Evaluation of New Cardiac Damage Biomarkers in Polytrauma: GDF-15, HFABP and uPAR for Predicting Patient Outcomes
Abstract
1. Introduction
2. Materials and Methods
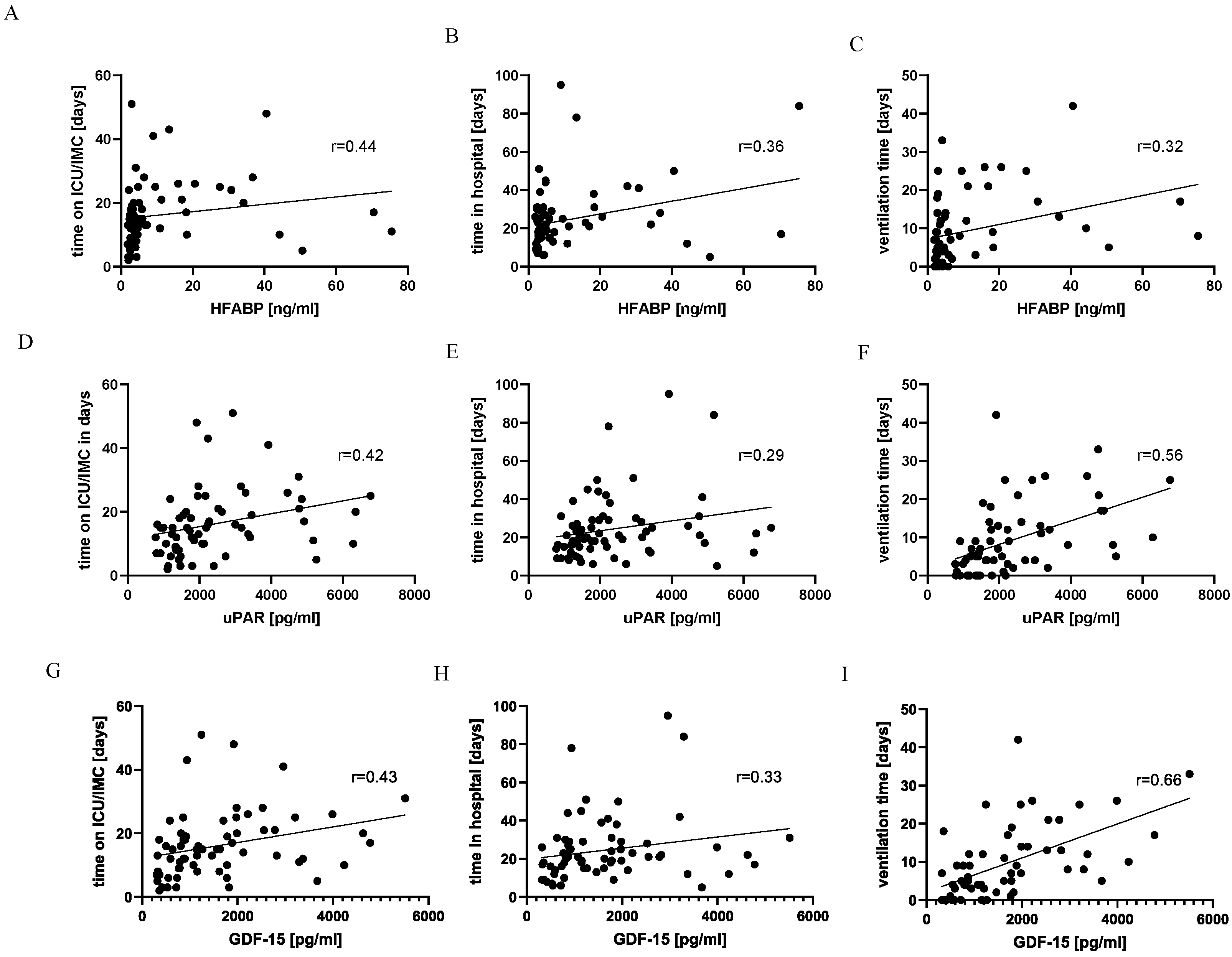
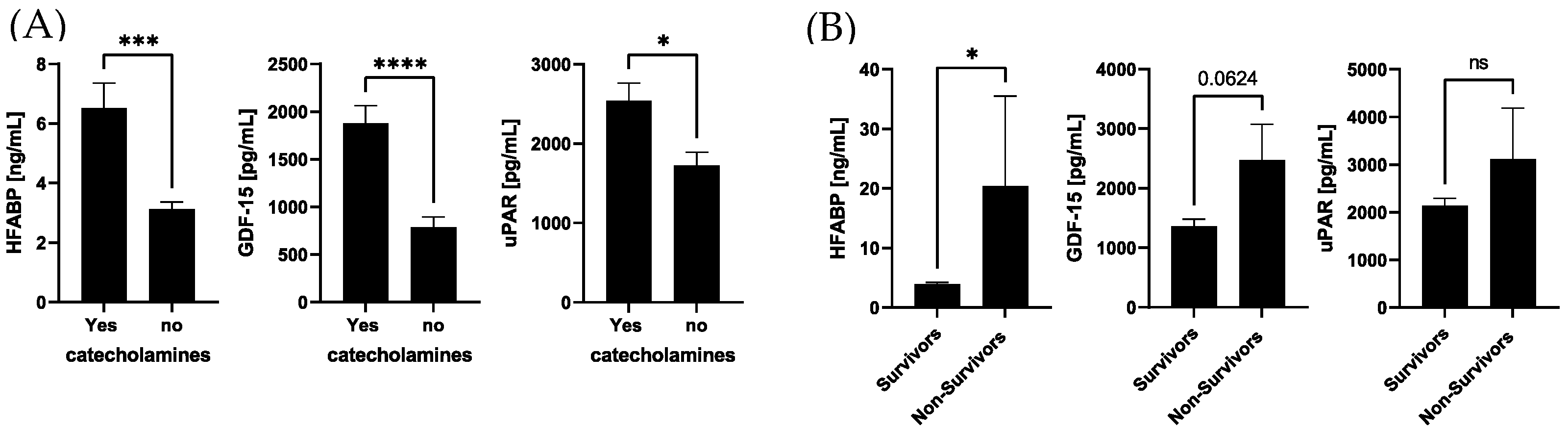
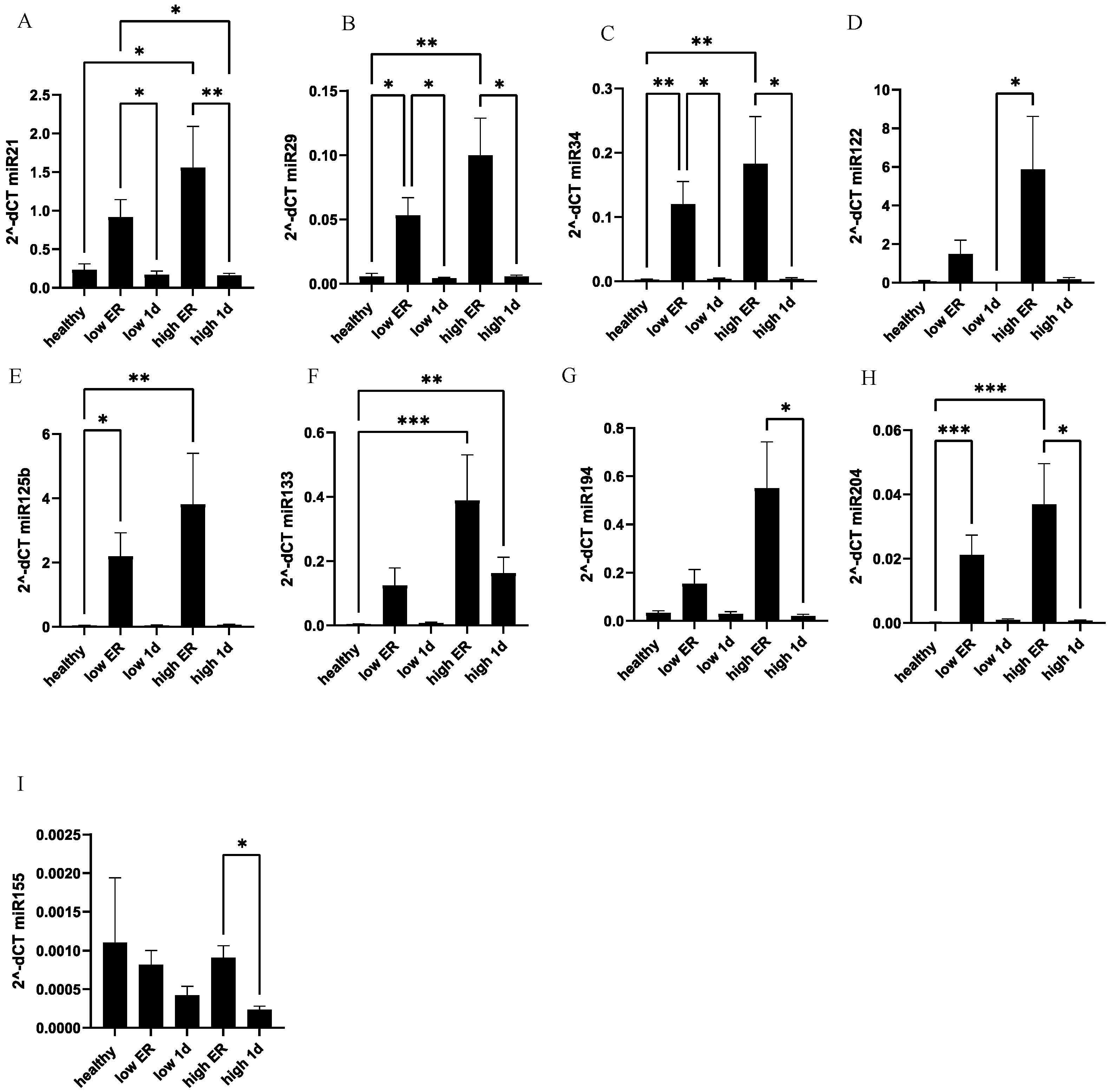
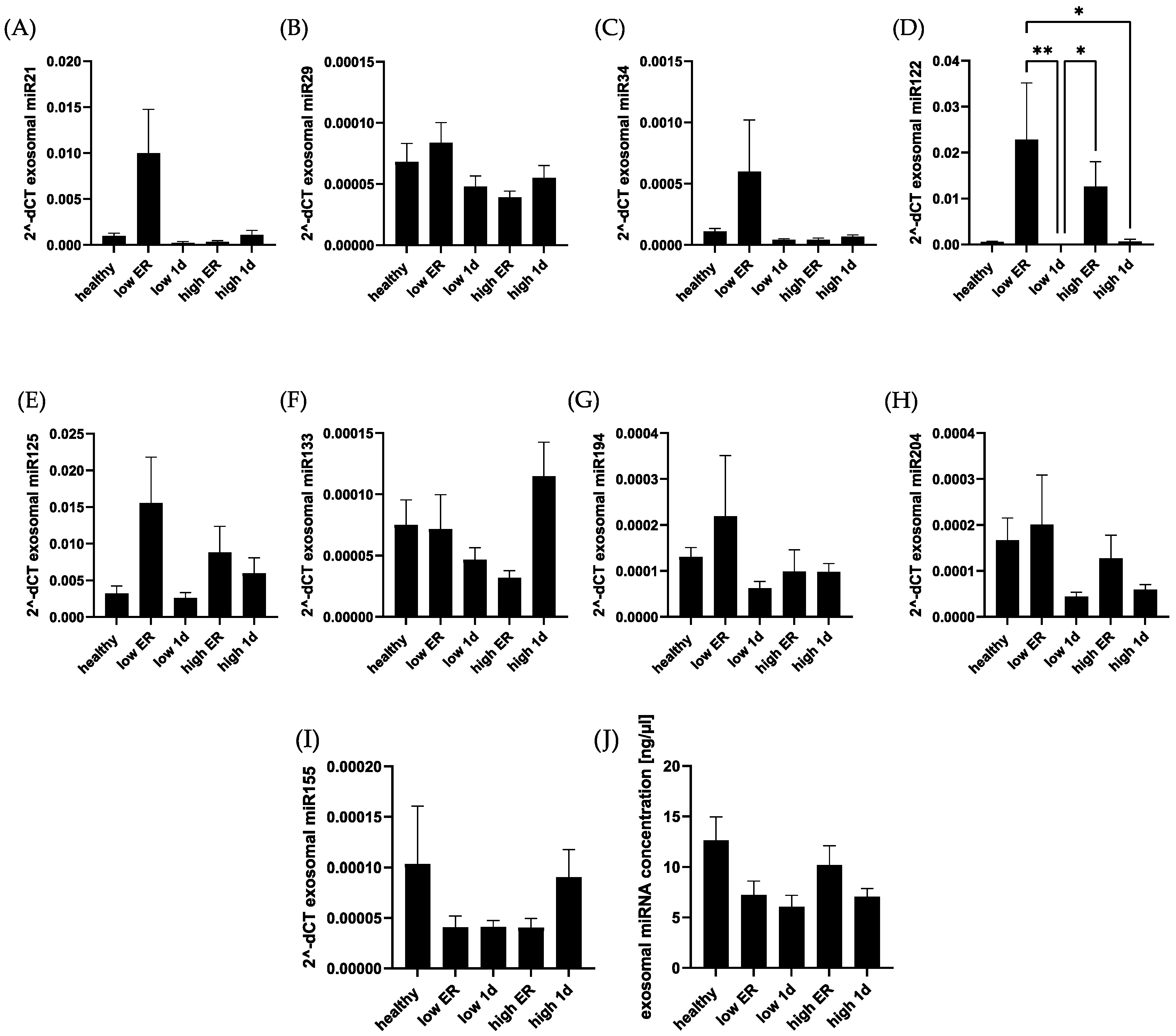
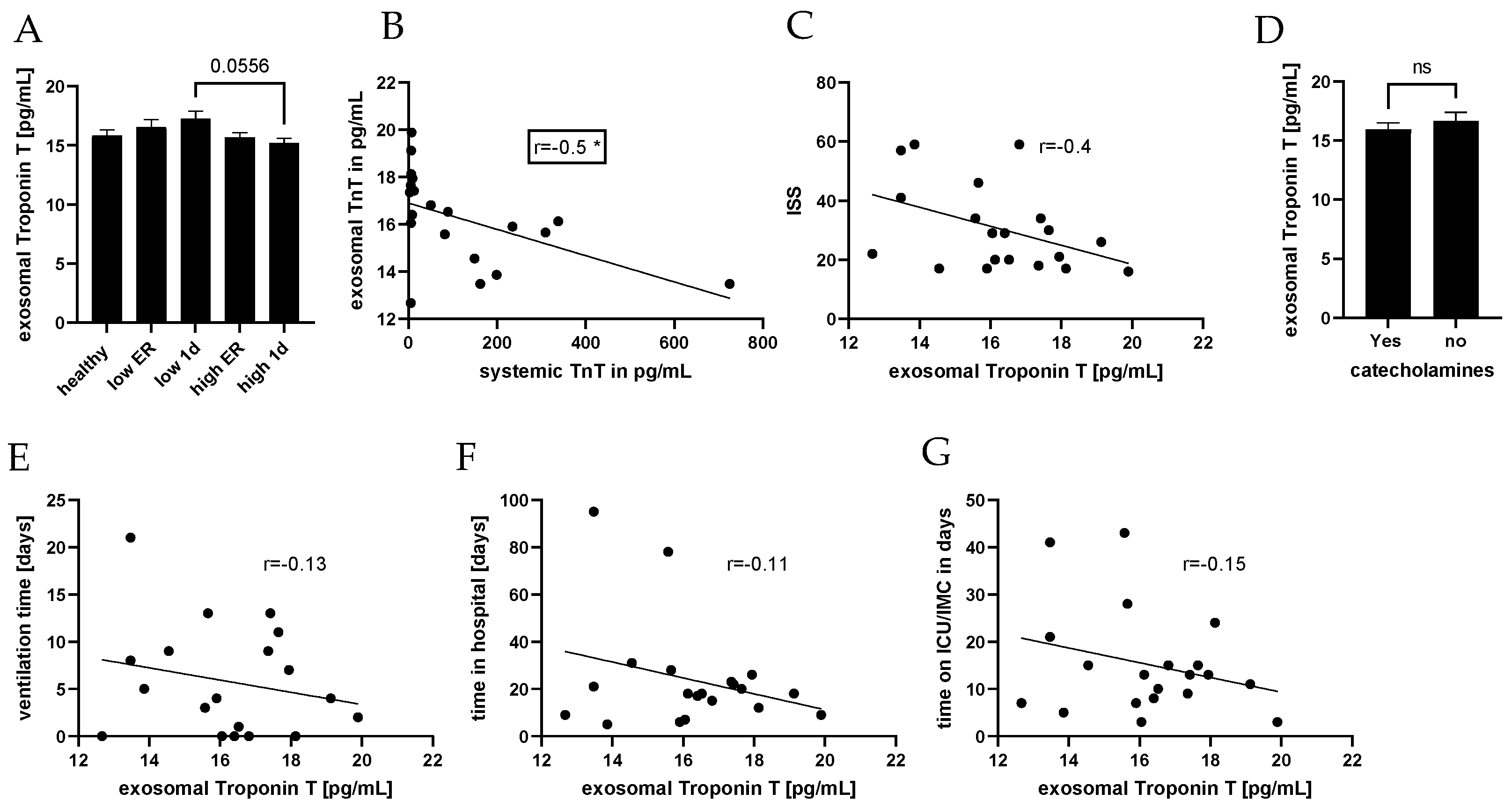
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hauser, C.J. Preclinical models of traumatic, hemorrhagic shock. Shock 2005, 24 (Suppl. S1), 24–32. [Google Scholar] [CrossRef] [PubMed]
- Butcher, N.; Balogh, Z.J. The definition of polytrauma: The need for international consensus. Injury 2009, 40 (Suppl. S4), S12–S22. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, M.; Kanz, K.-G.; Kirchhoff, C.; Khalil, P.N.; Wierer, M.; van Griensven, M.; Laugwitz, K.-L.; Biberthaler, P.; Lefering, R.; Huber-Wagner, S. Blunt Cardiac Injury in the Severely Injured—A Retrospective Multicentre Study. PLoS ONE 2015, 10, e0131362. [Google Scholar] [CrossRef]
- Huber, S.; Biberthaler, P.; Delhey, P.; Trentzsch, H.; Winter, H.; van Griensven, M.; Lefering, R.; Huber-Wagner, S. Predictors of poor outcomes after significant chest trauma in multiply injured patients: A retrospective analysis from the German Trauma Registry (Trauma Register DGU®). Scand. J. Trauma Resusc. Emerg. Med. 2014, 22, 52. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Lackner, I.; Gebhard, F.; Miclau, T.; Kalbitz, M. Trauma, a Matter of the Heart-Molecular Mechanism of Post-Traumatic Cardiac Dysfunction. Int. J. Mol. Sci. 2021, 22, 737. [Google Scholar] [CrossRef] [PubMed]
- Kalbitz, M.; Schwarz, S.; Weber, B.; Bosch, B.; Pressmar, J.; Hoenes, F.M.; Braun, C.K.; Horst, K.; Simon, T.P.; Pfeifer, R.; et al. Cardiac Depression in Pigs after Multiple Trauma—Characterization of Posttraumatic Structural and Functional Alterations. Sci. Rep. 2017, 7, 17861. [Google Scholar] [CrossRef] [PubMed]
- Fridén, V.; Starnberg, K.; Muslimovic, A.; Ricksten, S.-E.; Bjurman, C.; Forsgard, N.; Wickman, A.; Hammarsten, O. Clearance of cardiac troponin T with and without kidney function. Clin. Biochem. 2017, 50, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.; Melot, J.; Krinock, M.D.; Kumar, A.; Nadar, S.K.; Lip, G.Y.H. Heart-type fatty acid-binding protein: An overlooked cardiac biomarker. Ann. Med. 2020, 52, 444–461. [Google Scholar] [CrossRef]
- Gami, B.N.; Patel, D.S.; Haridas, N.; Chauhan, K.P.; Shah, H.; Trivedi, A. Utility of Heart-type Fatty Acid Binding Protein as a New Biochemical Marker for the Early Diagnosis of Acute Coronary Syndrome. J. Clin. Diagn. Res. 2015, 9, BC22–BC24. [Google Scholar] [CrossRef]
- Andersson, J.; Fall, T.; Delicano, R.; Wennberg, P.; Jansson, J.-H. GDF-15 is associated with sudden cardiac death due to incident myocardial infarction. Resuscitation 2020, 152, 165–169. [Google Scholar] [CrossRef]
- Kempf, T.; Zarbock, A.; Widera, C.; Butz, S.; Stadtmann, A.; Rossaint, J.; Bolomini-Vittori, M.; Korf-Klingebiel, M.; Napp, L.C.; Hansen, B.; et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat. Med. 2011, 17, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.E.; Amrein, M.L.F.; Schäfer, I.; Zimmermann, T.; Lopez-Ayala, P.; Boeddinghaus, J.; Twerenbold, R.; Puelacher, C.; Nestelberger, T.; Wussler, D.; et al. Soluble urokinase plasminogen activator receptor and functionally relevant coronary artery disease: A prospective cohort study. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2022, 27, 278–285. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, Q.; Zhou, X.; Li, J.; Li, Y.; Zhang, L.; Zhou, Q.; Tang, B. Circulating miRNA-21 is a promising biomarker for heart failure. Mol. Med. Rep. 2017, 16, 7766–7774. [Google Scholar] [CrossRef]
- Ali Sheikh, M.S. The mir-21 Inhibition Enhanced HUVEC Cellular Viability during Hypoxia-Reoxygenation Injury by Regulating PDCD4. Mediat. Inflamm. 2022, 2022, 9661940. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.T.; Teng, K.; Wu, C.; Adam, M.; Johnstone, R.M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985, 101, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Bard, M.P.; Hegmans, J.P.; Hemmes, A.; Luider, T.M.; Willemsen, R.; Severijnen, L.-A.A.; van Meerbeeck, J.P.; Burgers, S.A.; Hoogsteden, H.C.; Lambrecht, B.N. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am. J. Respir. Cell Mol. Biol. 2004, 31, 114–121. [Google Scholar] [CrossRef]
- Caby, M.-P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [CrossRef]
- Michael, A.; Bajracharya, S.D.; Yuen, P.S.T.; Zhou, H.; Star, R.A.; Illei, G.G.; Alevizos, I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010, 16, 34–38. [Google Scholar] [CrossRef]
- Pisitkun, T.; Shen, R.-F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef]
- Relja, B.; Huber-Lang, M.; van Griensven, M.; Hildebrand, F.; Maegele, M.; Nienaber, U.; Brucker, D.P.; Sturm, R.; Marzi, I. A nationwide fluidics biobank of polytraumatized patients: Implemented by the Network “Trauma Research” (NTF) as an expansion to the TraumaRegister DGU® of the German Trauma Society (DGU). Eur. J. Trauma Emerg. Surg. 2020, 46, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Ugur, K.; Aydin, S.; Sahin, İ.; Yardim, M. Biomarkers in acute myocardial infarction: Current perspectives. Vasc. Health Risk Manag. 2019, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef]
- Furuhashi, M.; Ura, N.; Hasegawa, K.; Yoshida, H.; Tsuchihashi, K.; Nakata, T.; Shimamoto, K. Serum ratio of heart-type fatty acid-binding protein to myoglobin: A Novel Marker of Cardiac Damage and Volume Overload in Hemodialysis Patients. Nephron Clin. Pract. 2003, 93, C69–C74. [Google Scholar] [CrossRef]
- Wall, J.; Naganathar, S.; Praditsuktavorn, B.; Bugg, O.F.; McArthur, S.; Thiemermann, C.; Tremoleda, J.L.; Brohi, K. Modeling Cardiac Dysfunction Following Traumatic Hemorrhage Injury: Impact on Myocardial Integrity. Front. Immunol. 2019, 10, 2774. [Google Scholar] [CrossRef] [PubMed]
- Baur, M.; Weber, B.; Lackner, I.; Gebhard, F.; Pfeifer, R.; Cinelli, P.; Halvachizadeh, S.; Teuben, M.; Lipiski, M.; Cesarovic, N.; et al. Structural alterations and inflammation in the heart after multiple trauma followed by reamed versus non-reamed femoral nailing. PLoS ONE 2020, 15, e0235220. [Google Scholar] [CrossRef]
- Lagerstedt, L.; Azurmendi, L.; Tenovuo, O.; Katila, A.J.; Takala, R.S.K.; Blennow, K.; Newcombe, V.F.J.; Maanpää, H.-R.; Tallus, J.; Hossain, I.; et al. Interleukin 10 and Heart Fatty Acid-Binding Protein as Early Outcome Predictors in Patients with Traumatic Brain Injury. Front. Neurol. 2020, 11, 376. [Google Scholar] [CrossRef]
- De’Ath, H.D.; Rourke, C.; Davenport, R.; Manson, J.; Renfrew, I.; Uppal, R.; Davies, L.C.; Brohi, K. Clinical and biomarker profile of trauma-induced secondary cardiac injury. Br. J. Surg. 2012, 99, 789–797. [Google Scholar] [CrossRef]
- Watanabe, K.; Wakabayashi, H.; Veerkamp, J.H.; Ono, T.; Suzuki, T. Immunohistochemical distribution of heart-type fatty acid-binding protein immunoreactivity in normal human tissues and in acute myocardial infarct. J. Pathol. 1993, 170, 59–65. [Google Scholar] [CrossRef]
- Rochette, L.; Dogon, G.; Zeller, M.; Cottin, Y.; Vergely, C. GDF15 and Cardiac Cells: Current Concepts and New Insights. Int. J. Mol. Sci. 2021, 22, 8889. [Google Scholar] [CrossRef]
- Hagström, E.; Held, C.; Stewart, R.A.H.; Aylward, P.E.; Budaj, A.; Cannon, C.P.; Koenig, W.; Krug-Gourley, S.; Mohler, E.R.; Steg, P.G.; et al. Growth Differentiation Factor 15 Predicts All-Cause Morbidity and Mortality in Stable Coronary Heart Disease. Clin. Chem. 2017, 63, 325–333. [Google Scholar] [CrossRef]
- Wollert, K.C.; Kempf, T.; Peter, T.; Olofsson, S.; James, S.; Johnston, N.; Lindahl, B.; Horn-Wichmann, R.; Brabant, G.; Simoons, M.L.; et al. Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation 2007, 115, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Myrmel, G.M.S.; Steiro, O.-T.; Tjora, H.L.; Langørgen, J.; Bjørneklett, R.O.; Skadberg, Ø.; Bonarjee, V.V.S.; Mjelva, Ø.R.; Pedersen, E.R.; Vikenes, K.; et al. Prognostic value of growth differentiation factor-15 3 months after an acute chest pain admission. Heart 2023, 69, 649–660. [Google Scholar] [CrossRef]
- May, B.M.; Pimentel, M.; Zimerman, L.I.; Rohde, L.E. GDF-15 como Biomarcador em Doenças Cardiovasculares. Arq. Bras. Cardiol. 2021, 116, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Gürgöze, M.T.; van Vark, L.C.; Baart, S.J.; Kardys, I.; Akkerhuis, K.M.; Manintveld, O.C.; Postmus, D.; Hillege, H.L.; Lesman-Leegte, I.; Asselbergs, F.W.; et al. Multimarker Analysis of Serially Measured GDF-15, NT-proBNP, ST2, GAL-3, cTnI, Creatinine, and Prognosis in Acute Heart Failure. Circ. Heart Fail. 2023, 16, e009526. [Google Scholar] [CrossRef]
- Valiño-Rivas, L.; Cuarental, L.; Ceballos, M.I.; Pintor-Chocano, A.; Perez-Gomez, M.V.; Sanz, A.B.; Ortiz, A.; Sanchez-Niño, M.D. Growth differentiation factor-15 preserves Klotho expression in acute kidney injury and kidney fibrosis. Kidney Int. 2022, 101, 1200–1215. [Google Scholar] [CrossRef]
- Putt, M.; Hahn, V.S.; Januzzi, J.L.; Sawaya, H.; Sebag, I.A.; Plana, J.C.; Picard, M.H.; Carver, J.R.; Halpern, E.F.; Kuter, I.; et al. Longitudinal Changes in Multiple Biomarkers Are Associated with Cardiotoxicity in Breast Cancer Patients Treated with Doxorubicin, Taxanes, and Trastuzumab. Clin. Chem. 2015, 61, 1164–1172. [Google Scholar] [CrossRef]
- Demissei, B.G.; Freedman, G.; Feigenberg, S.J.; Plastaras, J.P.; Maity, A.; Smith, A.M.; McDonald, C.; Sheline, K.; Simone, C.B.; Lin, L.L.; et al. Early Changes in Cardiovascular Biomarkers with Contemporary Thoracic Radiation Therapy for Breast Cancer, Lung Cancer, and Lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 851–860. [Google Scholar] [CrossRef]
- Andersson, C.; Preis, S.R.; Beiser, A.; DeCarli, C.; Wollert, K.C.; Wang, T.J.; Januzzi, J.L.; Vasan, R.S.; Seshadri, S. Associations of Circulating Growth Differentiation Factor-15 and ST2 Concentrations with Subclinical Vascular Brain Injury and Incident Stroke. Stroke 2015, 46, 2568–2575. [Google Scholar] [CrossRef]
- Desmedt, S.; Desmedt, V.; Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. The intriguing role of soluble urokinase receptor in inflammatory diseases. Crit. Rev. Clin. Lab. Sci. 2017, 54, 117–133. [Google Scholar] [CrossRef]
- Rasmussen, L.J.H.; Petersen, J.E.V.; Eugen-Olsen, J. Soluble Urokinase Plasminogen Activator Receptor (suPAR) as a Biomarker of Systemic Chronic Inflammation. Front. Immunol. 2021, 12, 780641. [Google Scholar] [CrossRef] [PubMed]
- Lyngbæk, S.; Marott, J.L.; Møller, D.V.; Christiansen, M.; Iversen, K.K.; Clemmensen, P.M.; Eugen-Olsen, J.; Jeppesen, J.L.; Hansen, P.R. Usefulness of soluble urokinase plasminogen activator receptor to predict repeat myocardial infarction and mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous intervention. Am. J. Cardiol. 2012, 110, 1756–1763. [Google Scholar] [CrossRef]
- Timmermans, K.; Vaneker, M.; Scheffer, G.J.; Maassen, P.; Janssen, S.; Kox, M.; Pickkers, P. Soluble urokinase-type plasminogen activator levels are related to plasma cytokine levels but have low predictive value for mortality in trauma patients. J. Crit. Care 2015, 30, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.M.; Kuster, N.; Bargnoux, A.S.; Aguilhon, S.; Huet, F.; Leclercq, F.; Pasquié, J.-L.; Roubille, F.; Cristol, J.P. Long term pronostic value of suPAR in chronic heart failure: Reclassification of patients with low MAGGIC score. Clin. Chem. Lab. Med. 2021, 59, 1299–1306. [Google Scholar] [CrossRef]
- Hayek, S.S.; Tahhan, A.S.; Ko, Y.-A.; Alkhoder, A.; Zheng, S.; Bhimani, R.; Hartsfield, J.; Kim, J.; Wilson, P.; Shaw, L.; et al. Soluble Urokinase Plasminogen Activator Receptor Levels and Outcomes in Patients with Heart Failure. J. Card. Fail. 2023, 29, 158–167. [Google Scholar] [CrossRef]
- Theilade, S.; Rossing, P.; Eugen-Olsen, J.; Jensen, J.S.; Jensen, M.T. suPAR level is associated with myocardial impairment assessed with advanced echocardiography in patients with type 1 diabetes with normal ejection fraction and without known heart disease or end-stage renal disease. Eur. J. Endocrinol. 2016, 174, 745–753. [Google Scholar] [CrossRef]
- Gryshkova, V.; Fleming, A.; McGhan, P.; de Ron, P.; Fleurance, R.; Valentin, J.-P.; Da Nogueira Costa, A. miR-21-5p as a potential biomarker of inflammatory infiltration in the heart upon acute drug-induced cardiac injury in rats. Toxicol. Lett. 2018, 286, 31–38. [Google Scholar] [CrossRef]
- Sadat-Ebrahimi, S.-R.; Rezabakhsh, A.; Aslanabadi, N.; Asadi, M.; Zafari, V.; Shanebandi, D.; Zarredar, H.; Enamzadeh, E.; Taghizadeh, H.; Badalzadeh, R. Novel diagnostic potential of miR-1 in patients with acute heart failure. PLoS ONE 2022, 17, e0275019. [Google Scholar] [CrossRef]
- Huang, W.; Tian, S.-S.; Hang, P.-Z.; Sun, C.; Guo, J.; Du, Z.-M. Combination of microRNA-21 and microRNA-146a Attenuates Cardiac Dysfunction and Apoptosis During Acute Myocardial Infarction in Mice. Molecular therapy. Nucleic Acids 2016, 5, e296. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Li, Z.; Zhi, Z.; Wang, S.; Xu, G. MiR-21 derived from the exosomes of MSCs regulates the death and differentiation of neurons in patients with spinal cord injury. Gene Ther. 2019, 26, 491–503. [Google Scholar] [CrossRef]
- May, S.M.; Abbott, T.E.F.; Del Arroyo, A.G.; Reyes, A.; Martir, G.; Stephens, R.C.M.; Brealey, D.; Cuthbertson, B.H.; Wijeysundera, D.N.; Pearse, R.M.; et al. MicroRNA signatures of perioperative myocardial injury after elective noncardiac surgery: A prospective observational mechanistic cohort study. Br. J. Anaesth. 2020, 125, 661–671. [Google Scholar] [CrossRef]
- Bostjancic, E.; Zidar, N.; Stajer, D.; Glavac, D. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology 2010, 115, 163–169. [Google Scholar] [CrossRef]
- Yin, Z.; Han, Z.; Hu, T.; Zhang, S.; Ge, X.; Huang, S.; Wang, L.; Yu, J.; Li, W.; Wang, Y.; et al. Neuron-derived exosomes with high miR-21-5p expression promoted polarization of M1 microglia in culture. Brain Behav. Immun. 2020, 83, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Qi, X.; Li, Y.; Liu, H.; Zhang, F.; Qin, C. Biventricular pacing cardiac contractility modulation improves cardiac contractile function via upregulating SERCA2 and miR-133 in a rabbit model of congestive heart failure. Cell. Physiol. Biochem. 2014, 33, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Mao, S.; Liu, X.; Li, S.; Zhou, H.; Gu, Y.; Liu, W.; Fu, L.; Liao, C.; Wang, P. MiR-125b inhibits cardiomyocyte apoptosis by targeting BAK1 in heart failure. Mol. Med. 2021, 27, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-P.; Tian, T.; Wang, J.-Y.; He, J.-N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.-X.; Qiu, X.-T.; Li, C.-C.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef]
- Luís, A.; Hackl, M.; Jafarmadar, M.; Keibl, C.; Jilge, J.M.; Grillari, J.; Bahrami, S.; Kozlov, A.V. Circulating miRNAs Associated With ER Stress and Organ Damage in a Preclinical Model of Trauma Hemorrhagic Shock. Front. Med. 2020, 7, 568096. [Google Scholar] [CrossRef]
- Sassi, Y.; Avramopoulos, P.; Ramanujam, D.; Grüter, L.; Werfel, S.; Giosele, S.; Brunner, A.-D.; Esfandyari, D.; Papadopoulou, A.S.; de Strooper, B.; et al. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nat. Commun. 2017, 8, 1614. [Google Scholar] [CrossRef]
- Zhang, W.-C.; Yang, J.-H.; Liu, G.-H.; Yang, F.; Gong, J.-L.; Jia, M.-G.; Zhang, M.-J.; Zhao, L.-S. miR-34b/c regulates doxorubicin-induced myocardial cell injury through ITCH. Cell Cycle 2019, 18, 3263–3274. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- He, Y.; Gu, X.; Hu, Y.; Jia, H.; Zhao, Z.; Jiang, H.; Zheng, H.; Zhu, F. Low-density lipoprotein receptor and apolipoprotein A 5, myocardial infarction biomarkers in plasma-derived exosomes. J. Cardiol. 2022, 79, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Emanueli, C.; Shearn, A.I.U.; Laftah, A.; Fiorentino, F.; Reeves, B.C.; Beltrami, C.; Mumford, A.; Clayton, A.; Gurney, M.; Shantikumar, S.; et al. Coronary Artery-Bypass-Graft Surgery Increases the Plasma Concentration of Exosomes Carrying a Cargo of Cardiac MicroRNAs: An Example of Exosome Trafficking Out of the Human Heart with Potential for Cardiac Biomarker Discovery. PLoS ONE 2016, 11, e0154274. [Google Scholar] [CrossRef] [PubMed]
- Mair, J.; Lindahl, B.; Hammarsten, O.; Müller, C.; Giannitsis, E.; Huber, K.; Möckel, M.; Plebani, M.; Thygesen, K.; Jaffe, A.S. How is cardiac troponin released from injured myocardium? Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 553–560. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritter, A.; Lötterle, L.; Han, J.; Kalbitz, M.; Henrich, D.; Marzi, I.; Leppik, L.; Weber, B. Evaluation of New Cardiac Damage Biomarkers in Polytrauma: GDF-15, HFABP and uPAR for Predicting Patient Outcomes. J. Clin. Med. 2024, 13, 961. https://doi.org/10.3390/jcm13040961
Ritter A, Lötterle L, Han J, Kalbitz M, Henrich D, Marzi I, Leppik L, Weber B. Evaluation of New Cardiac Damage Biomarkers in Polytrauma: GDF-15, HFABP and uPAR for Predicting Patient Outcomes. Journal of Clinical Medicine. 2024; 13(4):961. https://doi.org/10.3390/jcm13040961
Chicago/Turabian StyleRitter, Aileen, Lorenz Lötterle, Jiaoyan Han, Miriam Kalbitz, Dirk Henrich, Ingo Marzi, Liudmila Leppik, and Birte Weber. 2024. "Evaluation of New Cardiac Damage Biomarkers in Polytrauma: GDF-15, HFABP and uPAR for Predicting Patient Outcomes" Journal of Clinical Medicine 13, no. 4: 961. https://doi.org/10.3390/jcm13040961
APA StyleRitter, A., Lötterle, L., Han, J., Kalbitz, M., Henrich, D., Marzi, I., Leppik, L., & Weber, B. (2024). Evaluation of New Cardiac Damage Biomarkers in Polytrauma: GDF-15, HFABP and uPAR for Predicting Patient Outcomes. Journal of Clinical Medicine, 13(4), 961. https://doi.org/10.3390/jcm13040961






