abstract
(1) Background: The present scoping review aims to scrutinize all existing patient-reported outcomes and assess the perspectives of obstructive sleep apnea patients after maxillomandibular surgery. (2) Methods: The review was carried out according to the extensions for scoping reviews using the PRISMA-ScR guidelines. Several databases were used to carry out the initial search. This study included randomized controlled trials, cohort studies, cross-sectional and case-control studies. The included studies considered patients with obstructive sleep apnea who were submitted to orthognathic surgery as the main subjects, and the patient’s perception of quality of life, satisfaction, treatment experience and side effects were assessed. (3) Results: From 1407 examined articles, a total of 16 were included. Most of the included studies used more than one questionnaire to assess quality of life, except for five articles. The most commonly referred instruments were the Epworth Sleepiness Scale, SF-36, the Functional Outcomes of Sleep and Ottawa Sleep Apnea. The most commonly assessed outcomes were sleep quality, daytime function, facial aesthetics, dental function and emotional health. (4) Conclusions: The number of variables that can be evaluated from a patient’s perspective are endless, as are the tools available to assess them. Not all of these tools, which are generally questionnaires, assess all the various outcomes, and some do not compare the pre- and post-surgical situations. Most of them are generic and lack specificity for obstructive sleep apnea.
1. Introduction
The American Academy of Sleep Medicine updated the international classification of sleep disorders in June 2023 to define obstructive sleep apnea (OSA) as the presence of one or more of the following symptoms: fatigue, insomnia, sleepiness or other symptoms that alter sleep-related quality of life [1]. OSA is classified as a sleep-related breathing disorder, and it affects between 2% and 5% of the pediatric population and 9% to 38% of the adult population worldwide [2,3,4]. This pathology results in a partial or total obstruction of the upper airway while the individual sleeps, with repeated respiratory arrests during this period. Its etiology is the focus of debate as it is thought to be multifactorial and may vary according to sex and age [2]. The associated pathophysiological factors can vary from anatomical (skeletal or soft tissue) to biomechanical, such as increased airflow resistance [5]. Other predisposing factors such as obesity, some genetic syndromes and respiratory and inflammatory pathologies should also be considered when discussing OSA. This condition can hinder development in patients of pediatric age, resulting in a reduced intellectual performance in adulthood [2,6,7]. Scientific evidence suggests that OSA is a risk factor for other pathologies like high blood pressure, cardiac arrhythmia, ischemic heart disease, metabolic disorders and cognitive dysfunction, among others [8,9,10].
When the first signs and symptoms appear, the diagnosis and treatment of OSA should be led by a multidisciplinary team with the aid of various examinations and validated questionnaires [11]. There are numerous questionnaires available for OSA screening in both adults and children. The most common for adults are the Epworth Sleepiness Scale (ESS) [12], the STOP-Bang questionnaire [13] and the Berlin questionnaire [14]. For children, the Pediatric Sleep Questionnaire (PSQ) is the most used questionnaire [15]. Due to its simplicity, the ESS is the most frequently used survey; however, it also has a lower diagnostic value because it measures the general level of sleepiness. The STOP-Bang and Berlin questionnaires, on the other hand, determine the risk of sleep apnea. Both questionnaires assess the signs, symptoms and risk factors of OSA, including snoring, drowsiness, witnessed apnea, obesity and hypertension, among others. The PSQ is a targeted questionnaire used mostly to assess behavior and cognitive performance in pediatric populations. For a thorough diagnosis, other tests, such as an overnight polysomnography (PSG) or an overnight home sleep apnea test (HSAT), should also be performed [11,16].
After a positive diagnosis with OSA, the patient is then categorized using the Apnea-Hypopnea Index (AHI), which categorizes patients according to the number of breathing events that occur per hour during sleep, with the following classification levels: mild (AHI = 5–15 events per hour; oxyhemoglobin saturation (SpO2)—86–91%); moderate (AHI = 15–30 events per hour; SpO2 = 76–85%); severe (AHI > 30 events per hour; SpO2 ≤ 75%) [17]. Similar to its diagnosis, OSA treatment should also be multidisciplinary, with the gold standard in adulthood being Positive Airway Pressure therapy using Continuous Positive Airway Pressure (CPAP) devices [11,16]. This therapy is effective in eliminating respiratory obstruction events and improves oxygen saturation during sleep. Nonetheless, tolerance and compliance to the therapy are generally reduced [18,19]. In the pediatric population diagnosed with OSA, rapid maxillary expansion (RME), mandibular advancement devices (MADs) and reverse traction extraoral appliances (RPHG) are the preferred treatment options for mild-to-moderate cases as they seem to lessen some of the symptoms temporarily. There are several literature reviews that report a decrease in the Apnea-Hypopnea Index (AHI) of about six points after the use of RMEs and MADs [20,21,22]. In individuals with severe OSA who are intolerant to CPAP, orthodontic–surgical treatment may be an option to take into consideration. The maxillomandibular advancement surgery increases the size of the nasopharyngeal, retropalatal and hypopharyngeal airways due to the physical expansion of the facial skeletal structure. The advancement of the maxilla and mandible increases tension in the pharyngeal soft tissues, the base of the tongue and the soft palate, thereby enlarging the mediolateral and anteroposterior dimensions of the upper airway. Regarding surgical treatment for OSA, a recent meta-analysis demonstrated that this treatment presented a mean decrease in the AHI from 63.9 to 9.5 events/hour, with a combined surgical success rate of 86.0% and an overall OSA treatment success rate of 43.2% [23]. There are other studies that demonstrate the high effectiveness of this surgical approach, showing that it reduces the frequency of respiratory events and drowsiness, increasing overall quality of life [10,24]. Furthermore, when there is a narrowing of the oropharynx, velopharynx and/or hypopharynx and maxillary retrognathia or skeletal hypoplasia, surgery is the most adequate therapeutic option [10,25]. The pre-operative severity of OSA is the most reliable predictor of the outcome effect, dimension and likelihood of surgical success. The more severe the OSA, the more benefits are reaped [26]. Less severe patients achieve an improvement in the AHI or RDI (respiratory disturbance index) of lower magnitude post-operatively but have a higher chance of successful treatment. Patients with less severe cases of RDI and AHI (despite previous treatments such as uvulopalatopharyngoplasty, partial glossectomy and/or nasal surgery) are highly likely to benefit from surgical maxillomandibular advancement [26].
Despite evidence supporting the success of orthognathic surgery as a treatment for OSA, it is also important to consider patient-reported outcomes (PROs). Evaluating the adaptability, adverse effects, impact of the surgery on the quality of sleep and quality of life of patients and their families is of the utmost importance. This review aims to evaluate the current literature regarding Patient-Reported Outcomes Measurements (PROMs) for children and adults with OSA who have undergone maxillomandibular surgery, specifically quality of life (QoL), adverse effects, patient satisfaction, overall experience with the treatment and the perception of occlusal or dental changes after treatment. The present scoping review intends to scrutinize all existing PROMs to assess the perspective of the OSA patient after maxillomandibular surgery.
2. Materials and Methods
The present review was carried out according to the extensions for scoping reviews using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA-ScR) criteria.
2.1. Research Question
The research question was chosen considering the patient’s perspective regarding expectations, satisfaction and quality of life after orthognathic surgery. The research question is described in Table 1.

Table 1.
PICO question.
The aim of this study was to evaluate the perspectives of OSAS patients after undergoing orthognathic surgery.
2.2. Database Search Protocol
For the present scoping review, a search was carried out using several databases, such as Medline (PubMed), all Web of Science databases, Embase and Cochrane. Table 2 describes the search keys, which were used on 7 March 2023.

Table 2.
Search keys of several Databases.
Beyond the described databases, a search was also conducted in the gray literature on the following websites: OpenGrey Europe (https://opengrey.eu, accessed on 10 March 2023) and ProQuest (https://www.proquest.com, accessed on 10 March 2023).
2.3. Analysis of Eligibility Criteria, Selection of Studies and Data Collection
This study exclusively included randomized controlled trials (RCT), cohort studies (prospective and retrospective), cross-sectional studies and case–control studies. Inclusion and exclusion criteria were established based on the research question. The inclusion criteria adopted during the development of this systematic review were as follows: (1) patients with OSAS submitted orthognathic surgery and (2) studies that evaluated patients’ perceptions regarding quality of life (QoL), namely satisfaction and treatment experience, as well as side effects.
Other types of papers, such as umbrella reviews, systematic reviews, case series studies, case–control editorials, conference abstracts, book chapters, guidelines, protocols, and opinion papers were excluded from the analysis. Studies including patients with systematic diseases, known genetic syndromes, and participants without an OSAS diagnosis were also excluded.
Two calibrated researchers (M.M. and C.O.) were responsible for article selection, following predefined inclusion and exclusion criteria. In instances where there was disagreement, a calibrated investigator (C.N.) assessed the articles in question. The duplicated citations were removed using automated and manual tools. The initial evaluation of studies involved screening their titles and abstracts. Those that met the inclusion criteria proceeded to undergo a thorough reading in full.
The included studies were scrutinized, and the following data were gathered: author; year of publication; sample size; sex; mean age of patients; type of orthognathic surgery (unimaxilar or bimaxilar); evaluated parameters; evaluation instruments and their description (PROM’s); and final observations.
3. Results
3.1. Study Selection
The search, screening, and eligibility processes are described in Figure 1. Initially, 1407 articles were retrieved from the search, none of which were cross-referenced. After removing duplicates, 973 articles were analyzed by title and abstract, resulting in 17 articles being selected for full reading. After fully reading the articles, it was found that one article did not evaluate the patient’s perspective, as the quality of life assessment was carried out by family members, leading to the exclusion of this article. Thus, sixteen articles were included in this review (Figure 1).
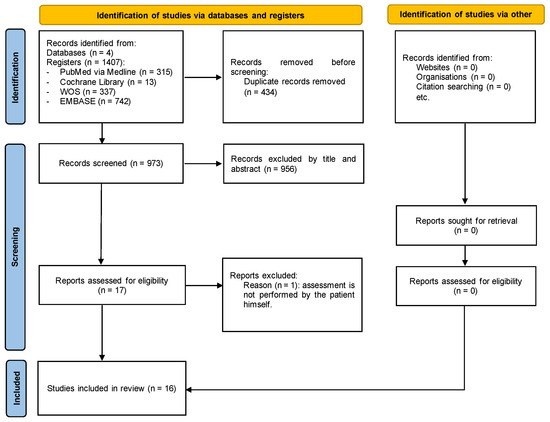
Figure 1.
Flow diagram.
3.2. Characteristics of the Included Studies
Sixteen articles assessed the impacts of orthognathic surgery on satisfaction and patient quality of life. The year of publication ranged from 2004 [27] to 2023 [28], given that five were published in 2020 [20,29,30,31] and three in 2022 [32,33,34]. Regarding the type of study, eight were retrospective cohort studies, four were cohort studies, three were prospective cohort studies and one was a retrospective case–control study. The sample size ranged from ten [34] to fifty-seven [27], resulting in a total sample of n = 413. The average age of patients was between 34.75 ± 11.33 [32] and 59.1 ± 11.7 years [29]. Apart from one study that did not mention the patients’ sex [35], all studies included more men than women. Regarding the instruments, five articles used a single questionnaire [27,29,30,36,37], while eleven employed multiple questionnaires, with the most extensive one using six [38]. The most commonly referred instruments were the Epworth Sleepiness Scale (nine studies) [27,28,31,34,35,37,38,39,40,41], SF-36 (four studies) [32,38,39,42], Functional Outcomes of Sleep (three studies) [31,39,41], Ottawa Sleep Apnea (two studies) [36,39], and Rustemever’s method (two studies) [32,33]. The most evaluated outcomes were sleep quality, daytime function, facial aesthetics, dental function and emotional health. Table 3 reports a summary of the characteristics of the included studies.

Table 3.
Characteristics of the Included Studies.
4. Discussion
The aim of this scoping review was to report on the current state of the art regarding the tools available to assess quality of life from the patient’s perspective after orthognathic surgery to manage OSAS. The qualitative analysis of the studies available in the literature allowed for a better understanding and evaluation of the studies published on this topic.
From the patient’s perspective, several outcomes can be assessed to rate quality of life. These can be related to aesthetics, function, mental health, sleep quality and post-operative condition. Regarding aesthetic outcomes, these are directly related to facial aesthetics and concern the facial changes resulting from orthognathic surgery. Mental health outcomes focus on general well-being, emotional status, mood, anxiety and depression. In relation to function, patients assess their general daily productivity, level of activity and focus, mobility, chewing, phonation, swallowing and intimacy, more specifically the quality of sexual life. Sleep quality includes outcomes such as insomnia, daytime sleepiness, frequent nocturnal diuresis and snoring. And, finally, some tools assess the post-operative condition, allowing the patient to describe their post-surgical recovery regarding the presence of signs and/or symptoms resulting from the surgery, such as pain or discomfort.
The best way to improve outcome reports is to develop and apply a core outcome set (COSs), that is, the minimum set of measurable and relevant outcomes that should be measured and reported in all disease-specific clinical trials [43]. Currently, the Core Outcome Measures in Effectiveness Trials (COMET) initiative promotes the development and implementation of COSs for the selection of health measurement instruments (COSMIN) [44]. However, on the topic of obstructive sleep apnea, there is still no defined COS, which makes the standardization of studies difficult. The heterogeneity in outcomes across studies poses significant challenges for drawing clear conclusions or generalizing findings, making informed decisions more complicated. This variability complicates the development of meta-analyses and consistent policies or guidelines, leading to inefficient resource allocation. To address these issues, standardization efforts are crucial. This includes establishing standardized outcome measures, protocol standardization, promoting data sharing and collaboration, utilizing advanced meta-analysis techniques and implementing quality improvement initiatives. These strategies will enable researchers to enhance research reliability and reproducibility, thus resulting in knowledge advancement and improved decision-making. This scoping review produced a synthesis of an existing body of literature, which can help to close the existing gap, since it verified the most evaluated results: sleep quality, daytime function, facial aesthetics, dental function and emotional health. In addition, the main focus of this review was assessing the outcomes from the patients’ perspectives, subsequently determining the relevance of the evaluated outcomes.
In the sixteen included studies, fifteen different tools for assessing quality of life from the patient’s perspective can be found, most of which consist of questionnaires completed by patients themselves before and/or after orthognathic surgery. Out of all the tools, five stand out: the Epworth Sleepiness Scale (ESS), SF-36, the Functional Outcomes of Sleep Questionnaire (FOSQ), Rustemeyer’s Questionnaire and the Ottawa Sleep Apnea Questionnaire (OSA-Q).
The ESS was used the most in the majority of included studies, having been employed in about 55% of the studies. It consists of a standardized and validated questionnaire that measures the level of daytime sleepiness. It consists of eight questions about subjective sleepiness in eight different everyday situations, each rated on a scale of 0 to 3, with a maximum score of 24. Normal values range between 2 and 10, while scores above 10 indicate a high level of excessive or pathological daytime sleepiness. In almost all studies, the questionnaire was administered at two different points in time: pre-surgery and post-surgery, allowing for the determination of long-term changes in subjective sleepiness after surgery. According to Matthew T Scharf [45], although the ESS is widely adopted and considered to be a useful tool for the assessment of excessive sleepiness, it should be applied and interpreted with caution within the appropriate clinical context, and it should be complemented with other assessment tools, especially when the test is negative [45,46]. The main advantages of the ESS are low cost, easy access and excellent validation. However, Matthew T Scharf [45] found some disadvantages, such as variability in the results obtained when the test was repeated and the fact that it measures different variables in different populations, meaning that a specific score in one group may present disparate variables when compared to a similar score in another group. Recently, Gonçalves et al. reinforced the need for further studies to investigate which variables may be the cause of the observed variability, making it important to include not only psychometric studies but also empirical studies [47].
The SF-36 questionnaire was the second most used tool in the included studies (25% of studies). Similar to the ESS, it is a questionnaire that needs to be completed at two different times: pre- and post-surgery, allowing for the measurement and comparison of surgical impacts on quality of life in patients with OSAS. This questionnaire assesses two components: physical and mental. The physical component includes the physical function, physical role, bodily pain and general health perception scales, while the mental component includes the vitality, social functioning, emotional role and general mental health scales. Scores range from 0 to 100, with 100 representing the highest level of function. These two components are divided into eight domains. The final scores in each domain represent an average of the scores obtained in the questions addressing that specific domain [48]. The frequent use of this tool may be related to some advantages such as its generic nature for assessing health status, its easy administration and comprehension and the fact that it can be used in groups of any age, pathology, treatment, ethnicity or gender. In fact, this tool is considered in the literature to be a reliable, valid and responsive method for a variety of medical diagnoses. Regarding OSAS, this method has been reported as sensitive to determine the effects of this pathology.
The third most commonly used tool was the Functional Outcomes of Sleep Questionnaire (FOSQ), appearing in 19% of the included studies. Similar to the questionnaires mentioned above, this survey is also conducted pre- and post-surgery. The FOSQ consists of 30 questions divided into 5 domains: activity level, vigilance, intimacy and sexual relationships, general productivity and social outcome. The scores for each domain are added together to give a total score, with a maximum of 20 points. A low score represents dysfunction due to excessive sleepiness during the day [31,48]. A major difference between this tool and the others is the fact that it assesses the sexual component. However, although this is an advantage, it can also sometimes be considered prejudicial, as some people may find the questions of this domain offensive and/or embarrassing, which may induce bias in the results [31,49].
In the present study, the three questionnaires mentioned above stood out. However, two others also stood out: the Ottawa Sleep Apnea Questionnaire (OSA-Q) and Rustemeyer’s Questionnaire. Rustemeyer’s Questionnaire, unlike the previously described tools, is performed only at one point, which is during the post-surgical period. Thus, it is only considered to be a post-operative tool. It consists of six questions, and the score ranges from 0 to 10. It aims to assess the patient’s overall satisfaction; changes in quality of life, aesthetics and masticatory function after undergoing orthognathic surgery; and the opinions of family and friends [32]. Finally, the OSA-Q consists of a questionnaire comprised of 38 questions regarding quality of life on a five-point Likert scale. The first 30 questions are related to sleep quality, daytime function and physical, mental, emotional and sexual health. However, as with the FOSQ, there is also a low response rate for questions related to intimacy and sexual health; so, in the study by Butterfield et al., these questions were made optional in order to encourage patients to complete the questionnaire [36] The remaining eight questions on the Likert scale are mainly aimed at assessing the impacts of side effects of orthognathic surgery on quality of life, taking into account surgical recovery and masticatory function [36].
Therefore, it can be noted that the number of outcomes that can be assessed from the patient’s perspective is countless, as are the tools available to assess them. Nevertheless, not all these tools, which are mostly questionnaires, assess all the various domains of outcomes, and some do not compare the pre-surgical situation with the post-surgical one. In future studies, it would be important to create a questionnaire that compiles all questions related to all outcomes and standardizes the assessment of quality of life in patients diagnosed with OSAS undergoing orthognathic surgery. Despite the variation in outcomes, most studies included were classified as good quality (scoring ≥ 7 points), facilitating a clearer interpretation of the results. This study could be the starting point for a more patient-centered precision medicine. In fact, patient-reported outcomes play a crucial role in clinical practice, patient care and future research directions in healthcare. Understanding the patient’s perspective on their health status, symptoms and quality of life provides valuable information about the effectiveness of treatments and interventions. Clinicians use these outcomes to tailor patient care plans, monitor treatment progress and make informed decisions about the most appropriate interventions for individual patients. Additionally, PROs enable OSA patients to actively participate in their care by articulating their concerns and preferences, fostering shared decision-making between patients and healthcare providers. Moreover, PRO data contribute to the advancement of research by identifying areas for improvement in OSA treatment, guiding the development of new interventions and evaluating the impact of interventions on patient outcomes. As healthcare continues to evolve to take on more patient-centered characteristics, the integration of PROs into OSA clinical practice and research will become increasingly essential for optimizing patient outcomes and enhancing the overall quality of care.
The best method for improving outcome reports is to develop and apply a core outcome set (COS), that is, the minimum set of measurable and relevant outcomes that should be measured and reported in all disease-specific clinical trials. However, for OSA, there is still no defined COS, which made it difficult to standardize the studies. Researchers should join the COMET Initiative, as it is a platform that allows the gathering of relevant resources, both applied and methodological, in order to facilitate the exchange in ideas and information and encourage methodological research in this area. Notwithstanding its limitations, this scoping review helps to fill the existing literature gap, as it has identified the five most-evaluated outcomes, which were sleep quality, daytime function, facial aesthetics, dental function and emotional health. It would be important to create a specific questionnaire for OSA/Orthognathic Surgery that compiles all questions related to all outcomes and standardizes the assessment of quality of life. Future studies should define the key results for characterizing quality of life, allowing for the development of a complete and effective questionnaire.
5. Conclusions
The number of outcomes that can be assessed from the patient’s perspective is countless, as are the tools available to assess them. The most evaluated outcomes were sleep quality, daytime function, facial aesthetics, dental function and emotional health. Collaborative endeavors from researchers are imperative to foster the widespread and uniform adoption of patient outcome reports in OSA research.
Author Contributions
Conceptualization, F.V. and I.F.; methodology, C.M.M. and A.B.P.; software, G.S.; validation, C.N., A.B.P. and R.T.; formal analysis, F.M.; investigation, M.P.R.; resources, M.M.; data curation, M.S.; writing—original draft preparation, C.O. and R.T.; visualization, E.C.; supervision, I.F. and G.S.; project administration, C.N.; funding acquisition, F.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
Since this manuscript is a review, the data in this manuscript were not created, as they already exist in other manuscripts.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Available online: https://aasm.org/clinical-resources/international-classification-sleep-disorders/ (accessed on 12 January 2024).
- Fagundes, N.C.F.; Minervini, G.; Furio Alonso, B.; Nucci, L.; Grassia, V.; d’Apuzzo, F.; Puigdollers, A.; Perillo, L.; Flores-Mir, C. Patient-reported outcomes while managing obstructive sleep apnea with oral appliances: A scoping review. J. Evid. Based Dent. Pract. 2023, 23, 101786. [Google Scholar] [CrossRef]
- Bixler, E.O.; Vgontzas, A.N.; Lin, H.-M.; Liao, D.; Calhoun, S.; Vela-Bueno, A.; Fedok, F.; Vlasic, V.; Graff, G. Sleep Disordered Breathing in Children in a General Population Sample: Prevalence and Risk Factors. Sleep 2009, 32, 731–736. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of Obstructive Sleep Apnea in the General Population: A Systematic Review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Arens, R.; Marcus, C.L. Pathophysiology of Upper Airway Obstruction: A Developmental Perspective. Sleep 2004, 27, 997–1019. [Google Scholar] [CrossRef]
- Morsy, N.E.; Farrag, N.S.; Zaki, N.F.W.; Badawy, A.Y.; Abdelhafez, S.A.; El-Gilany, A.-H.; El Shafey, M.M.; Pandi-Perumal, S.R.; Spence, D.W.; BaHammam, A.S. Obstructive Sleep Apnea: Personal, Societal, Public Health, and Legal Implications. Rev. Environ. Health 2019, 34, 153–169. [Google Scholar] [CrossRef]
- Jennum, P.; Rejkjær-Knudsen, M.; Ibsen, R.; Kiær, E.K.; von Buchwald, C.; Kjellberg, J. Long-Term Health and Socioeconomic Outcome of Obstructive Sleep Apnea in Children and Adolescents. Sleep Med. 2020, 75, 441–447. [Google Scholar] [CrossRef]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea. JAMA 2020, 323, 1389. [Google Scholar] [CrossRef]
- Alansari, R.A. The Role of Orthodontics in Management of Obstructive Sleep Apnea. Saudi Dent. J. 2022, 34, 194–201. [Google Scholar] [CrossRef]
- Levine, M.; Bennett, K.; Cantwell, M.; Postol, K.; Schwartz, D. Dental Sleep Medicine Standards for Screening, Treating, and Managing Adults with Sleep-Related Breathing Disorders. J. Dent. Sleep Med. 2018, 5, 61–68. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Chung, F.; Yegneswaran, B.; Liao, P.; Chung, S.A.; Vairavanathan, S.; Islam, S.; Khajehdehi, A.; Shapiro, C.M. STOP Questionnaire. Anesthesiology 2008, 108, 812–821. [Google Scholar] [CrossRef]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire To Identify Patients at Risk for the Sleep Apnea Syndrome. Ann. Intern. Med. 1999, 131, 485. [Google Scholar] [CrossRef]
- Chervin, R.D.; Hedger, K.; Dillon, J.E.; Pituch, K.J. Pediatric Sleep Questionnaire (PSQ): Validity and Reliability of Scales for Sleep-Disordered Breathing, Snoring, Sleepiness, and Behavioral Problems. Sleep Med. 2000, 1, 21–32. [Google Scholar] [CrossRef]
- Ramar, K.; Dort, L.C.; Katz, S.G.; Lettieri, C.J.; Harrod, C.G.; Thomas, S.M.; Chervin, R.D. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. J. Clin. Sleep Med. 2015, 11, 773–827. [Google Scholar] [CrossRef]
- Gurgel, M.; Cevidanes, L.; Pereira, R.; Costa, F.; Ruellas, A.; Bianchi, J.; Cunali, P.; Bittencourt, L.; Junior, C.C. Three-Dimensional Craniofacial Characteristics Associated with Obstructive Sleep Apnea Severity and Treatment Outcomes. Clin. Oral Investig. 2022, 26, 875–887. [Google Scholar] [CrossRef]
- Vanderveken, O.; Hoekema, A. How to Treat Patients That Do Not Tolerate Continuous Positive Airway Pressure. Breathe 2010, 7, 157–167. [Google Scholar] [CrossRef]
- Mansukhani, M.P.; Olson, E.J.; Caples, S.M. Upper Airway Surgery for Obstructive Sleep Apnea. JAMA 2020, 324, 1161. [Google Scholar] [CrossRef]
- Lin, S.; Su, Y.; Wu, Y.; Chang, J.Z.; Tu, Y. Management of Paediatric Obstructive Sleep Apnoea: A Systematic Review and Network Meta-analysis. Int. J. Paediatr. Dent. 2020, 30, 156–170. [Google Scholar] [CrossRef]
- Nazarali, N.; Altalibi, M.; Nazarali, S.; Major, M.P.; Flores-Mir, C.; Major, P.W. Mandibular Advancement Appliances for the Treatment of Paediatric Obstructive Sleep Apnea: A Systematic Review. Eur. J. Orthod. 2015, 37, 618–626. [Google Scholar] [CrossRef]
- Huynh, N.T.; Desplats, E.; Almeida, F.R. Orthodontics Treatments for Managing Obstructive Sleep Apnea Syndrome in Children: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2016, 25, 84–94. [Google Scholar] [CrossRef]
- Holty, J.-E.C.; Guilleminault, C. Maxillomandibular Advancement for the Treatment of Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2010, 14, 287–297. [Google Scholar] [CrossRef]
- Varghese, R.; Adams, N.G.; Slocumb, N.L.; Viozzi, C.F.; Ramar, K.; Olson, E.J. Maxillomandibular Advancement in the Management of Obstructive Sleep Apnea. Int. J. Otolaryngol. 2012, 2012, 373025. [Google Scholar] [CrossRef]
- Li, K.K.; Riley, R.W.; Powell, N.B.; Guilleminault, C. Maxillomandibular Advancement for Persistent Obstructive Sleep Apnea After Phase I Surgery in Patients without Maxillomandibular Deficiency. Laryngoscope 2000, 110, 1684–1688. [Google Scholar] [CrossRef]
- Zaghi, S.; Holty, J.-E.C.; Certal, V.; Abdullatif, J.; Guilleminault, C.; Powell, N.B.; Riley, R.W.; Camacho, M. Maxillomandibular Advancement for Treatment of Obstructive Sleep Apnea. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 58. [Google Scholar] [CrossRef]
- Dattilo, D.J.; Drooger, S.A. Outcome Assessment of Patients Undergoing Maxillofacial Procedures for the Treatment of Sleep Apnea: Comparison of Subjective and Objective Results. J. Oral Maxillofac. Surg. 2004, 62, 164–168. [Google Scholar] [CrossRef]
- Abdelwahab, M.; Huang, A.; Chou, C.; Fleury, T.; Riley, R.; Most, S.; Liu, S. Patient’s Perception of Nasal Function and Cosmesis After Maxillomandibular Advancement for Obstructive Sleep Apnea. Facial Plast. Surg. Aesthet. Med. 2023, 25, 132–140. [Google Scholar] [CrossRef]
- Cillo, J.E.; Dattilo, D.J. Maxillomandibular Advancement for Obstructive Sleep Apnea Produces Long-Term Horizontal Advancement of the Maxilla and Mandible. J. Oral Maxillofac. Surg. 2019, 77, 2524–2528. [Google Scholar] [CrossRef]
- Cillo, J.E.; Dattilo, D.J. Oral Functional Behavior and Neurosensation After Adult Maxillomandibular Advancement for Obstructive Sleep Apnea in the Long-Term. J. Oral Maxillofac. Surg. 2020, 78, 255–260. [Google Scholar] [CrossRef]
- De Ruiter, M.H.T.; Apperloo, R.C.; Milstein, D.M.J.; de Lange, J. Facial Esthetics and Subjective Impairment Assessed after Maxillomandibular Advancement Surgery for Patients with Obstructive Sleep Apnea. In CRANIO®; Taylor & Francis: Abingdon, UK, 2023; Volume 41, pp. 16–21. [Google Scholar] [CrossRef]
- Rossi, D.S.; Goker, F.; Cullati, F.; Baj, A.; Pignatelli, D.; Beltramini, G.; Russillo, A.; Giannì, A.B.; Lucchina, A.G.; Mortellaro, C.; et al. Analysis and Comparison of Quality of Life and Patients’ Satisfaction between Dental-Skeletal Dysmorphisms and Obstructive Sleep Apnea (OSA) Patients Following Orthognathic Surgery. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 62–77. [Google Scholar]
- Rossi, D.S.; Goker, F.; Cullati, F.; Baj, A.; Pignatelli, D.; Gianni, A.B.; Del Fabbro, M. Post-Operative Patients’ Satisfaction and Quality of Life Assessment in Adult Patients with Obstructive Sleep Apnea Syndrome (OSAS). Int. J. Environ. Res. Public Health 2022, 19, 6273. [Google Scholar] [CrossRef]
- Martin, M.J.; Khanna, A.; Srinivasan, D.; Sovani, M.P. Patient-Reported Outcome Measures Following Maxillomandibular Advancement Surgery in Patients with Obstructive Sleep Apnoea Syndrome. Br. J. Oral Maxillofac. Surg. 2022, 60, 963–968. [Google Scholar] [CrossRef]
- Goodday, R.H.; Bourque, S.E.; Edwards, P.B. Objective and Subjective Outcomes Following Maxillomandibular Advancement Surgery for Treatment of Patients with Extremely Severe Obstructive Sleep Apnea (Apnea-Hypopnea Index > 100). J. Oral Maxillofac. Surg. 2016, 74, 583–589. [Google Scholar] [CrossRef]
- Butterfield, K.J.; Marks, P.L.G.; McLean, L.; Newton, J. Quality of Life Assessment After Maxillomandibular Advancement Surgery for Obstructive Sleep Apnea. J. Oral Maxillofac. Surg. 2016, 74, 1228–1237. [Google Scholar] [CrossRef]
- Beranger, T.; Garreau, E.; Ferri, J.; Raoul, G. Morphological Impact on Patients of Maxillomandibular Advancement Surgery for the Treatment of Obstructive Sleep Apnea-Hypopnea Syndrome. Int. Orthod. 2017, 15, 40–53. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chin, W.-C.; Huang, Y.-S.; Wang, P.-F.; Li, K.K.; Pirelli, P.; Chen, Y.-H.; Guilleminault, C. Objective and Subjective Long Term Outcome of Maxillomandibular Advancement in Obstructive Sleep Apnea. Sleep Med. 2020, 74, 289–296. [Google Scholar] [CrossRef]
- Boyd, S.B.; Chigurupati, R.; Cillo, J.E.; Eskes, G.; Goodday, R.; Meisami, T.; Viozzi, C.F.; Waite, P.; Wilson, J. Maxillomandibular Advancement Improves Multiple Health-Related and Functional Outcomes in Patients with Obstructive Sleep Apnea: A Multicenter Study. J. Oral Maxillofac. Surg. 2019, 77, 352–370. [Google Scholar] [CrossRef]
- Pottel, L.; Neyt, N.; Hertegonne, K.; Pevernagie, D.; Veys, B.; Abeloos, J.; De Clercq, C. Long-Term Quality of Life Outcomes of Maxillomandibular Advancement Osteotomy in Patients with Obstructive Sleep Apnoea Syndrome. Int. J. Oral Maxillofac. Surg. 2019, 48, 332–340. [Google Scholar] [CrossRef]
- Boyd, S.B.; Walters, A.S.; Waite, P.; Harding, S.M.; Song, Y. Long-Term Effectiveness and Safety of Maxillomandibular Advancement for Treatment of Obstructive Sleep Apnea. J. Clin. Sleep Med. 2015, 11, 699–708. [Google Scholar] [CrossRef]
- González, M.B.; Casellas, J.B.; Fernández Mondragón, M.P.; Nuño, V.C.; Amezaga, J.A.; De Carlos Villafra, F. Clinical, Esthetic, and Quality of Life Outcomes after Telegnathic Surgery in Caucasian OSAS Patients. In CRANIO®; Taylor & Francis: Abingdon, UK, 2022; Volume 40, pp. 425–432. [Google Scholar] [CrossRef]
- Young, A.; Brookes, S.; Rumsey, N.; Blazeby, J. Agreement on What to Measure in Randomised Controlled Trials in Burn Care: Study Protocol for the Development of a Core Outcome Set. BMJ Open 2017, 7, e017267. [Google Scholar] [CrossRef]
- Mokkink, L.B.; Terwee, C.B.; Patrick, D.L.; Alonso, J.; Stratford, P.W.; Knol, D.L.; Bouter, L.M.; de Vet, H.C.W. The COSMIN Checklist for Assessing the Methodological Quality of Studies on Measurement Properties of Health Status Measurement Instruments: An International Delphi Study. Qual. Life Res. 2010, 19, 539–549. [Google Scholar] [CrossRef]
- Scharf, M.T. Reliability and Efficacy of the Epworth Sleepiness Scale: Is There Still a Place for It? Nat. Sci. Sleep 2022, 14, 2151–2156. [Google Scholar] [CrossRef]
- Puretić, H.; Bosnar Puretić, M.; Pavliša, G.; Jakopović, M. Revisiting the Epworth Sleepiness Scale. In Wiener Klinische Wochenschrift; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Gonçalves, M.T.; Malafaia, S.; Moutinho dos Santos, J.; Roth, T.; Marques, D.R. Epworth Sleepiness Scale: A Meta-Analytic Study on the Internal Consistency. Sleep Med. 2023, 109, 261–269. [Google Scholar] [CrossRef]
- Silva, G.; Goodwin, J.; Vana, K.; Quan, S. Obstructive Sleep Apnea and Quality of Life: Comparison of the SAQLI, FOSQ, and SF-36 Questionnaires. Southwest J. Pulm. Crit. Care 2016, 13, 137–149. [Google Scholar] [CrossRef]
- Izci, B.; Firat, F.; Ardic, S.; Kokturk, O.; Gelir, E.; Altinors, M. Adaptation of Functional Outcomes of Sleep Questionnaire (FOSQ) to Turkish Population. Tüberküloz Toraks Derg. 2004, 52, 224–230. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).