Seasonal and Treatment-Related Variation in 25-Hydroxy Vitamin D Concentration in Patients with Rheumatoid Arthritis
Abstract
1. Introduction
2. Materials and Methods
- Active RA diagnosis based on the 2010 American College of Rheumatology (ACR)—European League Against Rheumatism (EULAR) Classification Criteria for Rheumatoid Arthritis [17]: DAS28-ESR (Disease Activity Score-28 for Rheumatoid Arthritis with ESR) ≥ 2.6; DAS28-ESR was calculated on admission by the hospital’s personnel to check if the patient was in remission (score < 2.6 points) or had active disease. However, patients’ files collected for this study did not contain the score itself—only the descriptive information about the disease status (remission or active RA), which we used to include patients with active RA;
- RA treatment with MTX or LEF for at least one year;
- 25-OH-D concentration assessed;
- Supplementation with 25-OH-D (2000 IU daily) for at least one year.
- RA remission;
- 25-OH-D concentration not assessed;
- 25-OH-D not supplemented;
- RA treatment duration < 1 year or undetermined;
- Treatment that did not include MTX or LEF;
- Concurrent treatment with LEF and MTX;
- Gastrointestinal disorders that may affect 25-OH-D absorption;
- Ongoing oncological disease;
- Age < 18 years;
- Pregnancy.
- RA treatment: LEF vs. MTX.
- Assessment of seasonal effects:
- The comparisons between the astronomical seasons (spring, summer, autumn, and winter);
- The comparison between astronomical summer and the cumulative data for the remaining seasons (spring, autumn, and winter).
3. Results
3.1. Characteristics of the Studied Population
3.2. Analysis of Differences between Treatment Groups
3.3. Analysis of Seasonal Differences
3.4. Analysis of Seasonal Effects in Summer vs. the Rest of the Year
3.5. Analysis of 25-OH-D Deficiency in the Studied Population
- Deficient (<20 ng/mL);
- Suboptimal (20–30 ng/mL);
- Correct (30–50 ng/mL);
- Increased (>50 ng/mL).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, S.W.; Lee, H.C. Vitamin D and health—The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Casseb, G.A.S.; Kaster, M.P.; Rodrigues, A.L.S. Potential Role of Vitamin D for the Management of Depression and Anxiety. CNS Drugs 2019, 33, 619–637. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Falcini, F.; Stagi, S.; Fabbri, S.; Ciuffi, S.; Rigante, D.; Cerinic, M.M.; Brandi, M.L. Study of vitamin D status and vitamin D receptor polymorphisms in a cohort of Italian patients with juvenile idiopathic arthritis. Sci. Rep. 2020, 10, 17550. [Google Scholar] [CrossRef] [PubMed]
- Vitamin D. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/#disc (accessed on 26 June 2023).
- Yesil, H.; Sungur, U.; Akdeniz, S.; Gurer, G.; Yalcın, B.; Dundar, U. Association between serum vitamin D levels and neuropathic pain in rheumatoid arthritis patients: A cross-sectional study. Int. J. Rheum. Dis. 2018, 21, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J.; Razzaque, M.S.; Al-Daghri, N.M. Calcium and vitamin D in human health: Hype or real? J. Steroid Biochem. Mol. Biol. 2018, 180, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Płudowski, P.; Ducki, C.; Konstantynowicz, J.; Jaworski, M. Vitamin D status in Poland. Pol. Arch. Med. Wewn. 2016, 126, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Zgliczyński, W.S.; Rostkowska, O.M.; Sarecka-Hujar, B. Vitamin D Knowledge, Attitudes and Practices of Polish Medical Doctors. Nutrients 2021, 13, 2443. [Google Scholar] [CrossRef] [PubMed]
- Hamulka, J.; Jeruszka-Bielak, M.; Górnicka, M.; Drywień, M.E.; Zielinska-Pukos, M.A. Dietary Supplements during COVID-19 Outbreak. Results of Google Trends Analysis Supported by PLifeCOVID-19 Online Studies. Nutrients 2020, 13, 54. [Google Scholar] [CrossRef]
- Harrison, S.R.; Li, D.; Jeffery, L.E.; Raza, K.; Hewison, M. Vitamin D, Autoimmune Disease and Rheumatoid Arthritis. Calcif. Tissue Int. 2020, 106, 58–75. [Google Scholar] [CrossRef]
- Harrison, S.R.; Jutley, G.; Li, D.; Sahbudin, I.; Filer, A.; Hewison, M.; Raza, K. Vitamin D and early rheumatoid arthritis. BMC Rheumatol. 2020, 4, 38. [Google Scholar] [CrossRef]
- Lin, J.; Liu, J.; Davies, M.L.; Chen, W. Serum Vitamin D Level and Rheumatoid Arthritis Disease Activity: Review and Metaanalysis. PLoS ONE 2016, 11, e0146351. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Soldano, S.; Sulli, A.; Smith, V.; Gotelli, E. Influence of Seasonal Vitamin D Changes on Clinical Manifestations of Rheumatoid Arthritis and Systemic Sclerosis. Front. Immunol. 2021, 12, 683665. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.W.; Lee, A.M.C.; Xu, X.; Hua, B.; Tapp, H.; Wen, X.S.; Xian, C.J. Methotrexate Chemotherapy Causes Growth Impairments, Vitamin D Deficiency, Bone Loss, and Altered Intestinal Metabolism-Effects of Calcitriol Supplementation. Cancers 2023, 15, 4367. [Google Scholar] [CrossRef] [PubMed]
- Oosterom, N.; Dirks, N.F.; Heil, S.G.; de Jonge, R.; Tissing, W.J.E.; Pieters, R.; van den Heuvel-Eibrink, M.M.; Heijboer, A.C.; Pluijm, S.M.F. A decrease in vitamin D levels is associated with methotrexate-induced oral mucositis in children with acute lymphoblastic leukemia. Support Care Cancer 2019, 27, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Stawicki, M.K.; Abramowicz, P.; Góralczyk, A.; Młyńczyk, J.; Kondratiuk, A.; Konstantynowicz, J. Prevalence of Vitamin D Deficiency in Patients Treated for Juvenile Idiopathic Arthritis and Potential Role of Methotrexate: A Preliminary Study. Nutrients 2022, 14, 1645. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Badania Naukowe Niesponsorowane Komisja Bioetyczna przy Uniwersytecie Medycznym im. K. Marcinkowskiego w Pozaniu. Available online: https://bioetyka.ump.edu.pl/EKSPERYMENTY_MEDYCZNE_BADANIA_NAUKOWE_NIESPONSOROWANE.html (accessed on 25 January 2024).
- Verma, S.; Chaturvedi, V.; Ganguly, N.K.; Mittal, S.A. Vitamin D deficiency: Concern for rheumatoid arthritis and COVID-19? Mol. Cell Biochem. 2021, 476, 4351–4362. [Google Scholar] [CrossRef] [PubMed]
- Merlino, L.A.; Curtis, J.; Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Saag, K.G.; Iowa Women’s Health Study. Vitamin D intake is inversely associated with rheumatoid arthritis: Results from the Iowa Women’s Health Study. Arthritis Rheum. 2004, 50, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou-Athanassiou, I.; Athanassiou, P.; Lyraki, A.; Raftakis, I.; Antoniadis, C. Vitamin D and rheumatoid arthritis. Ther. Adv. Endocrinol. Metab. 2012, 3, 181–187. [Google Scholar] [CrossRef]
- Song, G.G.; Bae, S.C.; Lee, Y.H. Association between vitamin D intake and the risk of rheumatoid arthritis: A metaanalysis. Clin. Rheumatol. 2012, 31, 1733–1739. [Google Scholar] [CrossRef]
- Sharma, R.; Saigal, R.; Goyal, L.; Mital, P.; Yadav, R.N.; Meena, P.D.; Agrawal, A. Estimation of vitamin D levels in rheumatoid arthritis patients and its correlation with the disease activity. J. Assoc. Physicians India 2014, 62, 678–681. [Google Scholar] [PubMed]
- Matsumoto, Y.; Sugioka, Y.; Tada, M.; Okano, T.; Mamoto, K.; Inui, K.; Habu, D.; Koike, T. Relationships between serum 25-hydroxycalciferol, vitamin D intake and disease activity in patients with rheumatoid arthritis—TOMORROW study. Mod. Rheumatol. 2015, 25, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Azzeh, F.S.; Kensara, O.A. Vitamin D Is a Good Marker for Disease Activity of Rheumatoid Arthritis Disease. Dis. Markers 2015, 2015, 260725. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Bae, S.C. Vitamin D level in rheumatoid arthritis and its correlation with the disease activity: A metaanalysis. Clin. Exp. Rheumatol. 2016, 34, 827–833. [Google Scholar] [PubMed]
- Vojinovic, J.; Tincani, A.; Sulli, A.; Soldano, S.; Andreoli, L.; Dall’Ara, F.; Ionescu, R.; Pasalic, K.S.; Balcune, I.; Ferraz-Amaro, I.; et al. European multicentre pilot survey to assess vitamin D status in rheumatoid arthritis patients and early development of a new Patient Reported Outcome questionnaire (D-PRO). Autoimmun. Rev. 2017, 16, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Hajjaj-Hassouni, N.; Mawani, N.; Allali, F.; Rkain, H.; Hassouni, K.; Hmamouchi, I.; Dougados, M. Evaluation of Vitamin D Status in Rheumatoid Arthritis and Its Association with Disease Activity across 15 Countries: “The COMORA Study”. Int. J. Rheumatol. 2017, 2017, 5491676. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Moin, S.; Shahzad, S.; Khan, A.Q. Level of inflammatory cytokines in rheumatoid arthritis patients: Correlation with 25-hydroxy vitamin D and reactive oxygen species. PLoS ONE 2017, 12, e0178879. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekara, S.; Patted, A. Role of vitamin D supplementation in improving disease activity in rheumatoid arthritis: An exploratory study. Int. J. Rheum. Dis. 2017, 20, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Lahiry, S.; Thakur, S.; Chakraborty, D.S. Effect of 1,25 dihydroxy vitamin D3 supplementation on pain relief in early rheumatoid arthritis. J. Family Med. Prim. Care 2019, 8, 517–522. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, H. Impact of vitamin D deficiency on clinical parameters in treatment-naïve rheumatoid arthritis patients. Z. Rheumatol. 2018, 77, 833–840. [Google Scholar] [CrossRef]
- Adami, G.; Rossini, M.; Bogliolo, L.; Cantatore, F.P.; Varenna, M.; Malavolta, N.; Del Puente, A.; Muratore, M.; Orsolini, G.; Gatti, D.; et al. An exploratory study on the role of vitamin D supplementation in improving pain and disease activity in rheumatoid arthritis. Mod. Rheumatol. 2019, 29, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Hao, Y.; Guan, Y.; Bu, H.; Wang, H. The Effect of Vitamin D Supplementation on Rheumatoid Arthritis Patients: A Systematic Review and Metaanalysis. Front. Med. 2020, 7, 596007. [Google Scholar] [CrossRef] [PubMed]
- Mouterde, G.; Gamon, E.; Rincheval, N.; Lukas, C.; Seror, R.; Berenbaum, F.; Dupuy, A.M.; Daien, C.; Daurès, J.P.; Combe, B. Association Between Vitamin D Deficiency and Disease Activity, Disability, and Radiographic Progression in Early Rheumatoid Arthritis: The ESPOIR Cohort. J. Rheumatol. 2020, 47, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, E.; Oleröd, G.; Konar, J.; Petzold, M.; Hammarsten, O. Seasonal variations in serum 25-hydroxy vitamin D levels in a Swedish cohort. Endocrine 2015, 49, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B.; Haji Mirghassemi, M.B. Seasonal variations in serum vitamin D according to age and sex. Caspian J. Intern. Med. 2012, 3, 535–540. [Google Scholar] [PubMed]
- Herly, M.; Stengaard-Pedersen, K.; Vestergaard, P.; Christensen, R.; Möller, S.; Østergaard, M.; Junker, P.; Hetland, M.L.; Hørslev-Petersen, K.; Ellingsen, T. Impact of season on the association between vitamin D levels at diagnosis and one-year remission in early Rheumatoid Arthritis. Sci. Rep. 2020, 10, 7371. [Google Scholar] [CrossRef] [PubMed]
- Yazmalar, L.; Ediz, L.; Alpayci, M.; Hiz, O.; Toprak, M.; Tekeoglu, I. Seasonal disease activity and serum vitamin D levels in rheumatoid arthritis, ankylosing spondylitis and osteoarthritis. Afr. Health Sci. 2013, 13, 47–55. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smyczyńska, J.; Smyczyńska, U.; Stawerska, R.; Domagalska-Nalewajek, H.; Lewiński, A.; Hilczer, M. Seasonality of vitamin D concentrations and the incidence of vitamin D deficiency in children and adolescents from central Poland. Pediatr. Endocrinol. Diabetes Metab. 2019, 25, 54–59. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Hua, Z.; Luo, X.; Li, Y.; Yu, L.; Li, M.; Lu, C.; Zhao, T.; Liu, Y. Application and pharmacological mechanism of methotrexate in rheumatoid arthritis. Biomed. Pharmacother. 2022, 150, 113074. [Google Scholar] [CrossRef]
- Padda, I.S.; Goyal, A. Leflunomide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Bullock, J.; Rizvi, S.A.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 2018, 27, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Wu, C.; Dai, M.; Wang, S.; Xu, J.; Dai, L.; Li, Z.; He, L.; Zhu, X.; Sun, L.; et al. Leflunomide versus azathioprine for maintenance therapy of lupus nephritis: A prospective, multicentre, randomised trial and long-term follow-up. Ann. Rheum. Dis. 2022, 81, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Drug Safety Update Volume 17, Issue 1: August 2023: 4. Available online: https://assets.publishing.service.gov.uk/media/64eefdc36bc96d00104ed28b/August-2023-DSU.pdf (accessed on 25 January 2024).
- Chalcraft, J.R.; Cardinal, L.M.; Wechsler, P.J.; Hollis, B.W.; Gerow, K.G.; Alexander, B.M.; Keith, J.F.; Larson-Meyer, D.E. Vitamin D Synthesis Following a Single Bout of Sun Exposure in Older and Younger Men and Women. Nutrients 2020, 12, 2237. [Google Scholar] [CrossRef] [PubMed]
- Kmieć, P.; Żmijewski, M.; Lizakowska-Kmieć, M.; Sworczak, K. Widespread vitamin D deficiency among adults from northern Poland (54°N) after months of low and high natural UVB radiation. Endokrynol. Pol. 2015, 66, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Waszak, P.M.; Mędza, A.; Springer, J.; Zgłobicka, M.; Ogrodnik, P.; Kalinowska, P.; Kmieć, P.; Lizakowska-Kmieć, M.; Sworczak, K.; Żmijewski, M. Knowledge of Vitamin D and its Supplementation Among Students of Northern Poland. Eur. J. Transl. Clin. Med. 2018, 1, 46–54. [Google Scholar] [CrossRef]
- Kamiński, M.; Pankowska, M.; Wolska, M.; Uruska, A.; Zozulińska-Ziółkiewicz, D. Vitamin D supplementation among Polish medical university students: A cross-sectional study. Med. Res. J. 2020, 5, 9–14. [Google Scholar] [CrossRef]
- Salesi, M.; Farajzadegan, Z. Efficacy of vitamin D in patients with active rheumatoid arthritis receiving methotrexate therapy. Rheumatol. Int. 2012, 32, 2129–2133. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Hosoi, T.; Tanaka, E.; Nakajima, A.; Taniguchi, A.; Momohara, S.; Yamanaka, H. Prevalence of and factors associated with vitamin D deficiency in 4,793 Japanese patients with rheumatoid arthritis. Clin. Rheumatol. 2013, 32, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Singh, P.; Agrawal, P.; Saini, H. Efficacy of Vitamin D Supplementation among Newly Diagnosed Cases of Rheumatoid Arthritis Proposed to be Managed by Methotrexate Monotherapy: A Randomised Controlled Study. J. Clin. Diagn. Res. 2022, 16, RC06–RC09. [Google Scholar] [CrossRef]
- Lehmann, B. HaCaT cell line as a model system for vitamin D3 metabolism in human skin. J. Investig. Dermatol. 1997, 108, 78–82. [Google Scholar] [CrossRef]
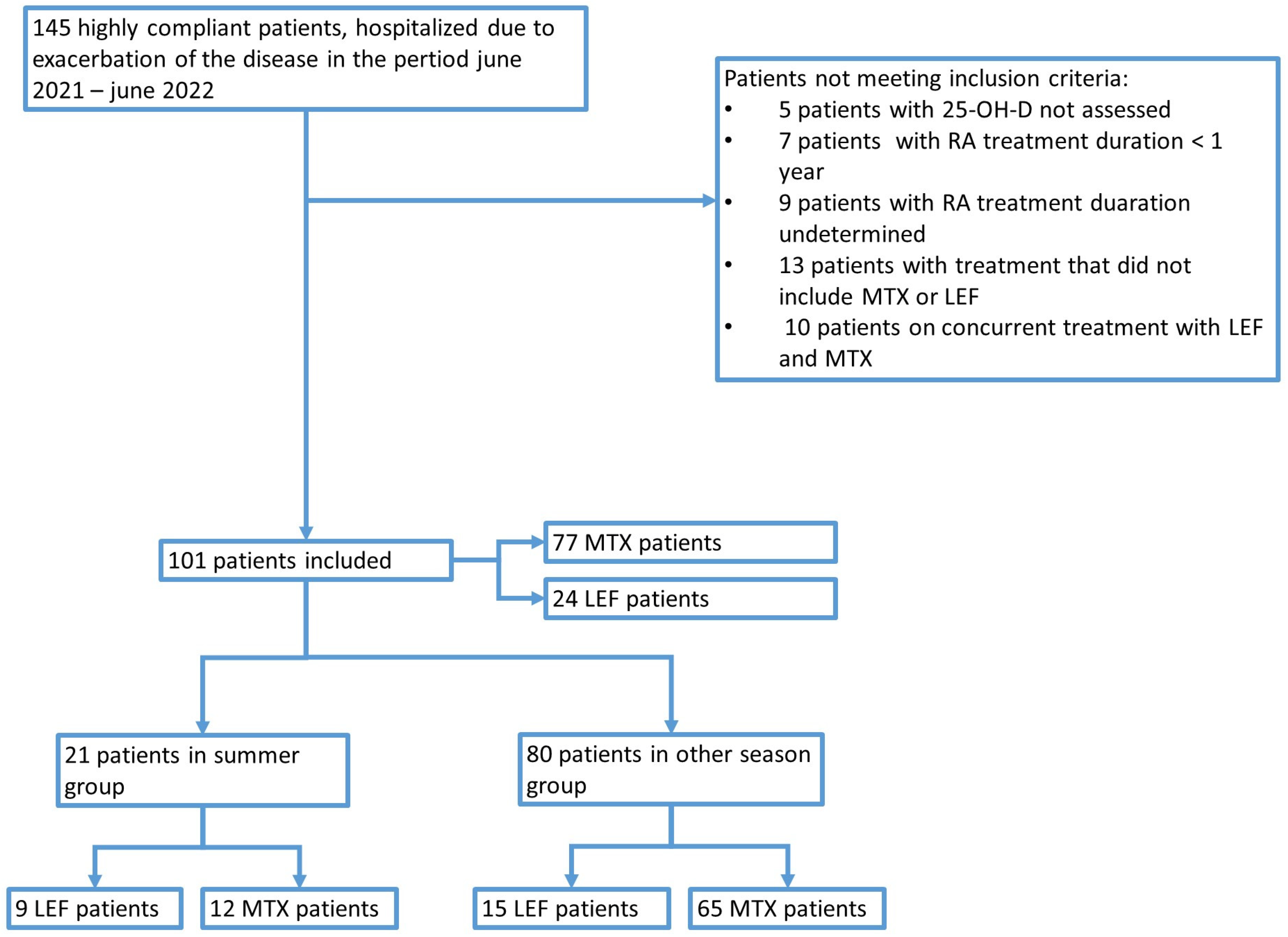



| Parameter [Unit] [Reference Range] | Valid N | Mean ± SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|
| Age [years] | 101 | 60 ± 11 | 62 | 28 | 82 |
| Duration of RA [years] | 101 | 12 ± 10 | 8 | 1 | 41 |
| Body mass [kg] | 101 | 77.47 ± 18.06 | 73.80 | 49.00 | 133.80 |
| BMI [kg/m2] [18.5–24.9] | 101 | 29.55 ± 7.13 | 28.00 | 18.70 | 53.40 |
| ESR [mm/one hour] [3–15] | 101 | 21.80 ± 17.24 | 17.00 | 2.00 | 94.00 |
| CRP [mg/L] [0.0–5.0] | 101 | 10.17 ± 15.43 | 4.00 | 0.00 | 87.50 |
| 25-OH-D [ng/mL] [30–100] | 101 | 31.98 ± 15.37 | 31.40 | 8.20 | 81.00 |
| TSH [uIU/mL] [0.35–4.94] | 99 | 1.79 ± 2.23 | 1.27 | 0.13 | 17.24 |
| Whole Population (n = 101) | MTX (n = 77) | LEF (n = 24) | |
|---|---|---|---|
| Paracetamol | 23 | 19 | 4 |
| NSAIDS 2 | 60 | 44 | 16 |
| Opioids 3 | 28 | 22 | 6 |
| Steroids 4 | 37 | 28 | 9 |
| Biological 5 | 9 | 8 | 1 |
| Janus kinase (JAK) inhibitors 6 | 3 | 3 | 0 |
| Variable | Valid N in LEF Group | Valid N in MTX Group | Mean (SD) Value in LEF Group | Mean (SD) Value in MTX Group | Median in LEF Group | Median in MTX Group | p |
|---|---|---|---|---|---|---|---|
| Whole population | |||||||
| Age [year] | 24 | 77 | 60 (13) | 60 (11) | 62 | 62 | 0.85 |
| Duration of RA 3 [years] | 24 | 77 | 11 (10) | 13 (10) | 6 | 9 | 0.43 |
| Body mass [kg] | 24 | 77 | 73.38 (19.03) | 78.74 (19.03) | 69.30 | 74.60 | 0.35 |
| BMI 4 [kg/m2] | 24 | 77 | 28.91 (7.43) | 29.75 (7.43) | 28.85 | 27.90 | 0.80 |
| ESR 5 [mm/one hour] | 24 | 77 | 24.54 (15.65) | 20.95 (15.65) | 17.50 | 17.00 | 0.63 |
| CRP 6 [mg/L] | 24 | 77 | 12.01 (13.46) | 9.60 (13.46) | 1.65 | 4.80 | 0.17 |
| 25-OH-D 7 [ng/mL] | 24 | 77 | 39.41 (12.38) | 29.66 (12.38) | 38.30 | 29.00 | 0.07 |
| TSH 8 [uIU/mL] | 23 | 76 | 1.88 (2.15) | 1.76 (2.15) | 1.27 | 1.24 | 0.76 |
| Summer population | |||||||
| Age [years] | 9 | 12 | 65 (7) | 63 (9) | 66 | 64 | 0.59 |
| Duration of RA [years] | 9 | 12 | 16 (12) | 16 (9) | 11 | 18 | 0.97 |
| Body mass [kg] | 9 | 12 | 74.69 (11.58) | 83.38 (23.96) | 74.00 | 77.75 | 0.78 |
| BMI [kg/m2] | 9 | 12 | 30.87 (5.64) | 33.21 (10.16) | 29.30 | 29.80 | 0.75 |
| ESR [mm/one hour] | 9 | 12 | 27.00 (28.08) | 23.75 (13.41) | 17.00 | 21.50 | 0.72 |
| CRP [mg/L] | 9 | 12 | 15.76 (26.02) | 12.89 (15.16) | 1.70 | 5.95 | 0.46 |
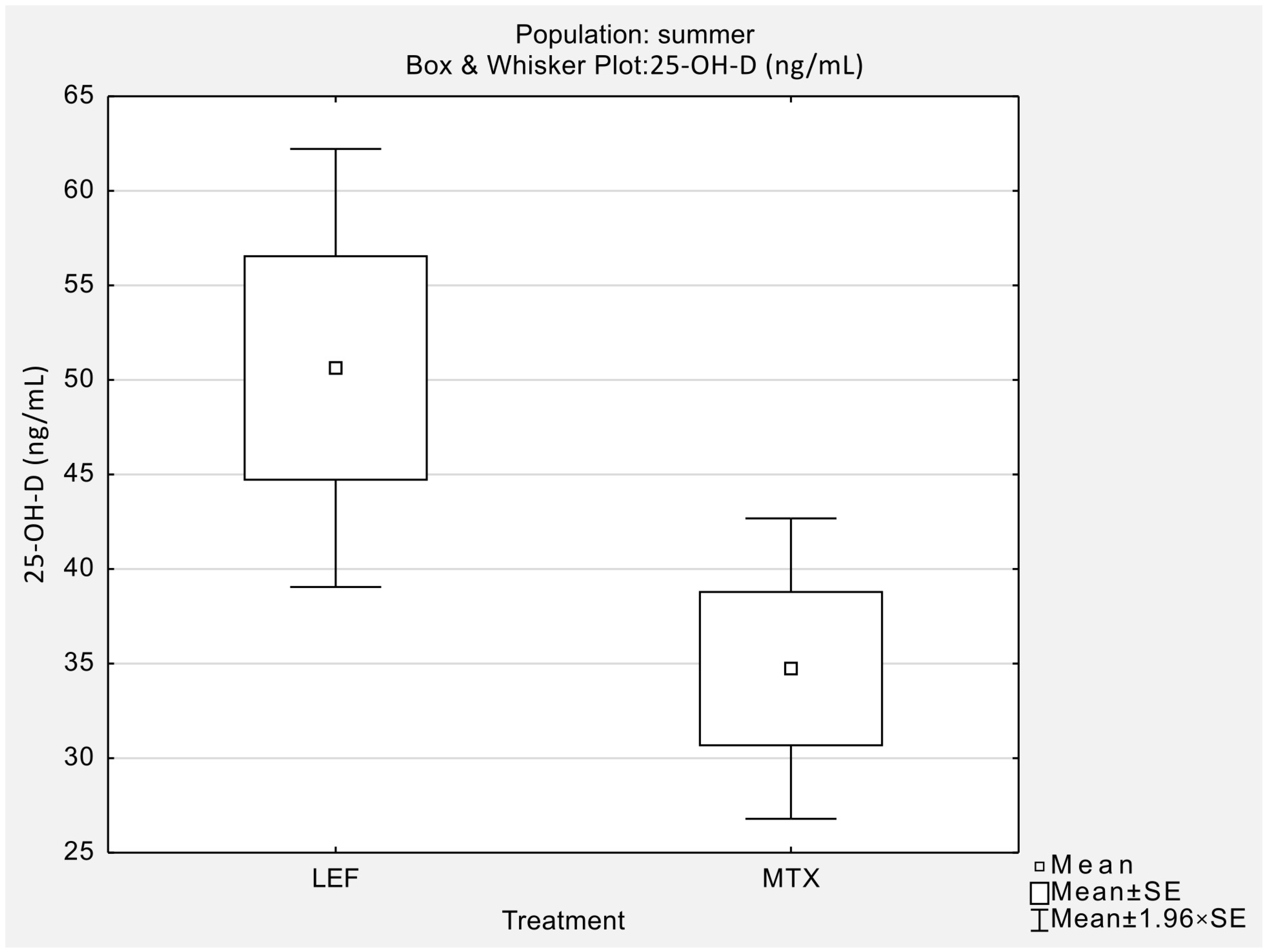
| 25-OH-D [ng/mL] | 9 | 12 | 50.63 (17.73) | 34.73 (14.04) | 51.30 | 35.60 | 0.03 |
| TSH [uIU/mL] | 9 | 12 | 2.84 (3.89) | 2.81 (4.68) | 1.37 | 1.35 | 0.97 |
| Other seasons’ population | |||||||
| Age [year] | 15 | 65 | 57 (15) | 60 (12) | 58 | 62 | 0.41 |
| Duration of RA [years] | 15 | 65 | 9 (9) | 12 (10) | 5 | 8 | 0.12 |
| Body mass [kg] | 15 | 65 | 72.60 (15.75) | 77.89 (18.08) | 67.80 | 74.60 | 0.36 |
| BMI [kg/m2] | 15 | 65 | 27.74 (6.31) | 29.12 (6.72) | 26.00 | 27.00 | 0.43 |
| ESR [mm/one hour] | 15 | 65 | 23.07 (17.85) | 20.43 (16.07) | 18.00 | 17.00 | 0.55 |
| CRP [mg/L] | 15 | 65 | 9.77 (17.55) | 8.99 (13.16) | 1.60 | 4.40 | 0.22 |
| 25-OH-D [ng/mL] | 15 | 65 | 32.67 (20.55) | 28.72 (11.93) | 30.10 | 27.50 | 0.99 |
| TSH [uIU/mL] | 14 | 64 | 1.26 (0.81) | 1.56 (1.19) | 1.07 | 1.24 | 0.60 |
| Season | Summer | Autumn | Winter |
|---|---|---|---|
| Autumn | 0.19 | ||
| Winter | 0.002 | 0.35 | |
| Spring | 0.03 | 0.91 | 0.67 |
| Group | Valid N in Summer Group | Valid N in Other Seasons Group | Mean 25-OH-D Concentration in Summer Group | Mean 25-OH-D Concentration in Other Seasons Group | Median in Summer Group | Median in Other Seasons Group | p |
|---|---|---|---|---|---|---|---|
| LEF | 9 | 15 | 50.63 ± 17.73 | 32.67 ± 20.55 | 51.30 | 30.10 | 0.04 |
| MTX | 12 | 65 | 34.73 ± 14.04 | 28.72 ± 11.93 | 35.60 | 27.50 | 0.12 |
| Whole Population | LEF 7 | MTX 8 | Summer | Other Seasons | Summer + LEF | Summer + MTX | Other Seasons + LEF | Other Seasons + MTX | |
|---|---|---|---|---|---|---|---|---|---|
| Age [years] | 0.17 | 0.21 | 0.16 | 0.09 | 0.15 | 0.00 | 0.07 | 0.09 | 0.16 |
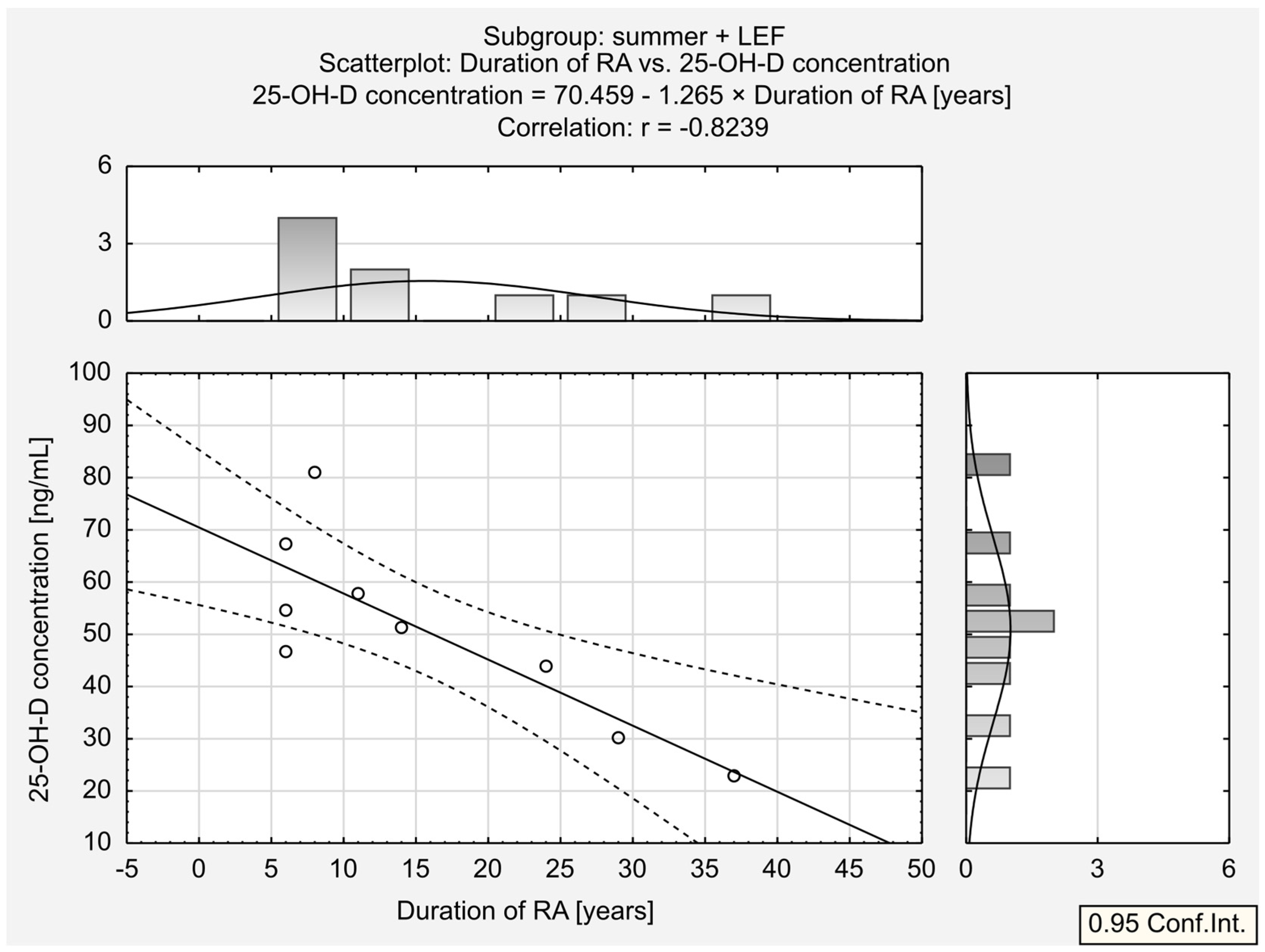
| Duration of RA [years] | 0.08 | −0.02 | 0.13 | −0.64 * | 0.14 | −0.82 * | −0.61 * | −0.19 | 0.18 |
| Body mass [kg] | −0.11 | −0.33 | 0.01 | 0.25 | −0.22 * | 0.17 | 0.47 | −0.66 * | −0.10 |
| BMI [kg/m2] | −0.03 | −0.11 | 0.05 | 0.28 | −0.17 | 0.31 | 0.50 | −0.57 * | −0.06 |
| ESR [mm/one hour] | 0.17 | 0.22 | 0.20 | 0.03 | 0.18 | 0.67 * | −0.45 | −0.03 | 0.26 * |
| CRP [mg/L] | 0.01 | −0.06 | 0.10 | −0.21 | 0.05 | −0.03 | −0.43 | −0.30 | 0.17 |
| TSH [uIU/mL] | −0.02 | 0.06 | −0.01 | 0.15 | −0.06 | 0.18 | 0.16 | −0.04 | −0.04 |
| Deficient | Suboptimal | Correct | Increased | Total | Likelihood Ratio p | |
|---|---|---|---|---|---|---|
| whole population | 29 (28.7) | 19 (18.8) | 40 (39.6) | 13 (12.9) | 101 | |
| LEF | 7 (29.2) | 1 (4.2) | 7 (29.2) | 9 (37.5) | 24 | 0.002 |
| MTX | 22 (28.6) | 18 (23.4) | 33 (42.9) | 4 (5.2) | 77 | |
| summer | 1 (4.8) | 4 (19.0) | 9 (42.9) | 7 (33.3) | 21 | 0.009 |
| other seasons | 28 (35.0) | 15 (18.8) | 31 (38.8) | 6 (7.5) | 80 |
| Reference Category | |||||
|---|---|---|---|---|---|
| Deficiency | Suboptimal | Correct | |||
| Estimated categories | suboptimal | treatment = LEF | 0.138 [0.015–1.291] | ||
| season = other seasons | 0.104 [0.010–1.071] | ||||
| correct | treatment = LEF | 0.531 [0.153–1.844] | 3.858 [0.432–34.420] | ||
| season = other seasons | 0.110 * [0.013–0.943] | 1.055 [0.271–4.103] | |||
| increased | treatment = LEF | 4.596 [0.962–21.964] | 33.389 * [3.156–353.218] | 8.655 * [1.989–37.654] | |
| season = other seasons | 0.043 * [0.004–0.437] | 0.415 [0.071–2.415] | 0.393 [0.091–1.702] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieślewicz, A.; Korzeniowska, K.; Grabańska-Martyńska, K.; Jabłecka, A.; Hrycaj, P. Seasonal and Treatment-Related Variation in 25-Hydroxy Vitamin D Concentration in Patients with Rheumatoid Arthritis. J. Clin. Med. 2024, 13, 973. https://doi.org/10.3390/jcm13040973
Cieślewicz A, Korzeniowska K, Grabańska-Martyńska K, Jabłecka A, Hrycaj P. Seasonal and Treatment-Related Variation in 25-Hydroxy Vitamin D Concentration in Patients with Rheumatoid Arthritis. Journal of Clinical Medicine. 2024; 13(4):973. https://doi.org/10.3390/jcm13040973
Chicago/Turabian StyleCieślewicz, Artur, Katarzyna Korzeniowska, Katarzyna Grabańska-Martyńska, Anna Jabłecka, and Paweł Hrycaj. 2024. "Seasonal and Treatment-Related Variation in 25-Hydroxy Vitamin D Concentration in Patients with Rheumatoid Arthritis" Journal of Clinical Medicine 13, no. 4: 973. https://doi.org/10.3390/jcm13040973
APA StyleCieślewicz, A., Korzeniowska, K., Grabańska-Martyńska, K., Jabłecka, A., & Hrycaj, P. (2024). Seasonal and Treatment-Related Variation in 25-Hydroxy Vitamin D Concentration in Patients with Rheumatoid Arthritis. Journal of Clinical Medicine, 13(4), 973. https://doi.org/10.3390/jcm13040973






