State-of-the-Art of Transcatheter Left Atrial Appendage Occlusion
Abstract
1. Patient Selection
1.1. Introduction
1.2. Left Atrial Appendage Occlusion for AF-Related Stroke Prevention
1.3. LAAO-Clinical Indications
1.4. Patients with Previous Spontaneous Intracerebral and Intracranial Hemorrhage
1.5. Patients at High-Risk of Intracranial Hemorrhage
1.6. Patients with Previous Major Gastrointestinal Bleeding
1.7. Patients with End-Stage Renal Disease or on Haemodialysis
- Hematologic disorders with a relative contraindication to antithrombotic therapy, such as myelodysplastic disorders, thrombocytopenia or hemophilia [38].
- Those patients for whom prolonged (and even double) antiplatelet therapy would be advisable, such as those with multiple coronary stents, multiple previous recurrent acute coronary syndromes or cerebral events secondary to carotid disease. Here, the combination of oral anticoagulation and antiplatelet therapy might result in a too-high long-term bleeding risk. In this specific category of patients, careful balancing risk factors and a multidisciplinary discussion including patient’s preference should all be taken into account when considering stroke prevention strategies.
- Recurrent stroke despite optimal anticoagulation. The potential indication for LAAO is represented by patients who experienced an ischemic stroke despite optimal anticoagulant therapy, then other plausible causes (e.g., carotid disease, severe mobile aortic arch atheromata) are excluded. ESC guidelines in this context recommend optimization of anticoagulant therapy [39] while adding an antiplatelet agent to OAC is another practice that may be encountered in the clinical arena, even if there are no available data supporting this approach. Seiffge et al. showed that the risk of stroke recurrence was very high (8–9%/yr) and a change of OAC strategy did not change the risk of stroke recurrence [38]. LAAO may be considered in this population as an alternative stroke preventive measure. Observational propensity-matched studies suggest a significantly lower risk of the composite outcome of stroke, major bleeding and all-cause mortality with LAAO therapy compared to DOAC but warrants confirmation in randomized trials [20,40]. Currently, two randomized clinical trials enroll patients within this category and compare outcomes to DOAC therapy; the OCCLUSION-AF trial (NCT03642509) randomizing between DOAC and LAAO combined with long-term SAPT, and the ELAN trial (NCT05976685) randomizing between DOAC and LAAO combined with continued DOAC-therapy.
- Non-compliant patients, including those not willing to take medications at all, those subjects with a specific lifestyle or profession leading to no or irregular drug intake or those presenting with non-compliance despite multiple measures to improve this. In any case, according to ESC guidelines, these patients should be strongly encouraged to take DOACs, which still represent the first option.
2. Contemporary Devices
2.1. The Watchman Device
2.2. Amplatzer Devices
2.3. Ultraseal Device
2.4. OMEGA Device
2.5. Lambre Device
2.6. CLAAS Device
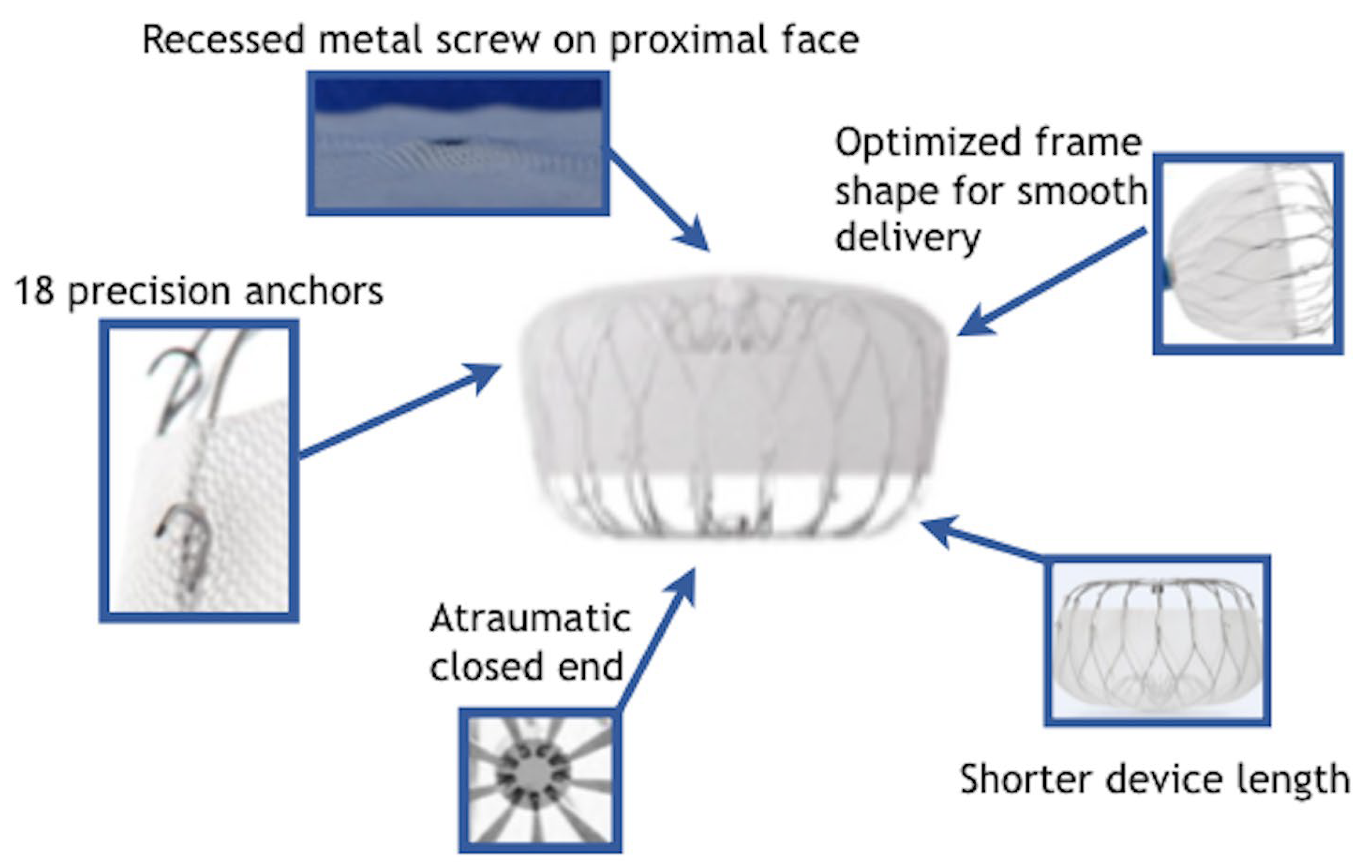
2.7. New Devices
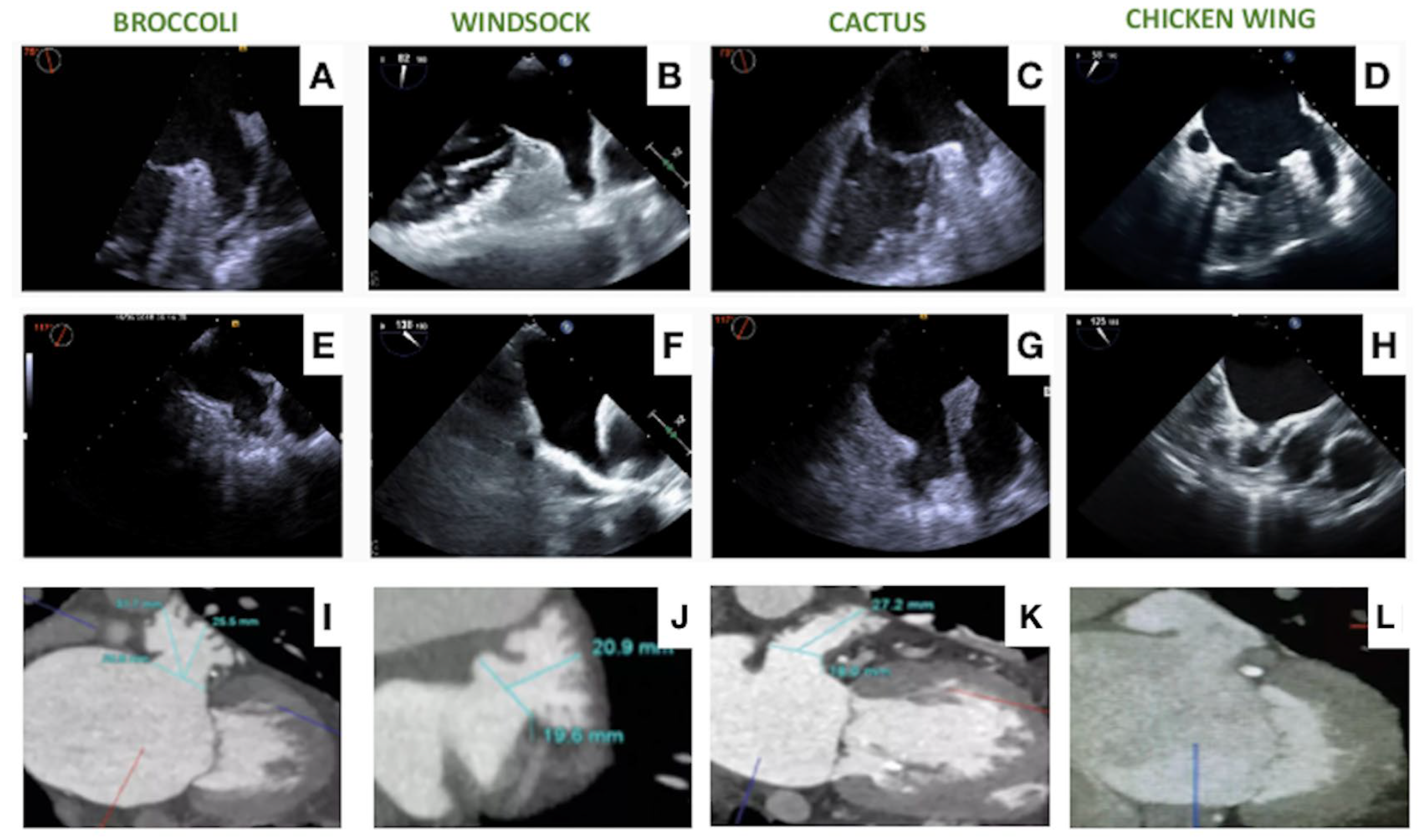
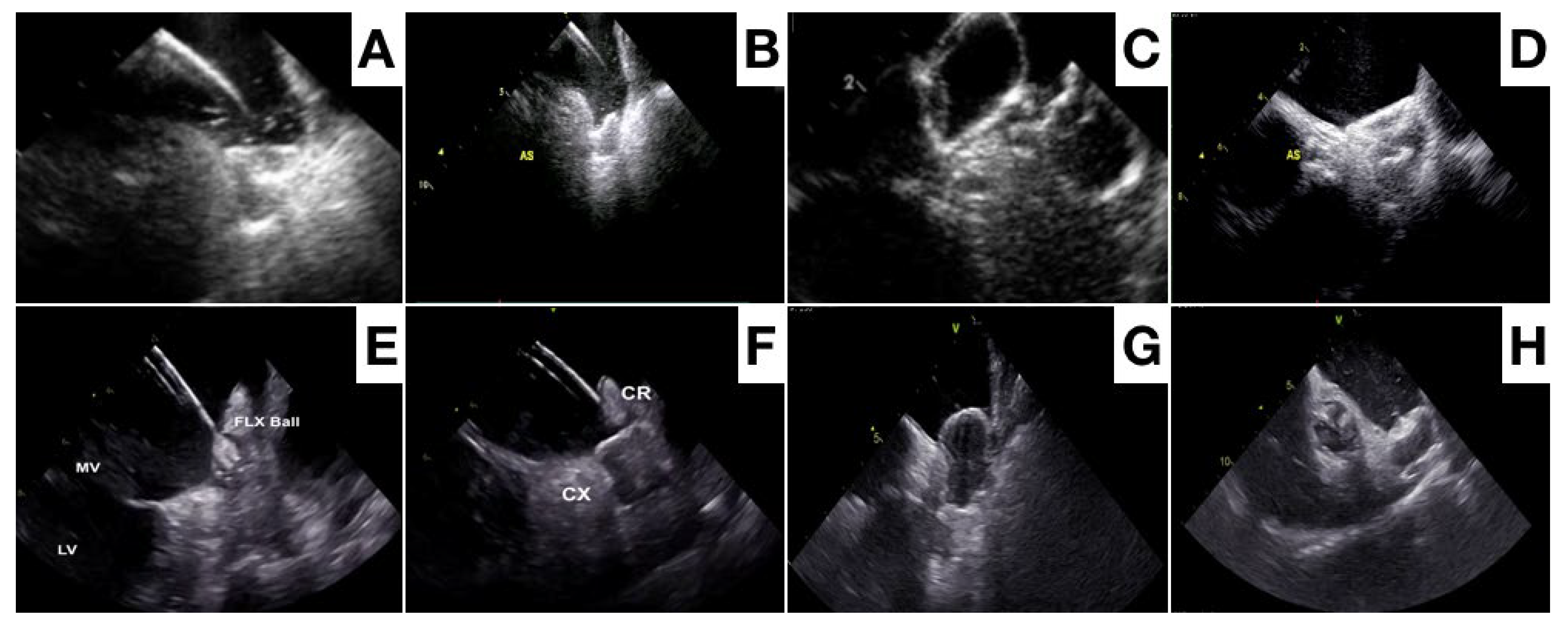
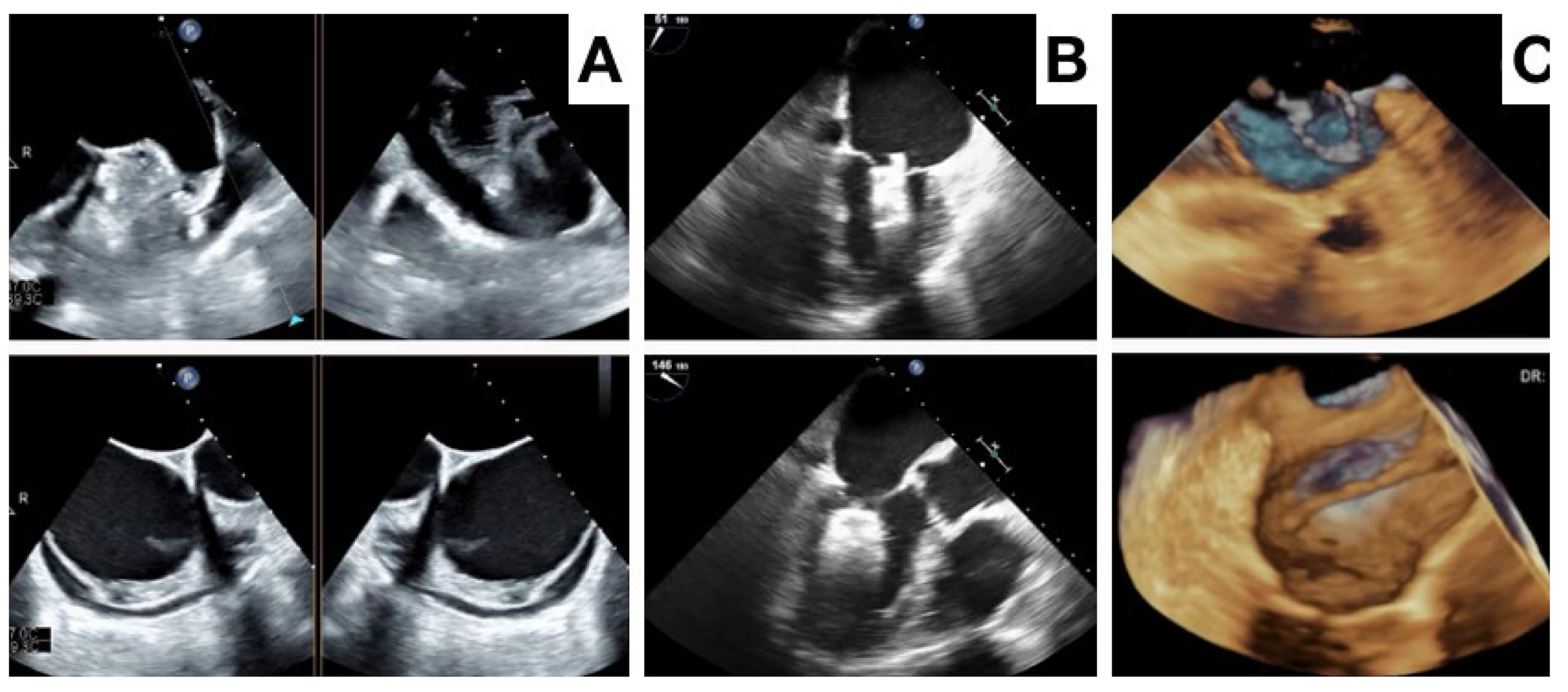
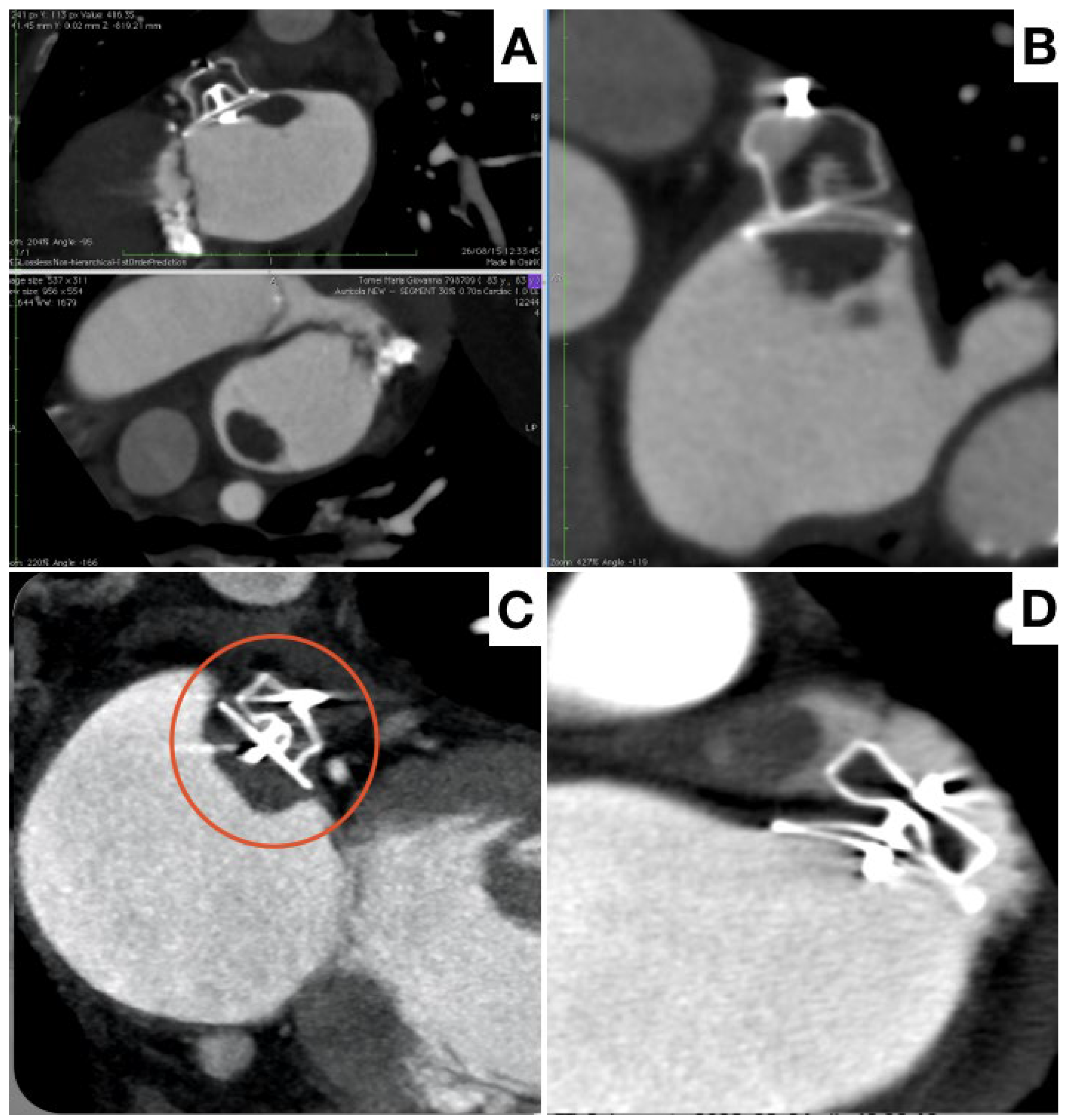
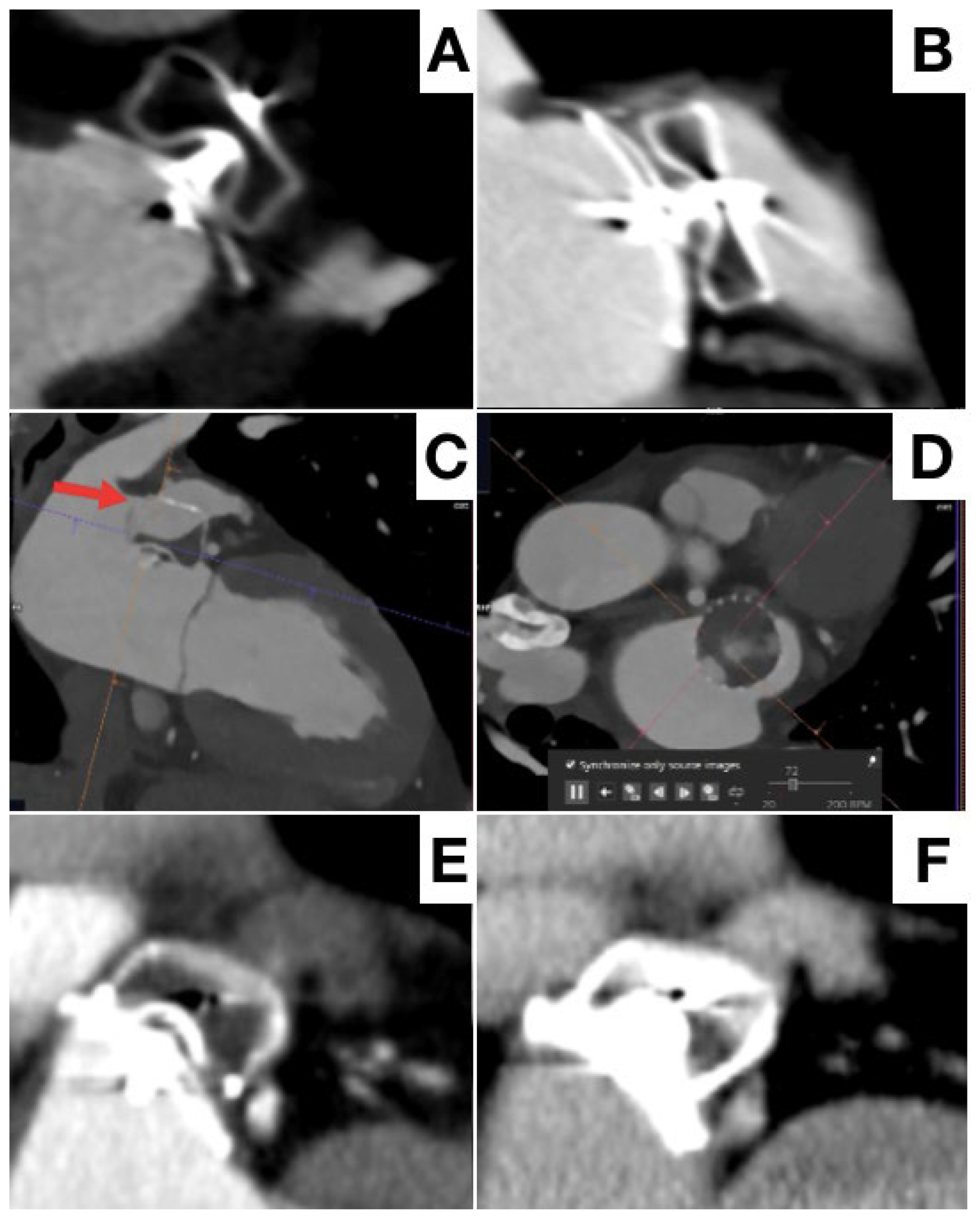
3. Procedural Planning and Execution
4. Follow-Up
5. Final Considerations
Author Contributions
Funding
Conflicts of Interest
References
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The anticoagulation and risk factors in atrial fibrillation (ATRIA) Study. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef]
- Flegel, K.M.; Shipley, M.J.; Rose, G. Risk of stroke in non-rheumatic atrial fibrillation. Lancet 1987, 1, 526–529. [Google Scholar] [CrossRef]
- Hart, R.G.; Pearce, L.A.; Aguilar, M.I. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007, 146, 857–867. [Google Scholar] [CrossRef]
- O’Brien, E.C.; Holmes, D.N.; Ansell, J.E.; Allen, L.A.; Hylek, E.; Kowey, P.R.; Gersh, B.J.; Fonarow, G.C.; Koller, C.R.; Ezekowitz, M.D.; et al. Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: Findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am. Heart J. 2014, 167, 601–609.e1. [Google Scholar] [CrossRef]
- Steinberg, B.A.; Greiner, M.A.; Hammill, B.G.; Curtis, L.H.; Benjamin, E.J.; Heckbert, S.R.; Piccini, J.P. Contraindications to anticoagulation therapy and eligibility for novel anticoagulants in older patients with atrial fibrillation. Cardiovasc. Ther. 2015, 33, 177–183. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. NEJM 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. NEJM 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. NEJM 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. NEJM 2013, 369, 20193–22104. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, A.K.; Mueller, I.; Bassand, J.P.; Fitzmaurice, D.A.; Goldhaber, S.Z.; Goto, S.; Haas, S.; Hacke, W.; Lip, G.Y.; Mantovani, L.G.; et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: Perspectives from the international, observational, prospective GARFIELD registry. PLoS ONE 2013, 8, e63479. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R.; Reddy, V.Y.; Turi, Z.G.; Doshi, S.K.; Sievert, H.; Buchbinder, M.; Mullin, C.M.; Sick, P. PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet 2009, 374, 534–542. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014, 130, e199–e267. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 3122–3135. [Google Scholar] [CrossRef]
- Boersma, L.V.; Ince, H.; Kische, S.; Pokushalov, E.; Schmitz, T.; Schmidt, B.; Gori, T.; Meincke, F.; Protopopov, A.V.; Betts, T.; et al. Evaluating real-world clinical outcomes in atrial fibrillation patients receiving the WATCHMAN left atrial appendage closure technology. Circ. Arrhythm. Electrophysiol. 2019, 12, e006841. [Google Scholar] [CrossRef]
- Tzikas, A.; Shakir, S.; Gafoor, S.; Omran, H.; Berti, S.; Santoro, G.; Kefer, J.; Landmesser, U.; Nielsen-Kudsk, J.E.; Cruz-Gonzalez, I.; et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: Multicentre experience with the AMPLATZER cardiac plug. EuroIntervention 2016, 11, 1170–1179. [Google Scholar] [CrossRef]
- Korsholm, K.; Valentin, J.B.; Damgaard, D.; Diener, H.C.; Camm, A.J.; Landmesser, U.; Hildick-Smith, D.; Johnsen, S.P.; Nielsen-Kudsk, J.E. Clinical outcomes of left atrial appendage occlusion versus direct oral anticoagulation in patients with atrial fibrillation and prior ischemic stroke: A propensity-score matched study. Int. J. Cardiol. 2022, 363, 56–63. [Google Scholar] [CrossRef]
- Nielsen-Kudsk, J.E.; Korsholm, K.; Damgaard, D.; Valentin, J.B.; Diener, H.C.; Camm, A.J.; Johnsen, S.P. Clinical Outcomes Associated With Left AtrialAppendageOcclusion Versus Direct OralAnticoagulation in AtrialFibrillation. JACC Cardiovasc. Interv. 2021, 14, 69–78. [Google Scholar] [CrossRef]
- Price, M.J.; Slotwiner, D.; Du, C.; Freeman, J.V.; Turi, Z.; Rammohan, C.; Kusumoto, F.M.; Kavinsky, C.; Akar, J.; Varosy, P.D.; et al. Clinical Outcomesat 1 Year Following Transcatheter Left AtrialAppendageOcclusion in the United States. JACC Cardiovasc. Interv. 2022, 15, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.J.; Du, C.; Wang, Y.; Agarwal, V.; Varosy, P.D.; Masoudi, F.A.; Holmes, D.R.; Reddy, V.Y.; Price, M.J.; Curtis, J.P.; et al. Patient-Level Analysis of Watchman Left AtrialAppendageOcclusion in Practice Versus Clinical Trials. JACC Cardiovasc. Interv. 2022, 15, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Bonde, A.N.; Blanche, P.; Staerk, L.; Gerds, T.A.; Gundlund, A.; Gislason, G.; Torp-Pedersen, C.; Lip, G.Y.H.; Hlatky, M.A.; Olesen, J.B. Oralanticoagulationamongatrialfibrillationpatients with anaemia: An observationalcohort study. Eur. Heart J. 2019, 40, 3782–3790. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.B.; Skjøth, F.; Nielsen, P.B.; Kjældgaard, J.N.; Lip, G.Y. Comparative effectiveness and safety of non-vitamin K antagonistoralanticoagulants and warfarin in patients with atrial fibrillation: Propensity weighted nationwide cohort study. BMJ 2016, 353, i3189. [Google Scholar] [CrossRef] [PubMed]
- Van den Ham, H.A.; Souverein, P.C.; Klungel, O.H.; Platt, R.W.; Ernst, P.; Dell’Aniello, S.; Schmiedl, S.; Grave, B.; Rottenkolber, M.; Huerta, C.; et al. Major bleeding in users of direct oral anticoagulants in atrial fibrillation: A pooled analysis of results from multiple population-based cohort studies. Pharmacoepidemiol. Drug Saf. 2021, 30, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Shatla, I.; El-Zein, R.S.; Kennedy, K.; Elkaryoni, A.; Ubaid, A.; Wimmer, A.P. Comparison of the Safety of Left Atrial Appendage Occlusion in Patients Aged <75 Versus Those Aged ≥75 Years (from a Nationwide Cohort Sample). Am. J. Cardiol. 2022, 172, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Nielsen-Kudsk, J.E.; Johnsen, S.P.; Wester, P.; Damgaard, D.; Airaksinen, J.; Lund, J.; De Backer, O.; Pakarinen, S.; Odenstedt, J.; Vikman, S.; et al. Left atrial appendage occlusion versus standard medical care in patients with atrial fibrillation and intracerebral haemorrhage: A propensity score-matched follow-up study. EuroIntervention 2017, 13, 371–378. [Google Scholar] [CrossRef]
- Schrag, M.; Mac Grory, B.; Nackenoff, A.; Eaton, J.; Mistry, E.; Kirshner, H.; Yaghi, S.; Ellis, C.R. Left Atrial Appendage Closure for Patients with Cerebral Amyloid Angiopathy and Atrial Fibrillation: The LAA-CAA Cohort. Transl. Stroke Res. 2021, 12, 259–265. [Google Scholar] [CrossRef]
- Revesz, T.; Holton, J.L.; Lashley, T.; Plant, G.; Rostagno, A.; Ghiso, J.; Frangione, B. Sporadic and familial cerebral amyloid angiopathies. Brain Pathol. 2002, 12, 343–357. [Google Scholar] [CrossRef]
- Triantafyllou, K.; Gkolfakis, P.; Gralnek, I.M.; Oakland, K.; Manes, G.; Radaelli, F.; Awadie, H.; Camus Duboc, M.; Christodoulou, D.; Fedorov, E.; et al. Diagnosis and management of acute lowergastrointestinalbleeding: European Society of GastrointestinalEndoscopy (ESGE) Guideline. Endoscopy 2021, 53, 850–868. [Google Scholar]
- Lempereur, M.; Aminian, A.; Freixa, X.; Gafoor, S.; Shakir, S.; Omran, H.; Berti, S.; Santoro, G.; Kefer, J.; Landmesser, U.; et al. Left atrial appendage occlusion in patients with atrial fibrillation and previous major gastrointestinal gleeding (from the Amplatzer Cardiac Plug Multicenter Registry). Am. J. Cardiol. 2017, 120, 414–420. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, A.S.; Caluwé, R.; Pyfferoen, L.; De Bacquer, D.; De Boeck, K.; Delanote, J.; De Surgeloose, D.; Van Hoenacker, P.; Van Vlem, B.; Verbeke, F. Multicenter Randomized Controlled Trial of Vitamin K Antagonist Replacement by Rivaroxaban with or without Vitamin K2 in Hemodialysis Patients with Atrial Fibrillation: The Valkyrie Study. J. Am. Soc. Nephrol. 2020, 31, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Pokorney, S.D.; Chertow, G.M.; Al-Khalidi, H.R.; Gallup, D.; Dignacco, P.; Mussina, K.; Bansal, N.; Gadegbeku, C.A.; Garcia, D.A.; Garonzik, S.; et al. Apixaban for Patients With Atrial Fibrillation on Hemodialysis: A Multicenter Randomized Controlled Trial. Circulation 2022, 146, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, H.; Engelbertz, C.; Bauersachs, R.; Breithardt, G.; Echterhoff, H.H.; Gerß, J.; Haeusler, K.G.; Hewing, B.; Hoyer, J.; Juergensmeyer, S.; et al. A Randomized Controlled Trial Comparing Apixaban With the Vitamin K Antagonist Phenprocoumon in Patients on Chronic Hemodialysis: The AXADIA-AFNET 8 Study. Circulation 2023, 147, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Luani, B.; Genz, C.; Herold, J.; Mitrasch, A.; Mitusch, J.; Wiemer, M.; Schmeißer, A.; Braun-Dullaeus, R.C.; Rauwolf, T. Cerebrovascular events, bleeding complications and device related thrombi in atrial fibrillation patients with chronic kidney disease and left atrial appendage closure with the WATCHMAN™ device. BMC Cardiovasc. Disord. 2019, 19, 112. [Google Scholar] [CrossRef]
- Kramer, A.D.; Korsholm, K.; Kristensen, A.; Poulsen, L.H.; Nielsen-Kudsk, J.E. Left atrialappendageocclusion in haemophilia patients with atrial fibrillation. J. Interv. Card. Electrophysiol. 2022, 64, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Seiffge, D.J.; De Marchis, G.M.; Koga, M.; Paciaroni, M.; Wilson, D.; Cappellari, M.; Macha Md, K.; Tsivgoulis, G.; Ambler, G.; Arihiro, S.; et al. RAF, RAF-DOAC, CROMIS-2, SAMURAI, NOACISP, Erlangen, and Verona registrycollaborators. Ischemic Stroke despiteOralAnticoagulant Therapy in Patients with AtrialFibrillation. Ann. Neurol. 2020, 87, 677–687. [Google Scholar] [CrossRef]
- Freixa, X.; Cruz-González, I.; Regueiro, A.; Nombela-Franco, L.; Estévez-Loureiro, R.; Ruiz-Salmerón, R.; Bethencourt, A.; Gutiérrez-García, H.; Fernández-Díaz, J.A.; Moreno-Samos, J.C.; et al. Left Atrial Appendage Occlusion as Adjunctive Therapy to Anticoagulation for Stroke Recurrence. J. Invasive Cardiol. 2019, 31, 212–216. [Google Scholar]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.H.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—An update. EuroIntervention 2020, 15, 1133–1180. [Google Scholar] [CrossRef] [PubMed]
- Marzec, L.N.; Wang, J.; Shah, N.D.; Chan, P.S.; Ting, H.H.; Gosch, K.L.; Hsu, J.C.; Maddox, T.M. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J. Am. Coll. Cardiol. 2017, 69, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Boersma, L.V.; Ince, H.; Kische, S.; Pokushalov, E.; Schmitz, T.; Schmidt, B.; Gori, T.; Meincke, F.; Protopopov, A.V.; Betts, T.; et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-Year follow-up outcome data of the EWOLUTION trial. Heart Rhythm 2017, 14, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Sievert, H.; Halperin, J.; Doshi, S.K.; Buchbinder, M.; Neuzil, P.; Huber, K.; Whisenant, B.; Kar, S.; Swarup, V.; et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: A randomized clinical trial. JAMA 2014, 312, 1988–1998. [Google Scholar] [CrossRef] [PubMed]
- Price, M.J.; Friedman, D.J.; Du, C.; Wang, Y.; Lin, Z.; Curtis, J.P.; Freeman, J.V. Comparative Safety of Transcatheter LAAO With the First-Generation Watchman and Next-Generation Watchman FLX Devices. J. Am. Coll. Cardiovasc. Interv. 2022, 15, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Doshi, S.K.; Sadhu, A.; Horton, R.; Osorio, J.; Ellis, C.; Stone, J., Jr.; Shah, M.; Dukkipati, S.R.; Adler, S.; et al. PrimaryOutcome Evaluation of a Next-Generation Left AtrialAppendageClosure Device: Results From the PINNACLE FLX Trial. Circulation 2021, 143, 1754–1762. [Google Scholar] [CrossRef]
- Saliba, W.I.; Kawai, K.; Sato, Y.; Kopesky, E.; Cheng, Q.; Ghosh, S.K.B.; Herbst, T.J.; Kawakami, R.; Konishi, T.; Virmani, R.; et al. Enhanced Thromboresistance and Endothelialization of a Novel Fluoropolymer-Coated Left Atrial Appendage Closure Device. JACC Clin. Electrophysiol. 2023, 9, 1555–1567. [Google Scholar] [CrossRef]
- Freeman, J.V.; Varosy, P.; Price, M.J.; Slotwiner, D.; Kusumoto, F.M.; Rammohan, C.; Kavinsky, C.J.; Turi, Z.G.; Akar, J.; Koutras, C.; et al. The NCDR Left AtrialAppendageOcclusionRegistry. J. Am. Coll. Cardiol. 2020, 75, 1503–1518. [Google Scholar] [CrossRef]
- Asmarats, L.; Rodes-Cabau, J. Percutaneous left atrial appendage closure: Current devices and clinical outcomes. Circ. Cardiovasc. Interv. 2017, 10, e005359. [Google Scholar] [CrossRef]
- Lakkireddy, D.; Thaler, D.; Ellis, C.R.; Swarup, V.; Sondergaard, L.; Carroll, J.; Gold, M.R.; Hermiller, J.; Diener, H.C.; Schmidt, B.; et al. Amplatzer amulet left atrial appendage occluder versus Watchman device for stroke prophylaxis (Amulet IDE): A randomized, controlled trial. Circulation 2021, 144, 1543–1552. [Google Scholar] [CrossRef]
- Galea, R.; De Marco, F.; Meneveau, N.; Aminian, A.; Anselme, F.; Gräni, C.; Huber, A.T.; Teiger, E.; Iriart, X.; Babongo Bosombo, F.; et al. Amulet or Watchman device for percutaneous left atrial appendage closure: Primary results of the SWISS-APERO randomized clinical trial. Circulation 2022, 145, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Pivato, C.A.; Liccardo, G.; Sanz-Sanchez, J.; Pelloni, E.; Pujdak, K.; Xuareb, R.G.; Cruz-Gonzalez, I.; Pisano, F.; Scotti, A.; Tarantini, G.; et al. Left atrial appendage closure with the II generation Ultraseal device: An international registry. The LIGATE study. Catheter. Cardiovasc. Interv. 2022, 100, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, B.; Srimahachota, S.; De Backer, O.; Boonyartavej, S.; Lertsuwunseri, V.; Tumkosit, M.; Søndergaard, L. First-in-human results of the Omega leftatrialappendage occluder for patients with non-valvularatrialfibrillation. EuroIntervention 2021, 17, e376–e379. [Google Scholar] [CrossRef] [PubMed]
- Chow, D.H.F.; Wong, Y.H.; Park, J.W.; Lam, Y.Y.; De Potter, T.; Rodés-Cabau, J.; Asmarats, L.; Sandri, M.; Sideris, E.; McCaw, T.; et al. An overview of current and emerging devices for percutaneous left atrial appendage closure. Trends Cardiovasc. Med. 2019, 29, 228–236. [Google Scholar] [CrossRef]
- De Backer, O.; Hafiz, H.; Fabre, A.; Lertsapcharoen, P.; Srimahachota, S.; Foley, D.; Sondergaard, L. State-of-the-art preclinical testing of the OMEGATM leftatrialappendage occluder. Catheter. Cardiovasc. Interv. 2021, 97, E1011–E1018. [Google Scholar] [CrossRef]
- Huang, H.; Liu, Y.; Xu, Y.; Wang, Z.; Li, Y.; Cao, K.; Zhang, S.; Yang, Y.; Yang, X.; Huang, D.; et al. Percutaneous left atrial appendage closure with the LAmbre device for stroke prevention in atrial fibrillation: A prospective, multicenter clinical study. JACC Cardiovasc. Interv. 2017, 10, 2188–2194. [Google Scholar] [CrossRef]
- Sommer, R.J.; Lamport, R.; Melanson, D.; Devellian, C.; Levine, A.; Cain, C.M.; Kaplan, A.V.; Gray, W.A. Preclinical assessment of a novel conformable foam-based left atrial appendage closure device. Biomed. Res. Int. 2021, 2021, 4556400. [Google Scholar] [CrossRef]
- Sommer, R.J.; Kim, J.H.; Szerlip, M.; Chandhok, S.; Sugeng, L.; Cain, C.; Kaplan, A.V.; Gray, W.A. Conformal Left Atrial Appendage Seal Device for Left Atrial Appendage Closure. J. Am. Coll. CardiolIntv. 2021, 14, 2368–2374. [Google Scholar] [CrossRef]
- Wong, G.X.; Kar, S.; Smith, T.W.; Spangler, T.; Bolling, S.F.; Rogers, J.H. Transcatheter Left Atrial Appendage Exclusion: Preclinical and Early Clinical Results With the Laminar Device. J. Am. Coll. Cardiol. Interv. 2023, 16, 1347–1357. [Google Scholar] [CrossRef]
- Bavishi, C. Transcatheter Left Atrial Appendage Closure: Devices Available, Pitfalls, Advantages, and Future Directions. US Cardiol. Rev. 2023, 17, e05. [Google Scholar] [CrossRef]
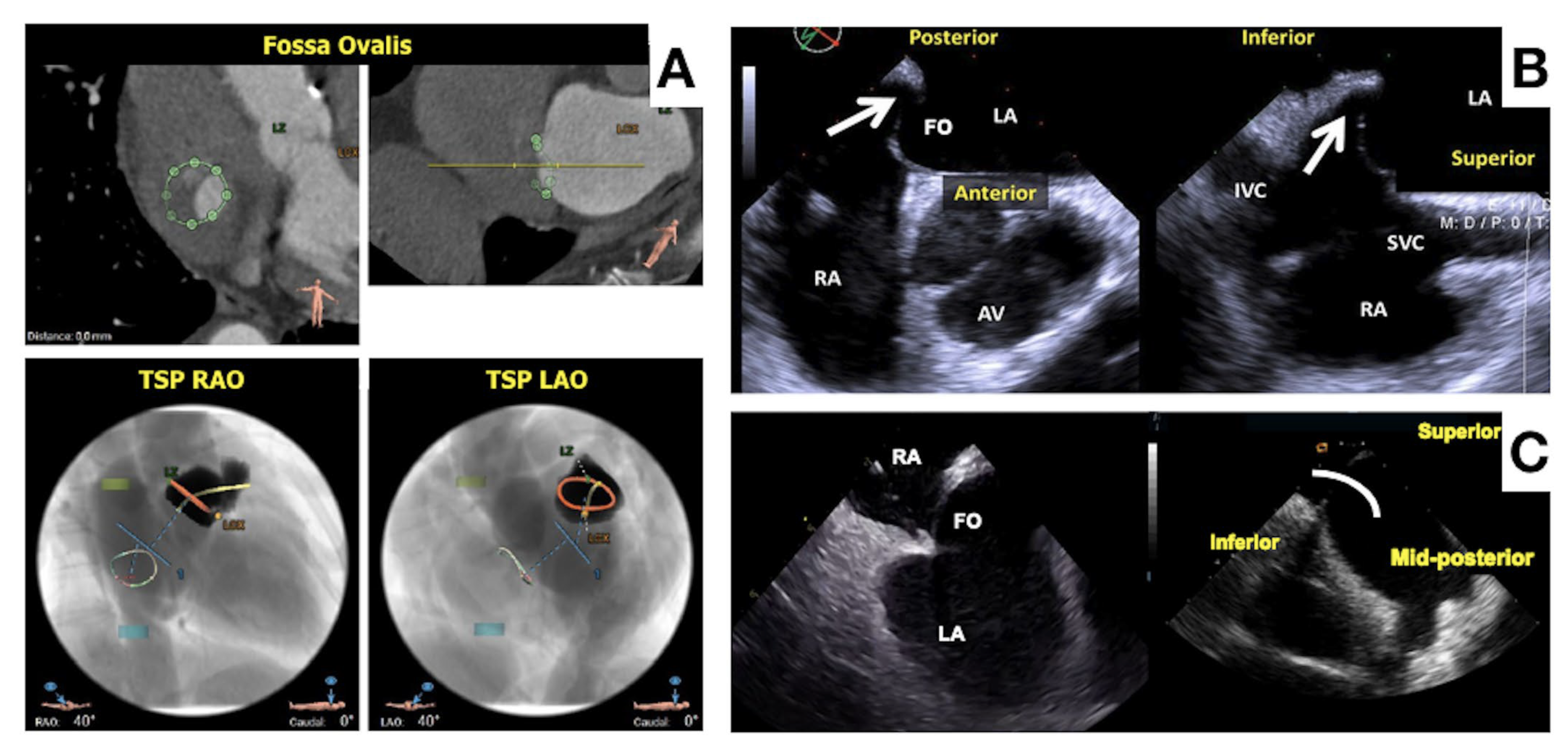
- Fukutomi, M.; Fuchs, A.; Bieliauskas, G.; Wong, I.; Kofoed, K.F.; Søndergaard, L.; De Backer, O. Computed tomography-based selection of transseptal puncture site for percutaneous left atrial appendage closure. EuroIntervention 2022, 17, e1435–e1444. [Google Scholar] [CrossRef]
- Wang, D.D.; Eng, M.; Kupsky, D.; Myers, E.; Forbes, M.; Rahman, M.; Zaidan, M.; Parikh, S.; Wyman, J.; Pantelic, M.; et al. Application of 3-Dimensional Computed Tomographic Image Guidance to WATCHMAN Implantation and Impact on Early Operator Learning Curve: Single-Center Experience. JACC Cardiovasc. Interv. 2016, 9, 2329–2340. [Google Scholar] [CrossRef]
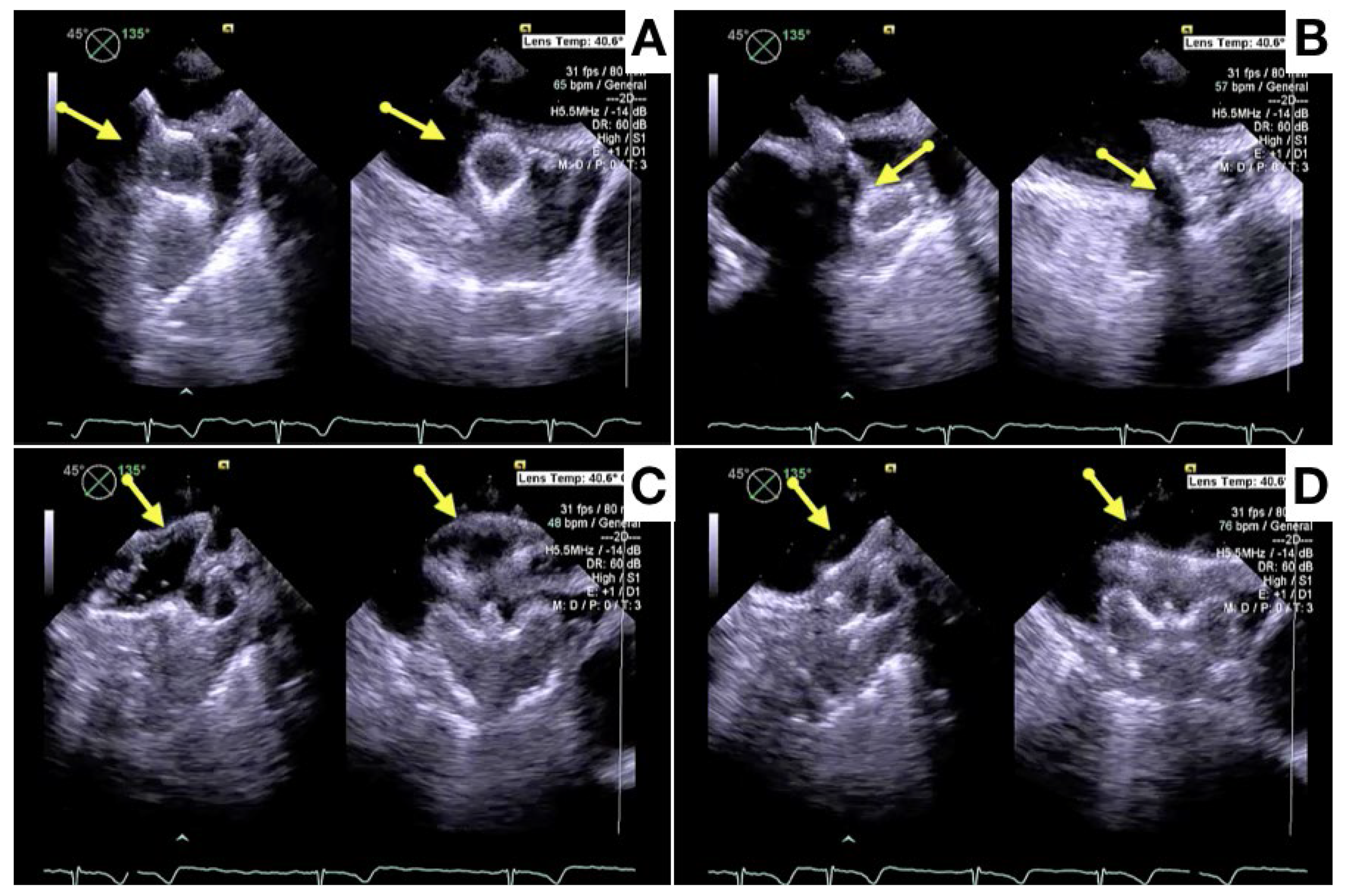
- Galea, R.; Räber, L.; Fuerholz, M.; Häner, J.D.; Siontis, G.C.M.; Brugger, N.; Moschovitis, A.; Heg, D.; Fischer, U.; Meier, B.; et al. Impact of Echocardiographic Guidance on Safety and Efficacy of Left Atrial Appendage Closure: An Observational Study. JACC Cardiovasc. Interv. 2021, 14, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Lennon, M.J.; Gibbs, N.M.; Weightman, W.M.; Leber, J.; Ee, H.C.; Yusoff, I.F. Transesophageal echocardiographyrelated gastrointestinal complications in cardiac surgical patients. J. Cardiothorac. Vasc. Anesth. 2005, 19, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Berti, S.; Pastormerlo, L.E.; Santoro, G.; Brscic, E.; Montorfano, M.; Vignali, L.; Danna, P.; Tondo, C.; Rezzaghi, M.; D’Amico, G.; et al. Intracardiac Versus Transesophageal Echocardiographic Guidance for Left Atrial Appendage Occlusion: The LAAO Italian Multicenter Registry. JACC Cardiovasc. Interv. 2018, 11, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Nielsen-Kudsk, J.E.; Berti, S.; De Backer, O.; Aguirre, D.; Fassini, G.; Cruz-Gonzalez, I.; Grassi, G.; Tondo, C. Use of Intracardiac Compared With Transesophageal Echocardiography for Left Atrial Appendage Occlusion in the Amulet Observational Study. JACC Cardiovasc. Interv. 2019, 12, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Chaker, Z.; Alqahtani, F.; Raslan, S.; Raybuck, B. Outcomes of Routine Intracardiac Echocardiography to Guide Left Atrial Appendage Occlusion. JACC Clin. Electrophysiol. 2020, 6, 393–400. [Google Scholar] [CrossRef]
- Ribeiro, J.M.; Teixeira, R.; Puga, L.; Costa, M.; Gonçalves, L. Comparison of intracardiac and transoesophageal echocardiography for guidance of percutaneous left atrial appendage occlusion: A meta-analysis. Echocardiography 2019, 36, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Nielsen-Kudsk, J.E.; Berti, S.; Caprioglio, F.; Ronco, F.; Arzamendi, D.; Betts, T.; Tondo, C.; Christen, T.; Allocco, D.J. Intracardiac Echocardiography to Guide Watchman FLX Implantation: The ICE LAA Study. JACC Cardiovasc. Interv. 2023, 16, 643–651. [Google Scholar] [CrossRef]
- Alkhouli, M.; Simard, T.; El Shaer, A.; Bird, J.; Nkomo, V.T.; Freidman, P.A.; Thaden, J.; Padang, R. First Experience With a Novel Live 3D ICE Catheter to Guide Transcatheter Structural Heart Interventions. JACC Cardiovasc. Imaging. 2022, 15, 1502–1509. [Google Scholar] [CrossRef]
- Dukkipati, S.R.; Kar, S.; Holmes, D.R.; Doshi, S.K.; Swarup, V.; Gibson, D.N.; Maini, B.; Gordon, N.T.; Main, M.L.; Reddy, V.K. Device-related thrombus after left atrial appendage closure. Circulation 2018, 138, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Busu, T.; Shah, K.; Osman, M.; Alqahtani, F.; Raybuck, B. Incidence and clinical impact of device-related thrombus following percutaneous left atrial appendage occlusion: A meta-analysis. J. Am. Coll. Cardiol. EP 2018, 4, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Korsholm, K.; Jensen, J.M.; Nørgaard, B.L.; Nielsen-Kudsk, J.E. Detection of Device-Related Thrombosis Following Left Atrial Appendage Occlusion: A Comparison Between Cardiac Computed Tomography and Transesophageal Echocardiography. Circ. Cardiovasc. Interv. 2019, 12, e008112. [Google Scholar] [CrossRef] [PubMed]
- Simard, T.; Jung, R.G.; Lehenbauer, K.; Piayda, K.; Pracoń, R.; Jackson, G.G.; Flores-Umanzor, E.; Faroux, L.; Korsholm, K.; Chun, J.K.R.; et al. Predictors of Device-Related Thrombus Following Percutaneous Left Atrial Appendage Occlusion. J. Am. Coll. Cardiol. 2021, 78, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Papazoglou, A.S.; Balomenakis, C.; Bekiaridou, A.; Moysidis, D.V.; Patsiou, V.; Orfanidis, A.; Giannakoulas, G.; Kassimis, G.; Fragakis, N.; et al. Residual leaks following percutaneous left atrial appendage occlusion and outcomes: A meta-analysis. Eur. Heart J. 2024, 45, 214–229. [Google Scholar] [CrossRef]
- Clemente, A.; Avogliero, F.; Berti, S.; Paradossi, U.; Jamagidze, G.; Rezzaghi, M.; Della Latta, D.; Chiappino, D. Multimodality imaging in preoperative assessment of left atrial appendage transcatheter occlusion with the Amplatzer Cardiac Plug. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1276–1287. [Google Scholar] [CrossRef]
- Korsholm, K.; Jensen, J.M.; Nørgaard, B.L.; Samaras, A.; Saw, J.; Berti, S.; Tzikas, A.; Nielsen-Kudsk, J.E. Peridevice Leak Following Amplatzer Left Atrial Appendage Occlusion: Cardiac Computed Tomography Classification and Clinical Outcomes. JACC Cardiovasc. Interv. 2021, 14, 83–93. [Google Scholar] [CrossRef]
- Alkhouli, M.; Du, C.; Killu, A.; Simard, T.; Noseworthy, P.A.; Friedman, P.A.; Curtis, J.P.; Freeman, J.V.; Holmes, D.R. Clinical Impact of Residual Leaks Following Left Atrial Appendage Occlusion: Insights From the NCDR-LAAO Registry. JACC Clin. Electrophysiol. 2022, 8, 766–778. [Google Scholar] [CrossRef]
| Primary Indications | |
| Previous intracranial hemorrhage | |
| High risk of intracranial hemorrhage | |
| Previous major GI bleeding | |
| End-stage renal disease | |
| Potential Indications | |
| Hematologic disorders | |
| Advisable prolonged anti-platelet therapy | |
| Recurrent events despite optimal anticoagulation | |
| Non-compliant patients | |
| Young patients (<55 years old, CHA2DS2-VASC > 1) |
| Device | Imagine | Design | Sizes (MM) | Sheat (F) | Company | Status CE |
|---|---|---|---|---|---|---|
| Watchman 2.5 | 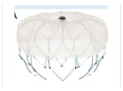 | Single (lobe) | 21, 24, 27, 30, 33 | 14 | Boston Scientific Corporation | CE Mark (2005) |
| Watchman FLX |  | Single (lobe) | 20, 24, 27, 31, 35 | 14 | Boston Scientific Corporation | CE Mark (2015) |
| Amplatzer cardiac Plug |  | Double (lobe and disc) | 16, 18, 20, 22, 24, 26, 28, 30 | 9, 13 | Abbott Vascular | CE Mark (2008) |
| Amplatzer Amulet |  | Double (lobe and disc) | 16, 18, 20, 22, 25, 28, 31, 34 | 12, 14 | Abbott Vascular | CE Mark (2013) |
| Ultraseal |  | Double (bull and sall) | 16, 18, 20, 22, 24, 26, 28, 30, 32 | 10, 12 | Cardia, Inc. | CE Mark (2016) |
| Omega |  | Double (lobe/cup and disc) | 14, 16, 18, 20, 22, 24, 26, 28, 30 | 14 | Eclipse Medical | CE Mark (2021) |
| LAmbre |  | Double (umbrella and cover) | 16, 18, 20, 22, 24, 26, 28, 30, 32, 34, 36 | 8, 10 | Lifetech Scientific, Co., Ltd. | CE Mark (2016) |
| CLAAS |  | Adaptable form | 27, 35 | 17 | Conformal Medical | Non approved |
| TEE | CCT | |
|---|---|---|
| ADVANTAGES | ||
| Standard of care in many centers | Higher spatial resolution | |
| No contrast medium | Low inter-observer variation | |
| No radiations | Optimal transeptal puncture planning | |
| Lower cost | Implant view planning | |
| Easily available | Potential for simulation/modeling | |
| LIMITS | ||
| Invasive | Contrast medium use | |
| Gastro-esophageal contraindications | Radiations | |
| Not optimal imaging in some cases | Higher cost |
| TEE | ICE | |
|---|---|---|
| ADVANTAGES | ||
| Standard of care in many centers | No need for orotracheal intubation | |
| Many cardiologists are familiar with TEE | Local anesthesia | |
| 3D evaluation | Better visualization of LAA from LA | |
| Higher quality | No need for dedicated operator | |
| Easily available | ||
| Low cost | ||
| Possible re-evaluation in the cathlab before vascular access | ||
| LIMITS | ||
| Invasive | 2D only (need for a preprocedural 3D evaluation) | |
| Gastro-esophageal contraindications | Higher use of contrast medium | |
| Not optimal imaging in some cases | Higher cost | |
| Interference with fluoroscopy | Limited operator experience | |
| Positioning in LA mandatory for good quality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastormerlo, L.E.; De Caterina, A.R.; Esposito, A.; Korsholm, K.; Berti, S. State-of-the-Art of Transcatheter Left Atrial Appendage Occlusion. J. Clin. Med. 2024, 13, 939. https://doi.org/10.3390/jcm13040939
Pastormerlo LE, De Caterina AR, Esposito A, Korsholm K, Berti S. State-of-the-Art of Transcatheter Left Atrial Appendage Occlusion. Journal of Clinical Medicine. 2024; 13(4):939. https://doi.org/10.3390/jcm13040939
Chicago/Turabian StylePastormerlo, Luigi Emilio, Alberto Ranieri De Caterina, Augusto Esposito, Kasper Korsholm, and Sergio Berti. 2024. "State-of-the-Art of Transcatheter Left Atrial Appendage Occlusion" Journal of Clinical Medicine 13, no. 4: 939. https://doi.org/10.3390/jcm13040939
APA StylePastormerlo, L. E., De Caterina, A. R., Esposito, A., Korsholm, K., & Berti, S. (2024). State-of-the-Art of Transcatheter Left Atrial Appendage Occlusion. Journal of Clinical Medicine, 13(4), 939. https://doi.org/10.3390/jcm13040939







