Efficacy and Safety of the Extreme Lateral Interbody Fusion (XLIF) Technique in Spine Surgery: Meta-Analysis of 1409 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Methods for Identification of Studies
2.4. Data Extraction and Data Items
2.5. Assessment of Risk of Bias in Included Studies
2.6. Assessment of Results
2.7. Risk of Bias across the Studies
2.8. Additional Analyses
3. Results
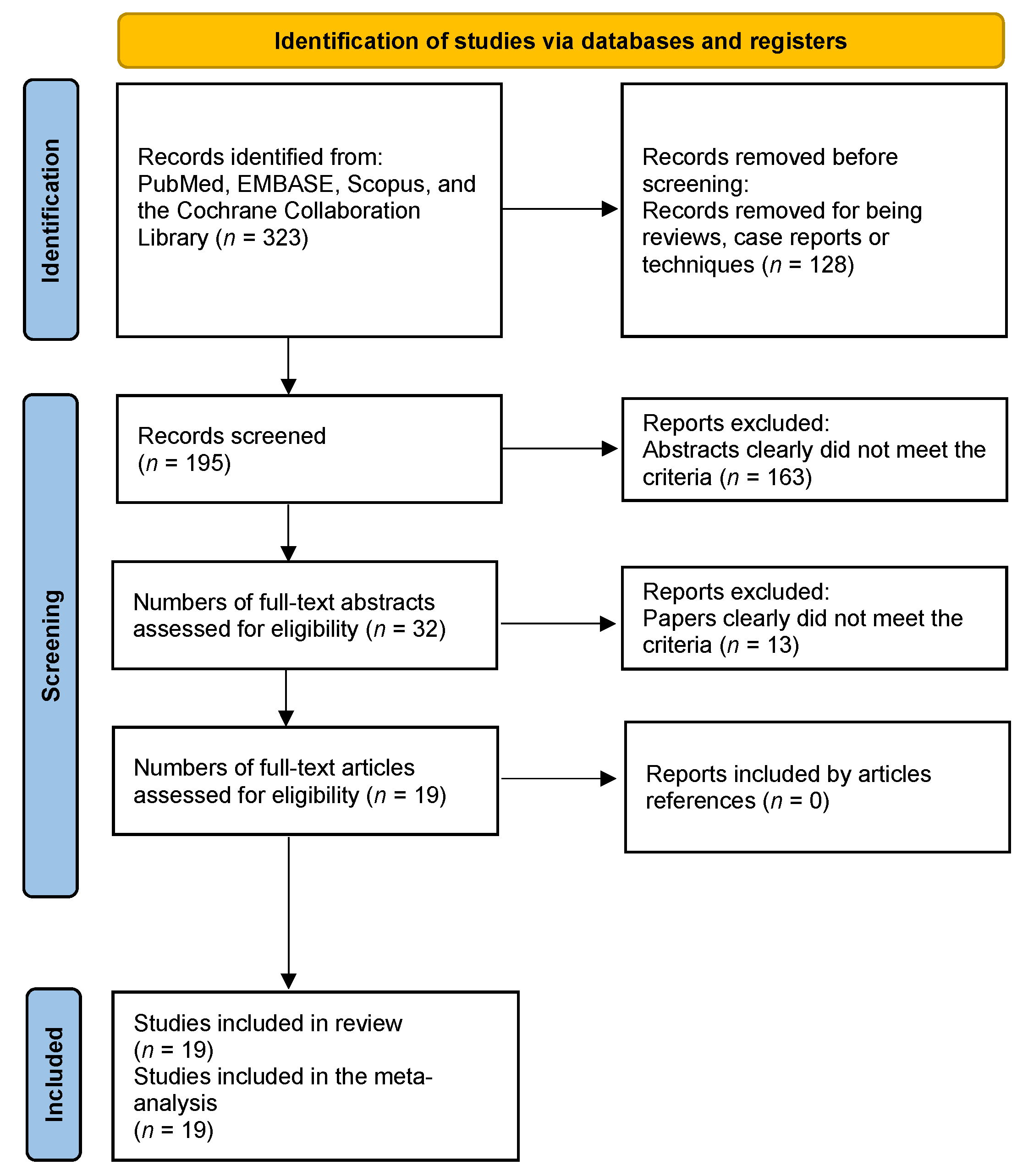
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
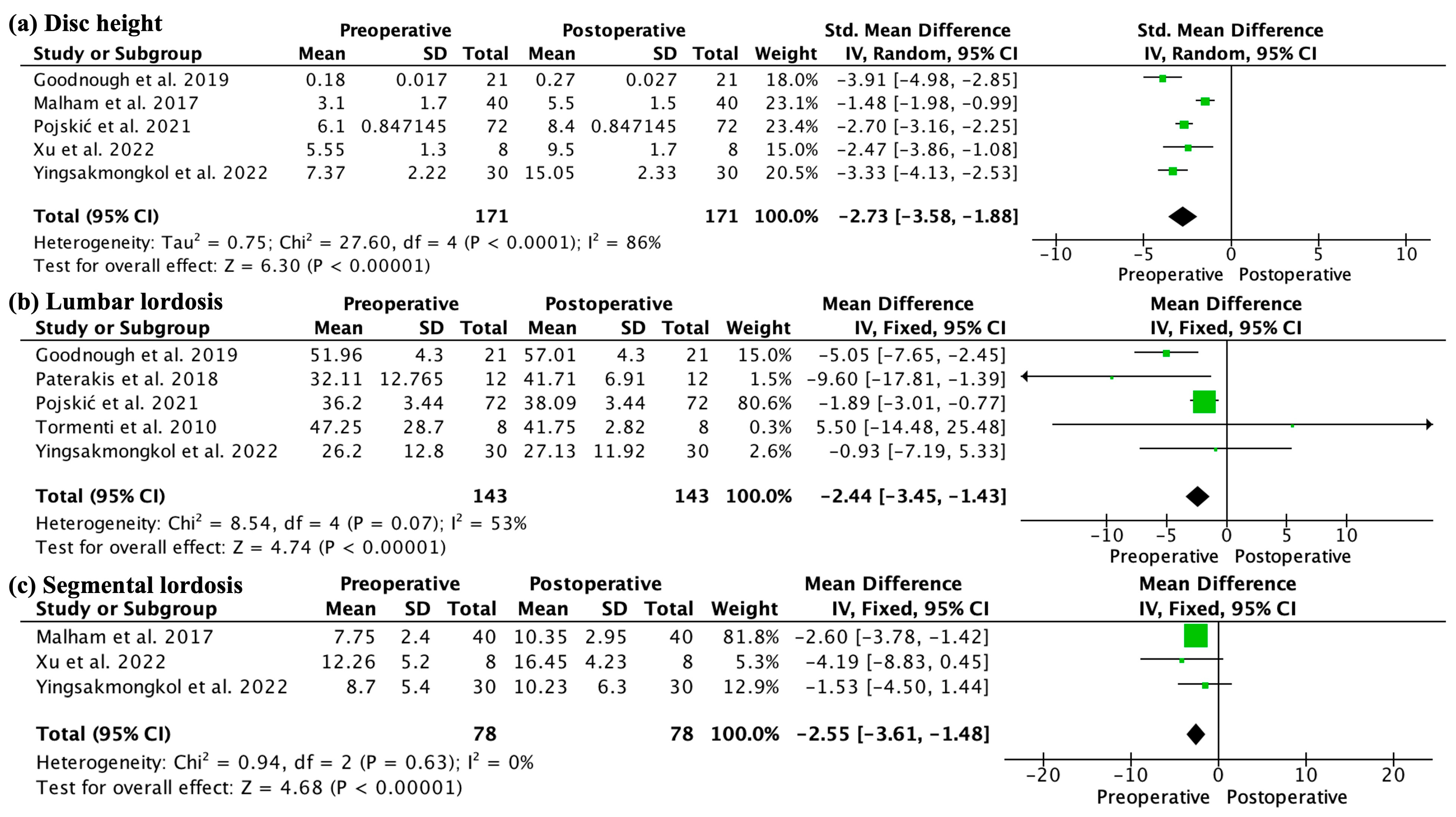
3.4. Outcomes
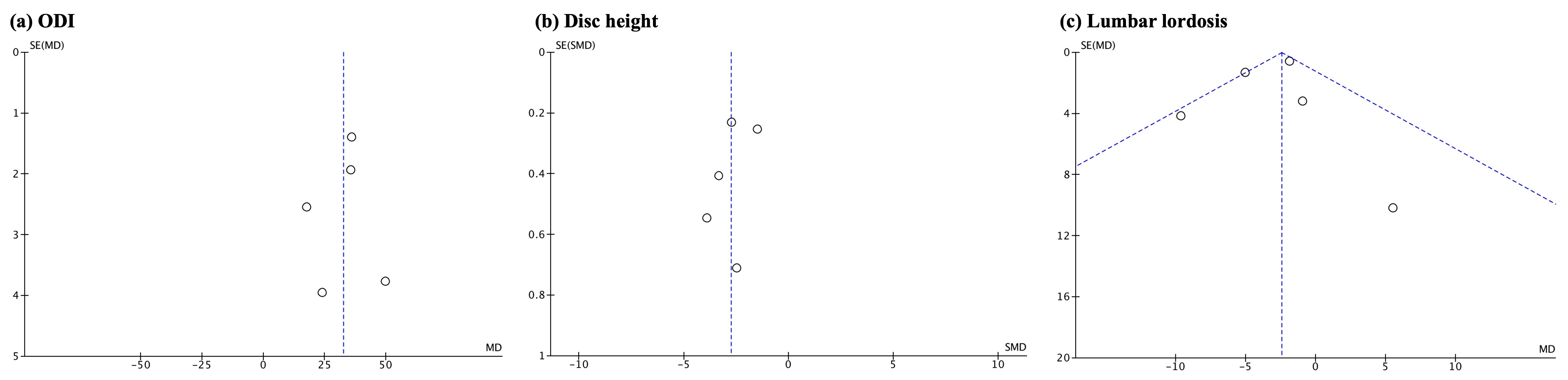
3.5. Additional Analyses
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boden, S.D.; McCowin, P.R.; Davis, D.O.; Dina, T.S.; Mark, A.S.; Wiesel, S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J. Bone Jt. Surg. Am. 1990, 72, 1178–1184. [Google Scholar] [CrossRef]
- Aoki, Y.; Takahashi, H.; Nakajima, A.; Kubota, G.; Watanabe, A.; Nakajima, T.; Eguchi, Y.; Orita, S.; Fukuchi, H.; Yanagawa, N.; et al. Prevalence of lumbar spondylolysis and spondylolisthesis in patients with degenerative spinal disease. Sci. Rep. 2020, 10, 6739. [Google Scholar] [CrossRef]
- Jensen, R.K.; Jensen, T.S.; Koes, B.; Hartvigsen, J. Prevalence of lumbar spinal stenosis in general and clinical populations: A systematic review and meta-analysis. Eur. Spine J. 2020, 29, 2143–2163. [Google Scholar] [CrossRef]
- Johnson, W.C.; Seifi, A. Trends of the neurosurgical economy in the United States. J. Clin. Neurosci. 2018, 53, 20–26. [Google Scholar] [CrossRef]
- Ozgur, B.M.; Aryan, H.E.; Pimenta, L.; Taylor, W.R. Extreme Lateral Interbody Fusion (XLIF): A novel surgical technique for anterior lumbar interbody fusion. Spine J. 2020, 6, 435–443. [Google Scholar] [CrossRef]
- Strom, R.G.; Bae, J.; Mizutani, J.; Valone, F., 3rd; Ames, C.P.; Deviren, V. Lateral interbody fusion combined with open posterior surgery for adult spinal deformity. J. Neurosurg. Spine 2016, 25, 697–705. [Google Scholar] [CrossRef]
- Goodnough, L.H.; Koltsov, J.; Wang, T.; Xiong, G.; Nathan, K.; Cheng, I. Decreased estimated blood loss in lateral trans-psoas versus anterior approach to lumbar interbody fusion for degenerative spondylolisthesis. J. Spine Surg. 2019, 5, 185–193. [Google Scholar] [CrossRef]
- Arnold, P.M.; Anderson, K.K.; McGuire, R.A., Jr. The lateral transpsoas approach to the lumbar and thoracic spine: A review. Surg. Neurol. Int. 2012, 3, S198–S215. [Google Scholar] [CrossRef] [PubMed]
- Grunert, P.; Drazin, D.; Iwanaga, J.; Schmidt, C.; Alonso, F.; Moisi, M.; Chapman, J.R.; Oskouian, R.J.; Tubbs, R.S. Injury to the Lumbar Plexus and its Branches After Lateral Fusion Procedures: A Cadaver Study. World Neurosurg. 2017, 105, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Guerin, P.; Obeid, I.; Bourghli, A.; Masquefa, T.; Luc, S.; Gille, O.; Pointillart, V.; Vital, J.M. The lumbosacral plexus: Anatomic considerations for minimally invasive retroperitoneal transpsoas approach. Surg. Radiol. Anat. 2012, 34, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N.E. High neurological complication rates for extreme lateral lumbar interbody fusion and related techniques: A review of safety concerns. Surg. Neurol. Int. 2016, 7 (Suppl. S25), S652–S655. [Google Scholar] [CrossRef]
- Tormenti, M.J.; Maserati, M.B.; Bonfield, C.M.; Okonkwo, D.O.; Kanter, A.S. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg. Focus 2010, 28, E7. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Guo, L.; Zhang, F.; Ding, W.; Zhang, W. Efficacy and safety of a modified lateral lumbar interbody fusion in L4-5 lumbar degenerative diseases compared with traditional XLIF and OLIF: A retrospective cohort study of 156 cases. BMC Musculoskelet. Disord. 2022, 23, 217. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Katoh, H.; Sakai, D.; Tanaka, M.; Sato, M.; Watanabe, M. Short-term comparison of preoperative and postoperative pain after indirect decompression surgery and direct decompression surgery in patients with degenerative spondylolisthesis. Sci. Rep. 2020, 10, 18887. [Google Scholar] [CrossRef] [PubMed]
- Yingsakmongkol, W.; Jitpakdee, K.; Varakornpipat, P.; Choentrakool, C.; Tanasansomboon, T.; Limthongkul, W.; Singhatanadgige, W.; Kotheeranurak, V. Clinical and Radiographic Comparisons among Minimally Invasive Lumbar Interbody Fusion: A Comparison with Three-Way Matching. Asian Spine J. 2022, 16, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Pojskic, M.; Saβ, B.; Völlger, B.; Nimsky, C.; Carl, B. Extreme lateral interbody fusion (XLIF) in a consecutive series of 72 patients. Bosn. J. Basic Med. Sci. 2021, 21, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, E.; Wang, L.; Zou, X.; Deng, C.; Chen, J.; Ma, R.; Ma, X.; Wu, Z. Extreme lateral interbody fusion (XLIF) approach for L5-S1: Preliminary experience. Front. Surg. 2022, 9, 995662. [Google Scholar] [CrossRef] [PubMed]
- Paterakis, K.N.; Brotis, A.G.; Paschalis, A.; Tzannis, A.; Fountas, K.N. Extreme lateral lumbar interbody fusion (XLIF) in the management of degenerative scoliosis: A retrospective case series. J. Spine Surg. 2018, 4, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.M.; McDonald, E.L.; Shakked, R.J.; Fuchs, D.; Raikin, S.M. Determination of Minimum Clinically Important Difference (MCID) in Visual Analog Scale (VAS) Pain and Foot and Ankle Ability Measure (FAAM) Scores After Hallux Valgus Surgery. Foot Ankle Int. 2009, 40, 687–693. [Google Scholar] [CrossRef]
- Copay, A.G.; Glassman, S.D.; Subach, B.R.; Berven, S.; Schuler, T.C.; Carreon, L.Y. Minimum clinically important difference in lumbar spine surgery patients: A choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008, 8, 968–974. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Schonauer, C.; Stienen, M.N.; Gautschi, O.P.; Schaller, K.; Tessitore, E. Endoscope-Assisted Extreme-Lateral Interbody Fusion: Preliminary Experience and Technical Note. World Neurosurg. 2017, 103, 869–875.e3. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, E.; Molliqaj, G.; Schaller, K.; Gautschi, O.P. Extreme lateral interbody fusion (XLIF): A single-center clinical and radiological follow-up study of 20 patients. J. Clin. Neurosci. 2016, 36, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Malham, G.M.; Ellis, N.J.; Parker, R.M.; Blecher, C.M.; White, R.; Goss, B.; Seex, K.A. Maintenance of Segmental Lordosis and Disk Height in Stand-alone and Instrumented Extreme Lateral Interbody Fusion (XLIF). Clin. Spine Surg. 2017, 30, E90–E98. [Google Scholar] [CrossRef] [PubMed]
- Timothy, J.; Pal, D.; Akhunbay-Fudge, C.; Knights, M.; Frost, A.; Derham, C.; Selvanathan, S. Extreme lateral interbody fusion (XLIF) as a treatment for acute spondylodiscitis: Leeds spinal unit experience. J. Clin. Neurosci. 2019, 59, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Sun, Y.; Zhang, F.; Gao, Y.; Li, Z.; Ding, W.; Shen, Y.; Zhang, W. Safety Analysis of Two Anterior Lateral Lumbar Interbody Fusions at the Initial Stage of Learning Curve. World Neurosurg. 2019, 127, e901–e909. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, K.; Shen, A.; Lagina, M.; Hutchison, A. Comparison of clinical outcomes following minimally invasive lateral interbody fusion stratified by preoperative diagnosis. Eur. Spine J. 2015, 24 (Suppl. S3), 322–330. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Ohmori, K.; Hori, T. Clinical and Radiological Outcomes of Corrective Surgery on Adult Spinal Deformity Patients: Comparison of Short and Long Fusion. Adv. Orthop. 2019, 2019, 9492486. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Huang, M.G.; Ou, D.Q.; Xu, Y.C.; Dong, J.W.; Yin, H.D.; Chen, W.; Rong, L.M. One-stage extreme lateral interbody fusion and percutaneous pedicle screw fixation in lumbar spine tuberculosis. J. Musculoskelet. Neuronal Interact. 2017, 17, 450–455. [Google Scholar]
- Rodgers, W.B.; Gerber, E.J.; Patterson, J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: An analysis of 600 cases. Spine 2011, 36, 26–32. [Google Scholar] [CrossRef]
- Malham, G.M.; Ellis, N.J.; Parker, R.M.; Seex, K.A. Clinical outcome and fusion rates after the first 30 extreme lateral interbody fusions. Sci. World J. 2012, 2012, 246989. [Google Scholar] [CrossRef]
- Tohmeh, A.G.; Khorsand, D.; Watson, B.B.; Zielinski, X. Radiographical and clinical evaluation of extreme lateral interbody fusion: Effects of cage size and instrumentation type with a minimum of 1-year follow-up. Spine 2014, 39, E1582–E1591. [Google Scholar] [CrossRef]
- Smith, W.D.; Youssef, J.A.; Christian, G.; Serrano, S.; Hyde, J.A. Lumbarized sacrum as a relative contraindication for lateral transpsoas interbody fusion at L5-6. J. Spinal Disord. Tech. 2012, 25, 285–291. [Google Scholar] [CrossRef]
- Formica, M.; Berjano, P.; Cavagnaro, L.; Zanirato, A.; Piazzolla, A.; Formica, C. Extreme lateral approach to the spine in degenerative and post traumatic lumbar diseases: Selection process, results and complications. Eur. Spine J. 2014, 23, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Taneichi, H. Update on pathology and surgical treatment for adult spinal deformity. J. Orthop. Sci. 2016, 21, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.; Patel, A.; Ungar, B.; Farcy, J.P.; Lafage, V. Adult spinal deformity-postoperative standing imbalance: How much can you tolerate? an overview of key parameters in assessing alignment and planning corrective surgery. Spine 2010, 35, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Marchi, L.; Coutinho, E.; Pimenta, L.A. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine 2010, 35 (Suppl. S26), S331–S337. [Google Scholar] [CrossRef] [PubMed]
- Alvi, M.A.; Alkhataybeh, R.; Wahood, W.; Kerezoudis, P.; Goncalves, S.; Murad, M.H.; Bydon, M. The impact of adding posterior instrumentation to transpsoas lateral fusion:A systematic review and meta-analysis. J. Neurosurg. Spine 2018, 30, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, G.; Nuñez, J.H.; Barrios, C.; Domenech-Fernández, P. A meta-analysis of bone morphogenetic protein-2 versus iliac crest bone graft for the posterolateral fusion of the lumbar spine. J. Bone Miner. Metab. 2020, 38, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, F.R.; Sherman, J.H.; Holmberg, A.; Louis, R.; Elias, J.; Vega-Bermudez, F. Protecting the genitofemoral nerve during direct/extreme lateral interbody fusion (DLIF/XLIF) procedures. Am. J. Electroneurodiagn. Technol. 2010, 50, 321–335. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BML 2021, 372, n71. [Google Scholar] [CrossRef]

| Study | Region | Period | n | Type of Study | Surgeon | Age | % Female | Fusion ≥ 2 Segments | BMI |
|---|---|---|---|---|---|---|---|---|---|
| Goodnough et al., 2019 [7] | USA | 2008 to 2012 | 21 | Retrospective cohort | Fellows | 65.7 | 66.6% | 26.3% | 28.6 |
| Hiyama et al., 2020 [14] | Japan | 2016 to 2019 | 62 | Retrospective cohort | - | 70.2 | 40.3% | 30.6% | - |
| Khajavi et al., 2015 [28] | USA | 2008 to 2012 | 187 | Retrospective cohort | - | 61.0 | 66.0% | - | - |
| Li et al., 2019 [27] | China | - | 30 | Retrospective cohort | - | 58.4 | 60.0% | 20.0%% | - |
| Li et al., 2022 [13] | China | 2017 to 2019 | 51 | Retrospective cohort | - | 56.7 | 62.7% | - | - |
| Malham et al., 2012 [32] | Australia | 2011 | 30 | Case series | - | 62.7 | 66.7% | - | 26.7 |
| Malham et al., 2017 [25] | Australia | 2011 to 2012 | 40 | Retrospective cohort | Expert | 63.5 | 70.0% | 32.5% | 26.9 |
| Ono et al., 2019 [29] | Japan | 2014 to 2017 | 21 | Retrospective cohort | - | 71.1 | 81.0% | 71.4% | - |
| Paterakis et al., 2018 [18] | Greece | 2008 to 2017 | 12 | Case series | - | 64.5 | 100.0% | 83.3% | - |
| Pojskić et al., 2021 [16] | Germany | 2010 to 2018 | 72 | Case series | - | 66.6 | 44.4% | 33.3% | - |
| Rodgers et al., 2011 [31] | USA | Since 2006 | 600 | Retrospective cohort | Senior | 61.4 | 62.0% | - | 31.1 |
| Schonauer et al., 2017 [23] | Switzerland | 2014 to 2016 | 41 | Retrospective cohort | - | 67.4 | 45.7% | 14.3% | 27.6 |
| Tessitore et al., 2016 [24] | Switzerland | 2014 to 2015 | 20 | Case series | - | 67.4 | 50.0% | 10.0% | 27.7 |
| Timothy et al., 2019 [26] | UK | 2008 to 2011 | 14 | Case series | - | 51.0 | NA | 14.3% | - |
| Tohmeh et al., 2014 [33] | USA | 2008 to 2012 | 140 | Prospective cohort | - | 60.7 | 44.6% | - | 29.1 |
| Tormenti et al., 2010 [12] | USA | 2007 to 2009 | 8 | Retrospective cohort | - | 60.0 | - | - | - |
| Wang et al., 2016 [30] | China | 2012 to 2014 | 22 | Case series | Experienced | - | 45.5% | - | - |
| Xu et al., 2022 [17] | China | - | 8 | Case series | Experienced | 60.4 | 87.6% | 50.0% | - |
| Yingsakmongkol et al., 2022 [15] | Thailand | 2016 to 2019 | 30 | Retrospective cohort | Senior | 63.5 | 73.3% | - | - |
| Study | Clearly Stated Aim | Consecutive Patients | Prospective Collection Data | Endpoints | Assessment Endpoint | Follow-Up Period | Loss Less than 5% | Study Size | Adequate Control Group | Contemporary Group | Baseline Control | Statistical Analyses | Minors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Goodnough et al., 2019 [7] | 2 | 2 | 0 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 21 |
| Hiyama et al., 2020 [14] | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 23 |
| Khajavi et al., 2015 [28] | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 22 |
| Li et al., 2019 [27] | 2 | 1 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 21 |
| Li et al., 2022 [13] | 1 | 2 | 0 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 20 |
| Malham et al., 2012 [32] | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | - | - | - | - | 15 |
| Malham et al., 2017 [25] | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 21 |
| Ono et al., 2019 [29] | 2 | 2 | 0 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 20 |
| Paterakis et al., 2018 [18] | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | - | - | - | - | 12 |
| Pojskić et al., 2021 [16] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | - | - | - | - | 14 |
| Rodgers et al., 2011 [31] | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | - | - | - | - | 11 |
| Schonauer et al., 2017 [23] | 1 | 2 | 0 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 20 |
| Tessitore et al., 2016 [24] | 1 | 2 | 0 | 2 | 2 | 2 | 1 | 1 | - | - | - | - | 11 |
| Timothy et al., 2019 [26] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 1 | - | - | - | - | 13 |
| Tohmeh et al., 2014 [33] | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 1 | 2 | 18 |
| Tormenti et al., 2010 [12] | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | - | - | - | - | 13 |
| Wang et al., 2016 [30] | 1 | 2 | 0 | 2 | 2 | 1 | 2 | 1 | - | - | - | - | 11 |
| Xu et al., 2022 [17] | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 0 | - | - | - | - | 10 |
| Yingsakmongkol et al., 2022 [15] | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 22 |
| Study | LOS (Days) | BL (mL) | OR Time (min) |
|---|---|---|---|
| Hiyama et al., 2020 [14] | - | 84.4 | 109.9 |
| Li et al., 2019 [27] | - | 122.7 | 106.2 |
| Li et al., 2022 [13] | - | 63.7 | 85.0 |
| Paterakis et al., 2018 [18] | - | 102.0 | 118.0 |
| Schonauer et al., 2017 [23] | - | 528.0 | 241.0 |
| Wang et al., 2016 [30] | 25.8 | 249.8 | 347.5 |
| Xu et al., 2022 [17] | 7.5 | 240.0 | 311.4 |
| Yingsakmongkol et al., 2022 [15] | 3.6 | 49.17 | 144.0 |
| Fusion Rate | 6 Months | 9 Months | 12 Months |
|---|---|---|---|
| Li et al., 2019 [27] | 63.3% | - | 93.3% |
| Li et al., 2022 [13] | - | - | 92.2% |
| Malham et al., 2012 [32] | 46.0% | 58.0% | 85.0% |
| Malham et al., 2017 [25] | 36.1% | 53.5% | 87.6% |
| Complications | n | % |
|---|---|---|
| Retroperitoneal hematoma | 1 | 0.08 |
| Hardware failure * | 24 | 1.92 |
| Wound problems | 14 | 1.12 |
| Adjacent segment disease | 17 | 1.36 |
| Numbness | 1 | 0.08 |
| Transient hypoesthesia | 3 | 0.24 |
| Permanent hypoesthesia | 1 | 0.08 |
| Transient pain | 23 | 1.84 |
| Transient neurologic conditions | 27 | 2.17 |
| Pleural tear | 3 | 0.24 |
| Dural tear | 6 | 0.48 |
| Nerve root injury ** | 12 | 0.96 |
| Gastrointestinal impairment † | 9 | 0.72 |
| Vertebral infection | 2 | 0.16 |
| Endplate injury | 4 | 0.32 |
| Psoas weak | 1 | 0.08 |
| Pneumonia | 6 | 0.48 |
| Infarction | 2 | 0.16 |
| Urinary retention | 4 | 0.32 |
| Urinary incontinence | 1 | 0.08 |
| Anemia requiring transfusion | 4 | 0.32 |
| Vertebral fracture †† | 8 | 0.64 |
| Deep vein thrombosis | 1 | 0.08 |
| Pulmonary embolism | 3 | 0.24 |
| Atrial fibrillation | 5 | 0.40 |
| Peritoneal catheter | 1 | 0.08 |
| Bowel injury | 2 | 0.16 |
| Meningitis | 1 | 0.08 |
| Hemodynamic instability | 2 | 0.16 |
| Cerebrospinal fluid leak | 1 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios, P.; Palacios, I.; Palacios, A.; Gutiérrez, J.C.; Mariscal, G.; Lorente, A. Efficacy and Safety of the Extreme Lateral Interbody Fusion (XLIF) Technique in Spine Surgery: Meta-Analysis of 1409 Patients. J. Clin. Med. 2024, 13, 960. https://doi.org/10.3390/jcm13040960
Palacios P, Palacios I, Palacios A, Gutiérrez JC, Mariscal G, Lorente A. Efficacy and Safety of the Extreme Lateral Interbody Fusion (XLIF) Technique in Spine Surgery: Meta-Analysis of 1409 Patients. Journal of Clinical Medicine. 2024; 13(4):960. https://doi.org/10.3390/jcm13040960
Chicago/Turabian StylePalacios, Pablo, Isabel Palacios, Ana Palacios, Juan Carlos Gutiérrez, Gonzalo Mariscal, and Alejandro Lorente. 2024. "Efficacy and Safety of the Extreme Lateral Interbody Fusion (XLIF) Technique in Spine Surgery: Meta-Analysis of 1409 Patients" Journal of Clinical Medicine 13, no. 4: 960. https://doi.org/10.3390/jcm13040960
APA StylePalacios, P., Palacios, I., Palacios, A., Gutiérrez, J. C., Mariscal, G., & Lorente, A. (2024). Efficacy and Safety of the Extreme Lateral Interbody Fusion (XLIF) Technique in Spine Surgery: Meta-Analysis of 1409 Patients. Journal of Clinical Medicine, 13(4), 960. https://doi.org/10.3390/jcm13040960







