Abstract
Diabetic foot ulcers (DFUs) pose a significant threat to individuals with diabetes mellitus (DM), such as lower limb amputation and severe morbidity. Bioengineered skin substitutes (BSS) are alternatives to traditional interventions for treating DFUs, but their efficacy compared to standard wound care (SWC) or other treatment types, such as allografts, remains unknown. A scoping review of human studies was conducted to identify current approaches in the treatment of DFUs using BSS as compared with other treatment options. Systematic searches in PubMed, Cochrane Library, and Web of Science were conducted to identify comparative studies that enrolled 10 or more patients and evaluated wound healing outcomes (closure, time-to-healing, and area reduction). Database searches isolated articles published from 1 December 2012 to 1 December 2022 and were conducted in accordance with PRISMA-ScR guidelines. The literature search yielded 1312 articles, 24 of which were included for the qualitative analysis. Findings in these studies demonstrated that BSS outperformed SWC in all measured outcomes, suggesting that BSS may be a superior treatment for DFUs. Of the 24 articles, 8 articles compared human amniotic membrane allografts (hAMA) to BSS. Conflicting evidence was observed when comparing BSS and hAMA treatments, highlighting the need for future research.
1. Introduction
Worldwide, 425 million people were affected by Diabetes Mellitus (DM) type 1 and 2 as of 2017. DM affects approximately 10% (34.2 million adults) of the United States population []. Diabetic foot ulcers (DFUs) can lead to challenges in maintaining lower extremities health, and they affect an estimated 15–25% of patients with diabetes mellitus (DM) []. When conventional treatments for chronic wounds prove ineffective, the likelihood of soft tissue infection followed by bone infection significantly increases. Infection may lead to lower limb amputation and mortality []. Approximately 85% of below-the-knee amputations in DM patients are a result of non-healing DFUs [] with a 5-year mortality of 40–50% post-amputation []. Furthermore, it is important to acknowledge that DFU complications not only impact morbidity and mortality but also pose a significant risk to the patient’s overall quality of life. Among those who have experienced amputation, the inevitable loss of mobility and independence will often contribute to anxiety and depression amongst that population []. Furthermore, non-healing DFUs and subsequent amputations not only adversely impact the patient’s physical and emotional well-being but also impose a significant financial burden. The direct financial burden of lower diabetic limb complications is conservatively estimated to exceed that of the five most costly cancers in the US, which include breast cancer at $16.5 billion and colorectal cancer at $14.14 billion annually [].
Given the considerable challenges DFUs pose and their consequences in patients’ lives, it is imperative to prioritize research initiatives and implement strategic interventions to ameliorate DFU healing. The development of biomaterials for treating DFUs may enhance patient outcomes by mitigating amputation rates and recurrence, finally elevating the overall quality of life among diabetic individuals. Herein, we conducted a scoping review of high-quality studies to identify current approaches and potential challenges associated with the treatment of DFU using bioengineered strategies, such as Bioengineered Skin Substitutes (BSS), as compared with other options, such as extracellular matrices and standard wound care (SWC). We also present an overview of the current understanding of DFU pathophysiology, the various types of treatments available, and the biomaterials currently being utilized in this context.
2. Background
2.1. Overview of the Current Understanding of DFU Pathophysiology
DFUs are considered any below-the-ankle full thickness (deep into the dermis) chronic wound, and normally occur under advancing distal sensory neuropathy or peripheral vascular disease (PVD) [], as demonstrated in [Figure 1]. In In brief, diabetic impairment of sensory, motor, and autonomic fibers will eventually cause the inability to perceive the protective sensations of pressure, pain, or heat and limit one’s range of motion (ROM) and mobility []. Together, these factors elevate plantar foot pressure and result in the formation of a “callus” or biomechanical hyperkeratotic lesion with something far more concerning underneath [,].
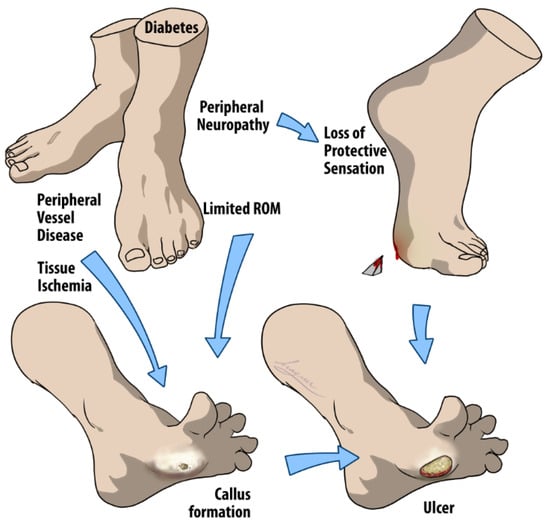
Figure 1.
Multifactorial pathways to DFU development. The arrows in the figure suggest a progression and/or causal relationship between the conditions listed, leading to the formation of a DFU. Initially, diabetes can cause peripheral neuropathy in the feet that leads to a loss of protective sensation, preventing individuals from feeling injuries. Concurrently, diabetes may induce peripheral vessel disease, which compromises blood circulation and causes tissue ischemia, depriving tissues of necessary oxygen and nutrients. The combination of these factors, along with limited ROM, contributes to abnormal pressure distribution on the feet. This abnormal pressure often results in callus formation, which, if left unchecked, can result in open wounds due to the body’s diminished ability to heal itself effectively, thus completing the multifactorial process of DFU development.
Furthermore, PVD is characterized by microvascular damage and endothelial dysfunction. Arterial basement membrane thickening and a decrease in capillary size, which are observed in diabetes, will restrict cellular exchange. Additionally, due to endothelial cell dysfunction (such as a nitric oxide synthetase deficiency), arteries and arterioles may not optimally dilate, leading to compromised blood flow and suboptimal healing [,].
The classification of wounds as acute or chronic is largely based on their persistence without showing signs of healing. Wounds that heal within 4 weeks are considered acute, whereas when a wound remains unhealed for more than 12 weeks, it is typically classified as chronic []. Of note, the cellular and molecular characteristics in acute healing versus chronic ulcers exhibit significant differences, and they are notably influenced by the inflammatory dysregulation induced by chronic hyperglycemia.
In brief, acute foot lesions in non-diabetic individuals follow a well-orchestrated healing process consisting of four distinct yet interrelated stages: hemostasis, inflammation, proliferation, and remodeling []. Immediately upon injury, hemostasis begins with the release of several growth factors, such as insulin-like growth factor (IGF), epidermal growth factor (EGF), transforming growth factor-β (TGF-β), and platelet-derived growth factor (PDGF), as well as numerous cytokines from platelets after they aggregate at the site of injury and begin the conversion of fibrinogen, forming a blood clot [,,]. Inflammation, the second stage, is characterized by the release of pro-inflammatory cytokines, such as tumor necrosis factor α (TNF-α) and interleukin-1 (IL-1), and the sequential recruitment of immune cells, such as neutrophils and monocytes/macrophages, to the wound site []. Neutrophils and macrophages are essential for removing eventual pathogens, while macrophages also clear degradation products and neutrophils [,]. In addition, macrophage polarization into either M1 (pro-inflammatory) or M2 (regenerative) phenotypes is a key event. M1 cells contribute to the prevention of infection and establish the immune response in the initial stages. At the same time, the M2 phenotype seems to be crucial for inflammation resolution, as well as stem cell proliferation and differentiation []. Lastly, the considerably overlapping stages of cell proliferation and tissue remodeling are crucial to healing completion. The proliferative phase is marked by angiogenesis, epithelialization, and the proliferation of fibroblasts for the deposition of an extracellular matrix, all mediated by regenerative factors, such as C-X-C Motif Chemokine Ligand-12 (CXCL12), TGFβ, Fibroblast Growth Factor-2 (FGF-2), and EGF []. This step also plays a role in epidermis regeneration by keratinocyte stem cells and the production of scar tissue, promoting wound closure []. Finally, M2 macrophages phagocytose excess cells during the remodeling phase and release MMPs for collagen degradation to remodel the newly formed extracellular matrix [].
While understanding the intricacies of acute wound healing in a non-diabetic environment is crucial for managing acute foot lesions, the current knowledge of the pathophysiology of chronic wounds is key for driving diagnostic modalities and more reliable treatment alternatives. Chronic ulceration results from a deficiency or malfunction in one or more of the described healing stages []. First, in DFUs, there is a lack of both stem cells, which have the ability to proliferate and differentiate into multiple lineages, and growth/vascular factors, which are largely sourced from stem cells []. Furthermore, it has been postulated that the dysregulated synthesis of essential growth factors and cytokines in diabetic patients affects the initiation of the wound healing cascade and leads to compromised inflammatory responses and weakened host defenses []. These events ultimately result in delayed healing, impaired pathogenic microbe clearance, and subsequent infections [].
Furthermore, a prolonged inflammatory stage is characterized by an imbalance in the polarization of M1/M2 macrophages, with a predominance and persistence of M1 macrophages []. Neutrophils accumulate and continue releasing cytotoxic granules, reactive oxygen species, and proteases, reducing the availability of crucial growth factors necessary for wound closure []. An overall pathophysiological aspect of the chronic DFU with non-diabetic acute wound healing is summarized in [Figure 2].
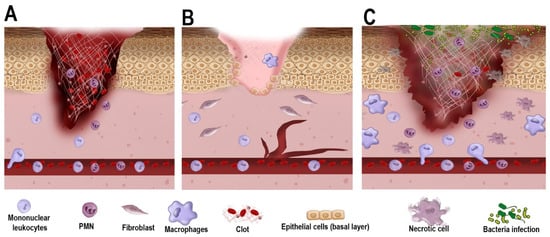
Figure 2.
Cellular and molecular mechanisms of wound healing in acute vs. chronic wound healing. (A). As trauma occurs, the acute wound undergoes blood clot formation with the early infiltration of polymorphonuclear (PMN) cells and mononuclear leukocytes (e.g., monocytes). The blood clot serves as a scaffold for cell migration and the temporary closure of the wound. (B). In a non-diabetic environment, as healing progresses, fibroblasts promote wound contraction, and epithelial cells from the basement membrane proliferate towards wound closure. Macrophages contribute to removing debris and secrete growth factors that further aid in wound closure. (C). In a diabetic environment, owing to poor vascularization and dysregulated inflammatory response, cell proliferation and wound contraction are delayed. PMN and macrophages also exhibit a defective response, favoring bacterial infection, necrosis, and impaired healing.
2.2. Types of Treatment of DFUs
The management of DFUs is a complex and challenging task for health care providers, involving multidisciplinary approaches to address various facets of the ailment, including fundamental causes of the DFU (e.g., perivascular diseases, pressure, repetitive trauma), regulation of glycemic levels, off-loading the affected area (the mainstay of DFU treatment), and mitigation of infections and other potential complications [,,]. Typical treatment of a chronic foot wound begins with the preparation of the wound bed and debridement of necrotic tissue []. Herein, we summarize the fundamental steps for DFU wound bed preparation before proceeding to other adjuvant strategies to improve tissue healing in DFUs. For instance, the quality and preparation of the wound bed, including removing necrotic tissues or any potential infection, is essential to a successful treatment plan and outcome [].
Debridement is a central component of wound care in DFUs []. It can be defined as removing necrotic or non-viable tissue and exudate, helping to reduce biofilm, minimize infection, and promote the production of granulation tissues [,]. The methods for debridement are diverse, falling into two main categories: selective and non-selective debridement [].
Non-selective debridement is a conventional method that can eliminate non-viable tissue; however, some viable, healthy tissue is also often damaged and lost [,]. Although this may initially seem counterintuitive, the underlying concept posits that the stalled healing process can be re-initiated to traverse the protracted stage of inflammation that sustains ulceration [,]. This strategy is practiced in various ways, including sharp/surgical debridement, mechanical debridement via wet-to-dry dressing, ultrasonic debridement, and aqueous high-pressure lavage []. Sharp/surgical debridement is currently considered the gold standard, making it the reference point to which all other techniques are compared [,]. For this technique, instruments, such as curettes, scissors, or scalpels, are used to remove unwanted tissue and debris from the wound bed but leave what still retains the potential to heal [,]. Wet-to-dry debridement involves applying wet gauze moistened with saline to the wound site and allowing it to dry []. Desiccation causes the gauze to adhere to the wound, and when removed forcefully, it can remove both necrotic and small amounts of viable tissues []. The wet-to-dry method comes with inherent disadvantages of its own. Aside from this technique being unfavorably time-consuming, patients who have retained peripheral sensation report that this technique is significantly more painful than other methods of debridement. The nature of the wet-to-dry debridement technique contributes to its disadvantages of being time-consuming and reportedly quite painful for patients [,]. Ultrasonic debridement is another nonselective method and utilizes low-frequency ultrasonic waves of 20–40 kHz to disrupt devitalized tissue through a mechanism called cavitation, where gas bubbles rapidly expand and implode within the targeted tissue fluid [].
Selective debridement methods include autolytic, enzymatic, and biodebridement []. Autolytic debridement refers to the induction of endogenous enzymes to simply digest and separate devitalized tissues from the wound bed []. This slow process is a component of the body’s innate ability to debride and is naturally occurring [,]. In contrast, enzymatic debridement is significantly faster and entails the introduction of external enzymes to necrotic tissue, facilitating their subsequent digestion [,,]. Biodebridement, which is also referred to as Maggot Debridement Therapy (MDT), makes use of larvae or “maggots” from two fly species, Lucilia sericata and Phaenicia sericata []. The sterile larvae are placed on the wound, either directly or within a permeable biobag, and it is covered and bandaged with a permeable dressing and absorbent pad, as MDT debrided wounds tend to produce large amounts of exudate []. Larvae tend to lose their rate of effective activity after about 48–72 h, in which time the dressing is removed, and a washout is performed []. The mechanism of MDT revolves around the secretion of collagenase, serine, and chymotrypsin/trypsin-like proteases, which work together to create a nutrient-rich food source for larval consumption from non-viable tissue and biofilm while leaving healthy tissue untouched and intact [,]. For these reasons, MDT has shown promise as a favorable option to significantly reduce DFU bacterial load while maximizing healthy tissue preservation [].
Once the wound bed is properly prepared, the healing process may return to its initial acute stage. However, it is important to note that the underlying pathology in DFUs remains, and often, achieving wound healing requires advanced therapy with adjunct materials. In the field of regenerative medicine, various strategies can be employed, including the use of skin grafts and bioengineered substitutes.
A skin graft consists of a piece of transplanted skin positioned over an open wound with the expectation that it will adhere to and integrate into the new site. The exact mechanism by which a skin graft stimulates the growth of new skin remains unclear; however, it is believed to be accomplished by harnessing the body’s utilization of extracellular matrices, growth factors, cytokines, and stem cells inherent to the graft, all of which are vital for achieving complete wound healing []. With numerous options available, skin grafts can typically be categorized into four main groups: autologous grafts, allografts, xenografts, and BSS [,]. Autologous skin grafts must be harvested from the same individual for whom they are intended []. A donor site is selected based on viability, appearance, availability, and patient preference. The harvested autograft is secured over the recipient wound bed, usually by suture or dressing []. However, the efficacy of these grafts is susceptible to failure, often attributed to complications such as hematoma, seroma, or infection []. Additionally, the procedure introduces a supplementary wound at the donor site, a concern particularly relevant for patients with compromised healing abilities, such as those with DFU [].
Allografts specifically involve the transplantation of tissues from a donor to a recipient. Unlike autografts, they circumvent the issue of patient donor site morbidity by sourcing the graft from a distinct human donor, commonly cadaveric or placental []. Because allografts originate from external sources, donors undergo screening for Human Immunodeficiency Virus, Hepatitis B and C, Cytomegalovirus, Human T-Lymphotropic Virus, Syphilis, and West Nile Virus []. Donor tissue is also analyzed histologically for appropriate structure, screened, and treated for immunogenicity [,]. Although these screening/treatment methods are extremely effective, complete microbe elimination is unlikely, and the risk of infection will persist []. While immuno-rejection vastly decreases after the graft undergoes pretreatment with 85% glycerol, rejection may still occur []. Nevertheless, allografts continue to be a viable option for chronic wound therapy.
A limitation in donor availability has led to xenografts, such as porcine skin, which involves removing sheets of animal skin, screening, and then applying directly onto a wound bed []. As with allografts, achieving complete microbial elimination is improbable in xenotransplantation, carrying the theoretical risk of zoonotic infection []. In addition to infection, hyperacute rejection, an immediate immune response, is also a clinical concern. In fact, three xeno-antigens have been identified in porcine skin alone []. When the body encounters xeno-antigens within the vascular endothelial cells of a graft, an antibody-mediated complement activation leads to the destruction of the transplant within hours [].
Over the past decade, tissue engineering has advanced significantly, resulting in alternative wound care strategies such as BSS [,]. Theoretically, BSSs hold great potential for enhancing the healing process of DFUs by providing a scaffold for cell growth and proliferation, immunomodulation, promoting angiogenesis, and stimulating tissue regeneration []. BSSs aim to address the primary challenges inherent in all three preceding methods—autografts, allografts, and xenografts—namely, issues related to donor morbidity and deficiency, susceptibility to infection, and the potential for immunogenic rejection [,]. These types of engineered grafts can be described as skin analogs created in a laboratory, and share some of the same biological and pharmacological properties as human skin [,]. In this scoping review, we will initially delineate the various BSSs employed as grafting alternatives for DFUs, followed by a thorough summarization of the evidence regarding their applications in human subjects.
3. Materials and Methods
3.1. Literature Search
A comprehensive search strategy was developed to identify relevant literature on tissue engineering strategies for treating diabetic foot ulcers. Searches were conducted in accordance with PRISMA-ScR guidelines in PubMed, Web of Science, and Cochrane Library []. The search strategy included controlled vocabulary, Medical Subject Headings (MeSH), and common keywords for the domains of bioengineering and diabetic ulcers. The search was performed using the following keywords: “diabetic foot ulcer”, “diabetic foot”, “chronic wound”, “bioengineering”, “bioengineered skin”, “bioengineered graft”, “scaffold-based tissue engineering”, “cell-based tissue engineering”, “bioengineered skin grafts”, ”bioengineered tissue”, “skin substitute”, “dermal substitute”, “synthetic graft”, “dermal graft”, “pressure ulcer”, “foot wound”, “venous stasis ulcer”, “venostasis”, and “ischemic ulcer”. The following MeSH terms were used to improve the accuracy of the search: “Diabetic Foot/therapy” [Mesh], “Regenerative Medicine” [Mesh], “Tissue Engineering” [Mesh], “Tissue Scaffolds” [Mesh], “Skin, Artificial” [Mesh], “Skin Transplantation” [Mesh], and “Bioengineering” [Mesh]. The final search was conducted until 25 July 2023, limited to studies published in English and conducted from 1 December 2012 to 1 December 2022. Boolean operators (AND, OR, NOT) were used to combine the keywords and broaden the search. Truncation was also used to capture variations of the keywords.
The search results were imported into Mendeley as reference management software to remove duplicates and screen for relevance. Three independent reviewers (NP, CCB, and PE) screened the titles and abstracts of all potential studies to determine eligibility for inclusion in the scoping review and to minimize bias. Subsequently, full-text articles were retrieved to verify the inclusion decision based on title and abstract screening. Any discrepancies were resolved through discussion or by a fourth reviewer (NS). Full-text articles of potentially eligible studies were obtained and assessed for inclusion using pre-defined inclusion and exclusion criteria.
3.2. Study Selection and Eligibility Criteria
Inclusion criteria were defined as follows: (1) clinical studies involving human subjects, (2) studies involving adult subjects, (3) clinical trials with a sample size of 10 or more patients, (4) studies evaluating bioengineered tissues for the treatment of diabetic foot ulcers, (5) studies reporting outcomes related to wound healing, such as wound closure, time-to-healing, or wound area reduction, (6) studies reporting safety and adverse events related to the use of tissue engineering strategies, (7) studies published in English, and (8) studies conducted between 1 December 2012 and 1 December 2022. Exclusion criteria consisted of (1) animal studies, (2) in vitro studies, (3) studies not related to tissue engineering strategies for treating diabetic foot ulcers, (4) studies that do not report outcomes related to wound healing, (5) studies that do not report safety and adverse events related to the use of tissue engineering strategies, (6) studies not published in English, and (7) studies conducted before 1 December 2012.
- Population: a sample size of 10 or more adult diabetic patients who received bioengineered skin substitutes compared to human skin allografts or standard wound care (SWC) in treating DFUs. No exclusionary or inclusionary criteria were set for patients’ sex or the presence and severity of DFU infection.
- Intervention: Application of bioengineered tissues for treatment of DFUs following wound debridement.
- Comparison: other alternate skin substitutes or SWC methods.
- Outcomes: sum and percentage of wounds achieving closure, time to wound closure (median and mean), incidence of amputation (post-treatment), and adverse events related to therapy.
3.3. Data Extraction and Synthesis
The following data items were extracted from the included studies: year of publication, study design, cohort size, ethnicity, race, age, sex, area of ulcer, number of wounds, duration of ulcer before treatment, incidence of wound closure, percentage of wound closures, median time to wound closure, mean time to wound closure, amputation rate post-treatment, and adverse events related to therapy.
4. Results and Discussion
The initial literature search yielded 1312 articles: 178 from PubMed, 863 from Cochrane Library, and 271 from Web of Science [Figure 3]. After removing duplicates, 1281 abstracts were screened for their eligibility, which resulted in the exclusion of 1249 articles. The remaining 32 were placed through a full-text screening, and 8 more were removed. 24 articles were ultimately included in this review. Seven studies were potentially relevant but excluded due to the small cohort size or comparing outcomes not listed in our inclusion criteria; in [] the authors compared a BSS to a fetal bovine collagen dressing for treating venous leg ulcers [Figure 3]. Other trials had the required number of patients; however, they did not investigate our outcomes of interest [].
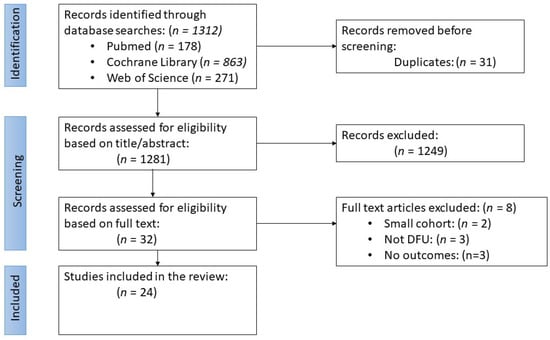
Figure 3.
Flow diagram for the literature search and study selection process according to the PRISMA-ScR guidelines.
4.1. Types of BSS Found in this Scoping Review
The flexibility of manufacturing strategies has led to a surge of options and varieties. BSS types are often categorized by their number of layers and the content therein [Figure 4] provides a breakdown of the different BSS varieties. Some are bi-layered and acellular, such as “Integra Bilayer Wound Matrix Dressing (Integra LifeSciences Corporation, Plainsboro, NJ, USA)”, consisting of a silicone membrane as the epidermal layer and a dermis made from a combination of bovine collagen and shark chondroitin-6-sulfate glycosaminoglycan []. Others, like “Apligraf® (Organogenesis Incorporated, Canton, MA, USA)”, are bi-layered and cellular, comprising a bioengineered dermis derived from neonatal foreskin fibroblasts and bovine type-1 collagen, along with a bioengineered epidermis from neonatal foreskin keratinocytes [,].
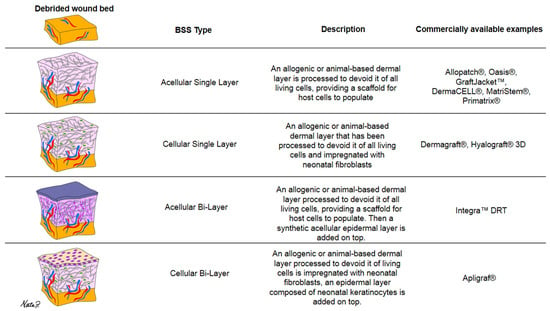
Figure 4.
Categorization of BSS varieties by number of layers and content description.
Additionally, some skin substitutes consist of just a single dermal/epidermal layer, which can be cellular or acellular. “Dermagraft® (Smith and Nephew, Largo, FL, USA)”, for example, is a mono-layered cellular substitute made from a bioengineered extracellular matrix impregnated with cultured neonatal fibroblasts []. “Oasis®® wound matrix (Smith & Nephew, Inc., Fort Worth, TX, USA)”, on the other hand, is an example of an acellular mono-layered dermal substitute []. It is important to note that this list is not exhaustive, as various other engineering strategies exist. Furthermore, it is crucial to understand that these skin grafts are artificially engineered rather than naturally occurring and harvested.
4.2. Subject Characteristics and Investigated Outcomes
All remaining studies investigated a cohort of adult patients with a history of DM and an existing DFU. Types of biomaterials and their descriptions are found in Table 1. The size and duration of pre-treatment ulceration were recorded as characteristics of the study population. Patient demographics are summarized in Table 2 and Table 3. Cohort sizes across all studies ranged from 23 to 24,823, with most patients in their fifth and sixth decades. Race or ethnicity were reported in 12 studies, and of those, seven consisted of treatment groups where over 90% of the participants were Caucasian [Table 3].Patient gender (biological sex) was documented in all studies except three, and in most cases, the majority of participants were male.

Table 1.
Types of BSS reported in the literature.

Table 2.
Clinical Outcomes and Cost for DFUs treatment modalities.

Table 3.
Demographic and Clinical Characteristics of Study Participants with DFUs.
4.3. Effectiveness of BSSs in Promoting Wound Closure and Improving Patient Outcomes
As mentioned above, the evaluated BSS strategies found in this search included Aligraf®®, Dermagraft®®, Oasis®®, Matristem Wound Matrix (Columbia, MD, USA), PriMatrix®® Dermal Repair Scaffold (Integra LifeSciences Corp., Plainsboro, NJ, USA), RaphaE, Integra®® Tebaderm (Teba Zist Polymer Co, Iran), AlloPatch®® (Musculoskeletal Transplant Foundation, Edison, NJ, USA), PRBM (Purified Reconstituted Bilayer Matrix, Geistlich Derma-Gide, Geistlich Pharma AG), GraftJacket™ RTM (Wright Medical Group N.V., Memphis, TN, USA), DermACELL®® Human Acellular Dermal Matrix (LifeNet Health, Virginia Beach, VA, USA), and Hyalograft 3D®® (Fidia Advanced Biopolymers, Abano Terme, Italy). Table 1 provides the details of each BSS description and its suppliers [,,,,,,,,,,,,,,,,,]. The reviewed studies compared BSSs with either another BSS variant, SWC (involving sharp debridement, offloading, infection prevention, and glycemic control), or a human amniotic membrane allograft (hAMA), which was available as a dehydrated human chorion amnion membrane (dHCAM) or a viable cryopreserved placental membrane (vCPM). When BSS was compared to SWC, every recorded outcome reported BSS to be the superior treatment option with superior outcomes [,,]. Comparison with hAMA yielded conflicting results. Five studies presented data favoring hAMA, indicating shorter healing times and a higher frequency of achieving complete wound closure [,,,,] [Table 2]. Conversely, three studies reported opposing results, with BSS associated with improved healing rates and shorter healing times [,,] [Table 2].
When comparing BSS varieties, there were a total of five articles, four of which compared cellular BSS to acellular BSS [,,,]. In those four, cellular BSSs demonstrated faster healing times and increased healing rates; however, they were vastly more expensive in two of those studies [,] [Table 2]. Cazzell et al. 2017 compared GraftJacket™ with DermACELL®®, two acellular dermal substitutes. In this particular trial, there were four groups, two of which received one treatment of either product (each group being assigned a BSS), while the other two groups received multiple applications of either dermal substitute (each group being assigned a BSS) []. Furthermore, adverse events related to treatment therapy were reported by five studies [,,,,]. The majority of adverse events were due to infection, either osteomyelitis or cellulitis. In two studies, adverse events occurred at a higher rate in control groups that only received SWC [,]. Cazzell et al., 2015 reported one “maceration” of the periwound area, possibly due to the BSS treatment []. A summary of recorded outcomes can be found in Table 3 [Table 3].
4.4. Cost Effectiveness
The cost of treatment was not widely recorded among studies. In the studies comparing BSS to hAMA, only half of them assessed the cost of treatment, and all four of these studies reported that BSS resulted in significantly higher treatment costs than hAMA [,,,]. Finally, one article did not match our inclusion criteria; however, it mentioned the cost-effectiveness of these treatments and factors that can influence the extent of financial burden []. According to Snyder 2020, the apparent increase in treatment costs may require further evaluation since some BSSs only come in one available size regardless of the wound area. In contrast, hAMA comes in multiple sizes, which enables a smaller graft to be used as the wound contracts, thus decreasing wastage and increasing cost-effectiveness [].
5. Limitations
One limitation to this scoping review is the potential exclusion of articles relevant to the topic. Studies published in a language other than English were excluded, as there are no formal translation service providers on our research team to screen these articles. In addition, while the search strategy aimed to be comprehensive, there is a possibility that studies were missed due to discrepancies in searching terminology or the databases searched. Given these limitations of the scoping review process, there may be insightful results within other studies that are not included in our synthesis of the literature. In addition, variations in the design and methodology across the included studies may introduce bias in the synthesis of the literature.
6. Conclusions and Perspectives
As a scoping review, the primary objective of this study was to provide an overview of the available literature on BSSs used for DFUs. As such, the focus was on the comprehensiveness of the literature rather than evaluating the quality and validity of these individual studies. We intended to identify the existing medical evidence on treatments being implemented in human subjects, providing a foundation for future research areas for further investigation. Time to wound closure and wound closure rate were the most recorded outcomes. The data suggested that any BSS might result in a superior outcome to SWC. On the other hand, BSSs, compared to hAMAs, provided contradictory results. In certain instances, the utilization of BSSs led to a reduction in wound closure time, while in some cases, BSSs yielded a lower rate of wound healing. As such, it is imperative to account for potential confounding factors in future studies.
It is also crucial to acknowledge that minor variations existed in the methods employed for SWC across different clinical trials, which might introduce a statistical bias. Notably, the choice of debridement techniques and off-loading methods, for instance, could significantly impact ulceration prognosis. Standardization of SWC across comparative studies can eliminate its potential to influence statistical outcomes.
Existing knowledge about BSSs in DFU treatment strongly suggests they have superior wound healing outcomes compared to SWC alone. Nevertheless, determination of the clinical superiority and cost-effectiveness of BSS over alternative treatment options remains inconclusive and continues to be a subject of ongoing research and comparative analysis.
Author Contributions
C.C.B., N.R.P. and P.T.E. drafted the manuscript. C.C.B., N.R.P. and H.L.A. created the figures. P.T.E. and N.R.P. performed the search. C.C.B. and N.S. verified the search. C.C.B., N.S., N.R.P., P.T.E., H.L.A., J.L.F. and K.V.C. critically reviewed and edited the manuscript. C.C.B., N.S. and K.V.C. contributed to the research design. C.C.B. and K.V.C. contributed to the development of the search strategy and definition of PICO criteria. C.C.B. and N.S. contributed to the funding acquisition and conception of the study. All authors have read and agreed to the published version of the manuscript.
Funding
C.B. and N.S. were supported by the SEED Grant, offered by the University of Texas Rio Grande Valley, Office of Faculty Success and Diversity. For the publication fee, we further acknowledge financial support by the School of Podiatric Medicine, UTRGV.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors will provide the raw data supporting the article’s conclusion without any unnecessary hesitation.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Jupiter, D.C.; Thorud, J.C.; Buckley, C.J.; Shibuya, N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: From ulceration to death, a systematic review. Int. Wound J. 2016, 13, 892–903. [Google Scholar] [CrossRef]
- Lavery, L.A.; Armstrong, D.G.; Wunderlich, R.P.; Mohler, M.J.; Wendel, C.S.; Lipsky, B.A. Risk Factors for Foot Infections in Individuals With Diabetes. Diabetes Care 2006, 29, 1288–1293. [Google Scholar] [CrossRef]
- Jeffcoate, W.J.; Harding, K.G. Diabetic foot ulcers. Lancet 2003, 361, 1545–1551. [Google Scholar] [CrossRef]
- Barshes, N.R.; Barshes, N.R.; Sigireddi, M.; Wrobel, J.S.; Mahankali, A.; Robbins, J.M.; Kougias, P.; Armstrong, D.G. The system of care for the diabetic foot: Objectives, outcomes, and opportunities. Diabet. Foot Ankle 2013, 4, 21847. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, L.; Nasiri, M.; Adarvishi, S. Literature review on the management of diabetic foot ulcer. World J. Diabetes 2015, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bardill, J.R.; Laughter, M.R.; Stager, M.; Liechty, K.W.; Krebs, M.D.; Zgheib, C. Topical gel-based biomaterials for the treatment of diabetic foot ulcers. Acta Biomater. 2022, 138, 73. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Bowers, D.T.; Liu, W.; Ma, M. Functional hydrogels for diabetic wound management. APL Bioeng. 2021, 5, 31503. [Google Scholar] [CrossRef]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and Treatment of Impaired Wound Healing in Diabetes Mellitus: New Insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef]
- Louiselle, A.E.; Niemiec, S.M.; Zgheib, C.; Liechty, K.W. Macrophage polarization and diabetic wound healing. Transl. Res. 2021, 236, 109–116. [Google Scholar] [CrossRef]
- Zarei, F.; Negahdari, B.; Eatemadi, A. Diabetic ulcer regeneration: Stem cells, biomaterials, growth factors. Artif. Cells Nanomed. Biotechnol. 2018, 46, 26–32. [Google Scholar] [CrossRef]
- Dovi, J.V.; Szpaderska, A.M.; DiPietro, L.A. Neutrophil function in the healing wound: Adding insult to injury? Thromb. Haemost. 2004, 92, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Everett, E.; Mathioudakis, N. Update on management of diabetic foot ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef]
- Crisologo, P.A.; Lavery, L.A.; La Fontaine, J. Conservative Offloading. Clin. Podiatr. Med. Surg. 2019, 36, 371–379. [Google Scholar] [CrossRef]
- Thomas, D.C.; Tsu, C.L.; Nain, R.A.; Arsat, N.; Fun, S.S.; Lah, N.A.S.N. The role of debridement in wound bed preparation in chronic wound: A narrative review. Ann. Med. Surg. 2021, 71, 102876. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, J.R.; Carter, M.J.; Covington, S. Frequency of Debridements and Time to Heal. JAMA Dermatol. 2013, 149, 1050. [Google Scholar] [CrossRef]
- Debridement Procedures for Managing Diabetic Foot Ulcers: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines. September 2014. Available online: https://www.ncbi.nlm.nih.gov/books/NBK253769/ (accessed on 3 January 2024).
- Dayya, D.; O’Neill, O.J.; Huedo-Medina, T.B.; Habib, N.; Moore, J.; Iyer, K. Debridement of Diabetic Foot Ulcers. Adv. Wound Care 2022, 11, 666. [Google Scholar] [CrossRef]
- Atkin, L. Understanding methods of wound debridement. Br J Nurs. 2014, 23, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Madhok, B.M.; Vowden, K.; Vowden, P. New techniques for wound debridement. Int. Wound J. 2013, 10, 247–251. [Google Scholar] [CrossRef]
- Rayman, G.; Vas, P.; Dhatariya, K.; Driver, V.; Hartemann, A.; Londahl, M.; Piaggesi, A.; Driver, V.; Apelqvist, J.; Attinger, C.; et al. Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. S1), e3283. [Google Scholar] [CrossRef]
- Anghel, E.L.; DeFazio, M.V.; Barker, J.C.; Janis, J.E.; Attinger, C.E. Current Concepts in Debridement. Plast. Reconstr. Surg. 2016, 138, 82S–93S. [Google Scholar] [CrossRef]
- Tombulturk, F.K.; Kanigur-Sultuybek, G. A molecular approach to maggot debridement therapy with Lucilia sericata and its excretions/secretions in wound healing. Wound Repair Regen. 2021, 29, 1051–1061. [Google Scholar] [CrossRef]
- Smith, F.; Dryburgh, N.; Donaldson, J.; Mitchell, M. Debridement for surgical wounds. Cochrane Database Syst. Rev. 2013, 2013, CD006214. [Google Scholar] [CrossRef]
- Santema, T.B.; Poyck, P.P.C.; Ubbink, D.T. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst. Rev. 2016, 2016, CD011255. [Google Scholar] [CrossRef]
- Kohlhauser, M.; Luze, H.; Nischwitz, S.P.; Kamolz, L.P. Historical Evolution of Skin Grafting—A Journey through Time. Medicina 2021, 57, 348. [Google Scholar] [CrossRef]
- Ramanujam, C.L.; Zgonis, T. An Overview of Autologous Skin Grafts and Advanced Biologics for the Diabetic Foot. Clin. Podiatr. Med. Surg. 2012, 29, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Rizzuto, A.; Rossi, A.; Perri, P.; Barbetta, A.; Abdalla, K.; Caroleo, S.; Longo, C.; Amantea, B.; Sammarco, G.; et al. Skin grafting for the treatment of chronic leg ulcers—A systematic review in evidence-based medicine. Int. Wound J. 2017, 14, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Goverman, J.; Kraft, C.T.; Fagan, S.; Levi, B. Back Grafting the Split-Thickness Skin Graft Donor Site. J. Burn. Care Res. 2017, 38, e443–e449. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mohapatra, D.P.; Chittoria, R.K.; Subbarayan, E.; Reddy, S.K.; Chavan, V.; Aggarwal, A.; Reddy, L.C. Human skin allograft: Is it a viable option in management of burn patients? J. Cutan. Aesthetic Surg. 2019, 12, 132. [Google Scholar] [CrossRef]
- Kearney, J.N. Guidelines on processing and clinical use of skin allografts. Clin. Dermatol. 2005, 23, 357–364. [Google Scholar] [CrossRef]
- Roy, A.; Griffiths, S. Intermediate layer contribution in placental membrane allografts. J. Tissue Eng. Regen. Med. 2020, 14, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Baganizi, D.R.; Sahu, R.; Dosunmu, E.; Chaudhari, A.; Vig, K.; Pillai, S.R.; Singh, S.R.; Dennis, V.A. Immunological challenges associated with artificial skin grafts: Available solutions and stem cells in future design of synthetic skin. J. Biol. Eng. 2017, 11, 49. [Google Scholar] [CrossRef]
- Yamamoto, T.; Iwase, H.; King, T.W.; Hara, H.; Cooper, D.K.C. Skin xenotransplantation: Historical review and clinical potential. Burns 2018, 44, 1738–1749. [Google Scholar] [CrossRef]
- Cooper, D.K.; Ezzelarab, M.B.; Hara, H.; Iwase, H.; Lee, W.; Wijkstrom, M.; Bottino, R. The pathobiology of pig-to-primate xenotransplantation: A historical review. Xenotransplantation 2016, 23, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Rijal, N.P.; Narmoneva, D.A. Biomaterials for diabetic wound-healing therapies. In Wound Healing, Tissue Repair, and Regeneration in Diabetes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 273–304. [Google Scholar] [CrossRef]
- Oualla-Bachiri, W.; Fernández-González, A.; Quiñones-Vico, M.I.; Arias-Santiago, S. From Grafts to Human Bioengineered Vascularized Skin Substitutes. Int. J. Mol. Sci. 2020, 21, 8197. [Google Scholar] [CrossRef] [PubMed]
- Alrubaiy, L. Skin Substitutes: A Brief Review of Types and Clinical Applications. Oman Med. J. 2009, 24, 4–6. [Google Scholar] [CrossRef]
- Tchanque-Fossuo, C.N.; Dahle, S.E.; Lev-Tov, H.; West, K.I.M.; Li, C.S.; Rocke, D.M.; Isseroff, R.R. Cellular versus acellular matrix devices in the treatment of diabetic foot ulcers: Interim results of a comparative efficacy randomized controlled trial. J. Tissue Eng. Regen. Med. 2019, 13, 1430–1437. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Sabolinski, M.L.; Gibbons, G. Comparative effectiveness of a bilayered living cellular construct and an acellular fetal bovine collagen dressing in the treatment of venous leg ulcers. J. Comp. Eff. Res. 2018, 7, 797–805. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Sisakht, M.M.; Amirkhani, M.A.; Seifalian, A.M.; Banafshe, H.R.; Verdi, J.; Nouradini, M. Engineered skin graft with stromal vascular fraction cells encapsulated in fibrin–collagen hydrogel: A clinical study for diabetic wound healing. J. Tissue Eng. Regen. Med. 2020, 14, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Orgill, D.P.; Galiano, R.D.; Glat, P.M.; Kaufman, J.P.; Carter, M.J.; DiDomenico, L.A.; Zelen, C.M. Use of a purified reconstituted bilayer matrix in the management of chronic diabetic foot ulcers improves patient outcomes vs standard of care: Results of a prospective randomised controlled multi-centre clinical trial. Int. Wound J. 2022, 19, 1197–1209. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Tettelbach, W.H.; Chang, T.J.; De Jong, J.L.; Glat, P.M.; Hsu, J.H.; Kelso, M.R.; Niezgoda, J.A.; Tucker, T.L.; Labovitz, J.M. Observed impact of skin substitutes in lower extremity diabetic ulcers: Lessons from the Medicare Database (2015–2018). J. Wound Care 2021, 30 (Suppl. S7), S5–S16. [Google Scholar] [CrossRef]
- Campitiello, F.; Mancone, M.; Della Corte, A.; Guerniero, R.; Canonico, S. To evaluate the efficacy of an acellular Flowable matrix in comparison with a wet dressing for the treatment of patients with diabetic foot ulcers: A randomized clinical trial. Updates Surg. 2017, 69, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Djavid, G.E.; Tabaie, S.M.; Tajali, S.B.; Totounchi, M.; Farhoud, A.; Fateh, M.; Taghizadeh, S. Application of a collagen matrix dressing on a neuropathic diabetic foot ulcer: A randomised control trial. J. Wound Care 2020, 29 (Suppl. S3), S13–S18. [Google Scholar] [CrossRef] [PubMed]
- Cazzell, S.M.; Lange, D.L.; Dickerson, J.E.; Slade, H.B. The Management of Diabetic Foot Ulcers with Porcine Small Intestine Submucosa Tri-Layer Matrix: A Randomized Controlled Trial. Adv. Wound Care 2015, 4, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Driver, V.R.; Lavery, L.A.; Reyzelman, A.M.; Dutra, T.G.; Dove, C.R.; Kotsis, S.V.; Chung, K.C. A clinical trial of Integra Template for diabetic foot ulcer treatment. Wound Repair Regen. 2015, 23, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Cazzell, S.M.; Arroyo-Rivera, J.; Tallis, A.; Reyzelman, A.M.; Saba, F.; Gilbert, T.W. Evaluation of tissue engineering products for the management of neuropathic diabetic foot ulcers: An interim analysis. J. Wound Care 2016, 25 (Suppl. S7), S18–S25. [Google Scholar] [CrossRef]
- Rosa, S.S.R.F.; Rosa, M.F.F.; Marques, M.P.; Guimarães, G.A.; Motta, B.C.; Macedo, Y.C.L.; Inazawa, P.; Dominguez, A.; Macedo, F.S.; Lopes, C.A.P.; et al. Regeneration of Diabetic Foot Ulcers Based on Therapy with Red LED Light and a Natural Latex Biomembrane. Ann. Biomed. Eng. 2019, 47, 1153–1164. [Google Scholar] [CrossRef]
- You, H.J.; Han, S.K.; Rhie, J.W. Randomised controlled clinical trial for autologous fibroblast-hyaluronic acid complex in treating diabetic foot ulcers. J. Wound Care 2014, 23, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Zelen, C.M.; Serena, T.E.; Snyder, R.J. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int. Wound J. 2014, 11, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Zelen, C.M.; Gould, L.; Serena, T.E.; Carter, M.J.; Keller, J.; Li, W.W. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int. Wound J. 2015, 12, 724–732. [Google Scholar] [CrossRef]
- Zelen, C.M.; Serena, T.E.; Gould, L.; Le, L.; Carter, M.J.; Keller, J.; Li, W.W. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: A prospective, randomised, controlled, multi-centre comparative study examining clinical efficacy and cost. Int. Wound J. 2016, 13, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Zelen, C.M.; Orgill, D.P.; Serena, T.; Galiano, R.; Carter, M.J.; DiDomenico, L.A.; Keller, J.; Kaufman, J.; Li, W.W. A prospective, randomised, controlled, multicentre clinical trial examining healing rates, safety and cost to closure of an acellular reticular allogenic human dermis versus standard of care in the treatment of chronic diabetic foot ulcers. Int. Wound J. 2017, 14, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Cazzell, S.; Vayser, D.; Pham, H.; Walters, J.; Reyzelman, A.; Samsell, B.; Moore, M. A randomized clinical trial of a human acellular dermal matrix demonstrated superior healing rates for chronic diabetic foot ulcers over conventional care and an active acellular dermal matrix comparator. Wound Repair Regen. 2017, 25, 483–497. [Google Scholar] [CrossRef]
- Glat, P.; Orgill, D.P.; Galiano, R.; Armstrong, D.; Serena, T.; DiDomenico, L.A.; Kaufman, J.; Carter, M.J.; Jacobs, A.M.; Zelen, C.M. Placental Membrane Provides Improved Healing Efficacy and Lower Cost Versus a Tissue-Engineered Human Skin in the Treatment of Diabetic Foot Ulcerations. Plast. Reconstr. Surg. Glob. Open 2019, 7, 2371. [Google Scholar] [CrossRef]
- Sanders, L.; Landsman, A.S.; Landsman, A.; Keller, N.; Cook, J.; Cook, E.; Hopson, M. Prospective, multicenter, randomized, controlled clinical trial comparing a bioengineered skin substitute to a human skin allograft. Ostomy/Wound Manag. 2014, 60, 26–38. [Google Scholar]
- Kirsner, R.S.; Sabolinski, M.L.; Parsons, N.B.; Skornicki, M.; Marston, W.A. Comparative effectiveness of a bioengineered living cellular construct vs. a dehydrated human amniotic membrane allograft for the treatment of diabetic foot ulcers in a real-world setting. Wound Repair Regen. 2015, 23, 737–744. [Google Scholar] [CrossRef]
- Kraus, I.; Sabolinski, M.L.; Skornicki, M.; Parsons, N.B. The Comparative Effectiveness of a Human Fibroblast Dermal Substitute versus a Dehydrated Human Amnion/Chorion Membrane Allograft for the Treatment of Diabetic Foot Ulcers in a Real-world Setting. Wounds A Compend. Clin. Res. Pract. 2017, 29, 125–132. [Google Scholar]
- Fitzgerald, R.H.; Sabolinski, M.L.; Skornicki, M. Evaluation of Wound Closure Rates Using a Human Fibroblast-derived Dermal Substitute Versus a Fetal Bovine Collagen Dressing: A Retrospective Study. Wound Manag. Prev. 2019, 65, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, A.M.; Waycaster, C.R.; Landsman, A.L. Wound closure in patients with DFU: A cost-effectiveness analysis of two cellular/tissue-derived products. J. Wound Care 2015, 24, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Ananian, C.E.; Dhillon, Y.S.; Van Gils, C.C.; Lindsey, D.C.; Otto, R.J.; Dove, C.R.; Pierce, J.T.; Saunders, M.C. A multicenter, randomized, single-blind trial comparing the efficacy of viable cryopreserved placental membrane to human fibroblast-derived dermal substitute for the treatment of chronic diabetic foot ulcers. Wound Repair Regen. 2018, 26, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R. A Comparative Analysis of the Cost Effectiveness of Five Advanced Skin Substitutes in the Treatment of Foot Ulcers in Patients with Diabetes. Ann. Rev. Res. 2020, 6, 1–14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).