Longitudinal Measurements of Blood Biomarkers in Patients with Crohn’s Disease or Ulcerative Colitis Treated with Infliximab: Only the Latest Values in the Induction Period Predict Treatment Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
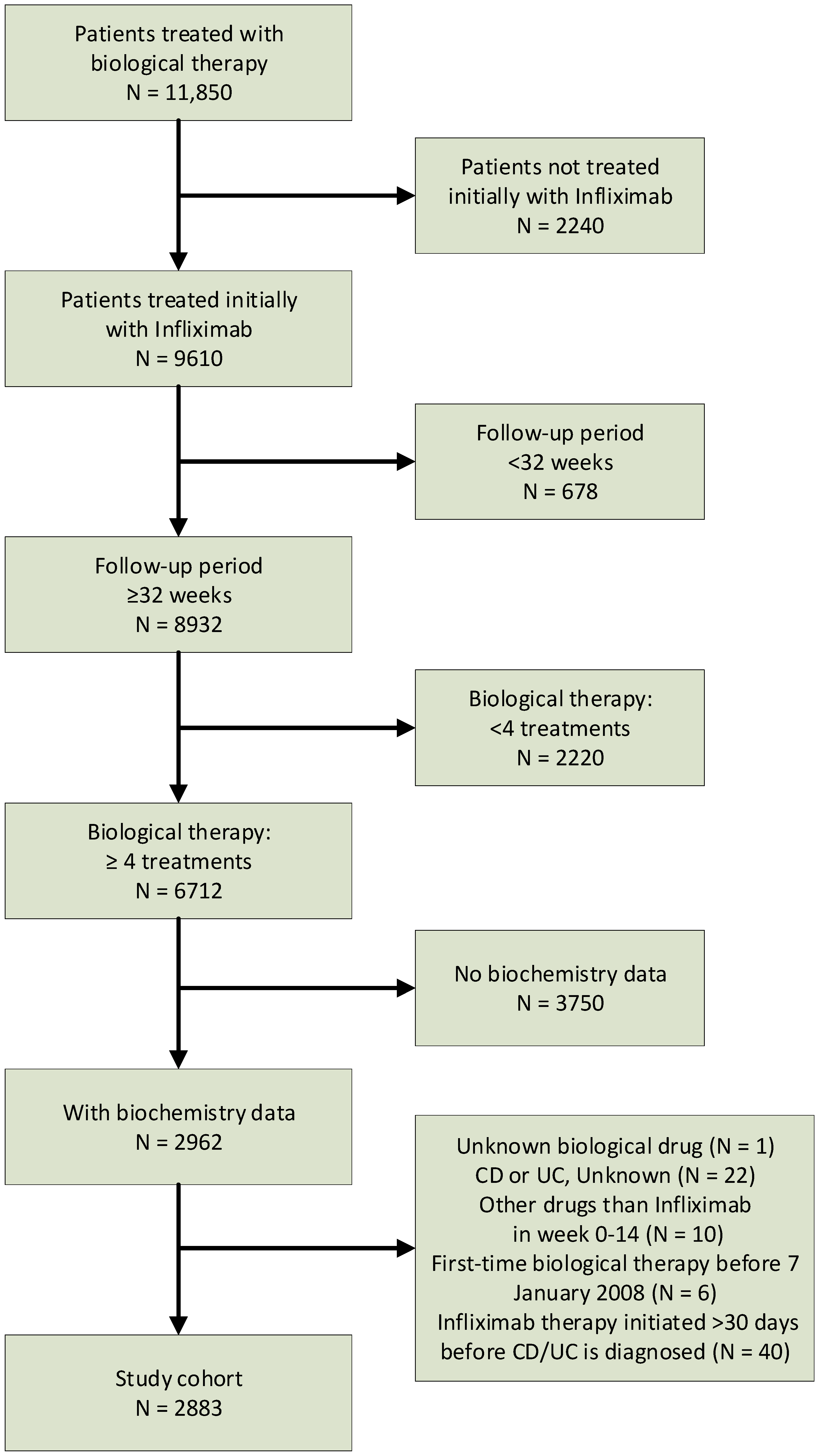
2.2. Study Population
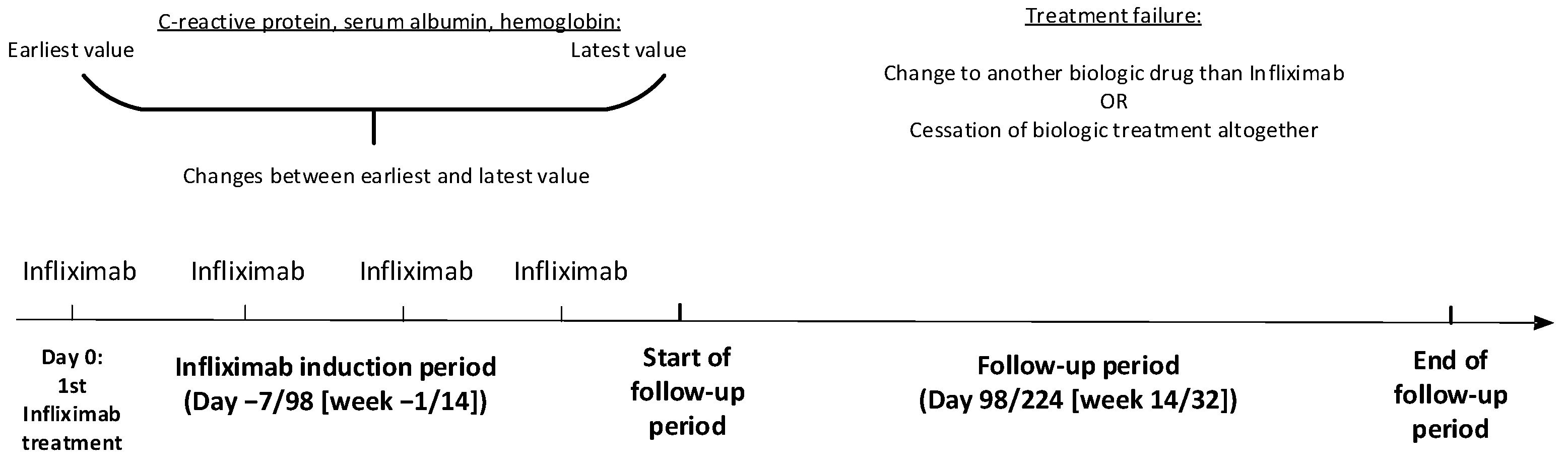
2.3. Outcome
2.4. Statistical Analyses
- Model A: the latest level of CRP10, serum albumin, and hemoglobin
- Model B: Model A + changes in CRP10, serum albumin, and hemoglobin levels
- Model C: Model A + changes in hemoglobin levels
- Model D: the latest levels of CRP10 and hemoglobin + changes in hemoglobin levels
- Model E: Model D + gender, age groups (0–16, 17–39, 40–59, ≥60 years), body mass index (BMI) (<18.5, ≥18.5 and <25, ≥25 and <30, ≥30 and <35, ≥35, missing), and quartiles of time from diagnosis of IBD to first-time treatment with infliximab (0–187, 188–808, 809–3053, 3054–14,652 days).
3. Results
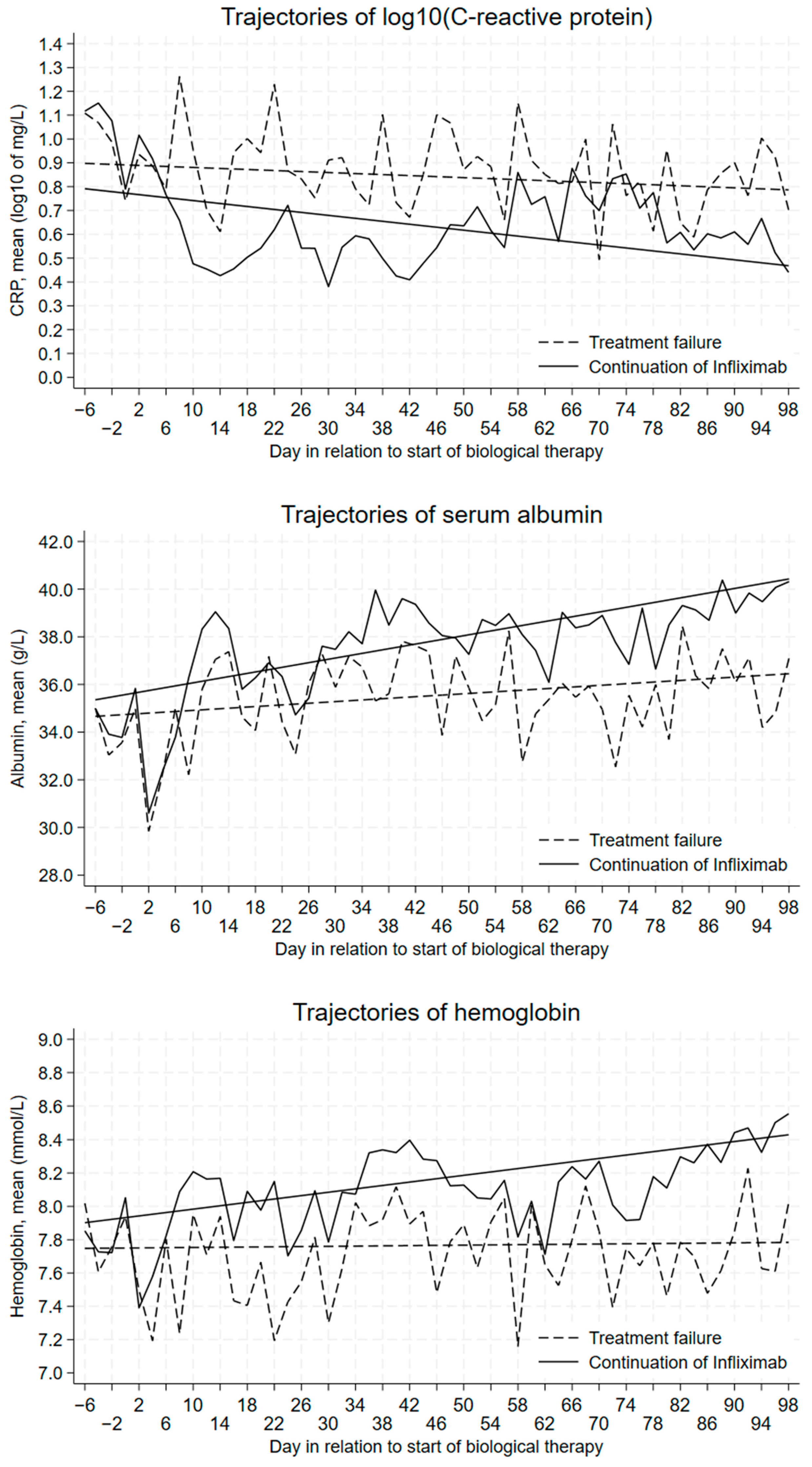
3.1. Trajectories of Mean Daily Levels
3.2. Logistic Regression Analyses Separately for the Three Biomarkers
3.3. Multivariate Logistic Regression Analyses
3.4. AUROCs for Models A–E
3.5. Subgroup Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnar, T.; Raine, T.; Sebastian, S.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohn’s Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Tarapatzi, G.; Filidou, E.; Kandilogiannakis, L.; Vradelis, S.; Kolios, G. Biomarkers in Inflammatory Bowel Diseases: Predicting the Indication and the Effect of Biologics. J. Gastrointest. Liver Dis. 2022, 31, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, C.J. Hypoalbuminemia as Surrogate and Culprit of Infections. Int. J. Mol. Sci. 2021, 22, 4496. [Google Scholar] [CrossRef] [PubMed]
- Levitt, D.G.; Levitt, M.D. Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int. J. Gen. Med. 2016, 9, 229–255. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Dubois, M.J.; Navickis, R.J.; Wilkes, M.M. Hypoalbuminemia in acute illness: Is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann. Surg. 2003, 237, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Koutroubakis, I.E.; Ramos-Rivers, C.; Regueiro, M.; Koutroumpakis, E.; Click, B.; Schoen, R.E.; Hashash, J.G.; Schwartz, M.; Swoger, J.; Baidoo, L.; et al. Persistent or Recurrent Anemia Is Associated with Severe and Disabling Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2015, 13, 1760–1766. [Google Scholar] [CrossRef]
- Truelove, S.C.; Witts, L.J. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br. Med. J. 1955, 2, 1041–1048. [Google Scholar] [CrossRef]
- Best, W.R.; Becktel, J.M.; Singleton, J.W.; Kern, F., Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976, 70, 439–444. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Chaparro, M. Predictors of Primary Response to Biologic Treatment [Anti-TNF, Vedolizumab, and Ustekinumab] in Patients with Inflammatory Bowel Disease: From Basic Science to Clinical Practice. J. Crohn’s Colitis 2020, 14, 694–709. [Google Scholar] [CrossRef]
- Dragoni, G.; Innocenti, T.; Galli, A. Biomarkers of Inflammation in Inflammatory Bowel Disease: How Long before Abandoning Single-Marker Approaches? Dig. Dis. 2021, 39, 190–203. [Google Scholar] [CrossRef]
- Reinisch, W.; Wang, Y.; Oddens, B.J.; Link, R. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn’s disease: A post-hoc analysis from ACCENT I. Aliment. Pharmacol. Ther. 2012, 35, 568–576. [Google Scholar] [CrossRef]
- Cornillie, F.; Hanauer, S.B.; Diamond, R.H.; Wang, J.; Tang, K.L.; Xu, Z.; Rutgeerts, P.; Vermeire, S. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: A retrospective analysis of the ACCENT I trial. Gut 2014, 63, 1721–1727. [Google Scholar] [CrossRef]
- Ma, C.; Battat, R.; Khanna, R.; Parker, C.E.; Feagan, B.G.; Jairath, V. What is the role of C-reactive protein and fecal calprotectin in evaluating Crohn’s disease activity? Best Pract. Res. Clin. Gastroenterol. 2019, 38–39, 101602. [Google Scholar] [CrossRef]
- Schmidt, M.; Schmidt, S.A.J.; Adelborg, K.; Sundboll, J.; Laugesen, K.; Ehrenstein, V.; Sorensen, H.T. The Danish health care system and epidemiological research: From health care contacts to database records. Clin. Epidemiol. 2019, 11, 563–591. [Google Scholar] [CrossRef]
- Christiansen, T. Organization and financing of the Danish health care system. Health Policy 2002, 59, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Fonager, K.; Sørensen, H.T.; Rasmussen, S.N.; Moller-Petersen, J.; Vyberg, M. Assessment of the diagnoses of Crohn’s disease and ulcerative colitis in a Danish hospital information system. Scand. J. Gastroenterol. 1996, 31, 154–159. [Google Scholar] [CrossRef]
- Schmidt, M.; Schmidt, S.A.; Sandegaard, J.L.; Ehrenstein, V.; Pedersen, L.; Sørensen, H.T. The Danish National Patient Registry: A review of content, data quality, and research potential. Clin. Epidemiol. 2015, 7, 449–490. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J.F.H.; Hansen, A.T.; Ladefoged, S.A.; Sorensen, H.T.; Pedersen, L.; Adelborg, K. Existing Data Sources in Clinical Epidemiology: Laboratory Information System Databases in Denmark. Clin. Epidemiol. 2020, 12, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef]
- Singer, J.D.; Willett, J.B. Applied Longitudinal Data Analysis—Modeling Change and Event Occurrence, 1st ed.; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S. Assessing the fit of the model. In Applied Logistic Regression, 2nd ed.; Hosmer, D.W., Lemeshow, S., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2000; Volume 1, pp. 143–202. [Google Scholar]
- Larsen, M.D.; Nørgård, B.M.; Kjeldsen, J. Does Disease Activity After Induction Treatment with Biologics Predict Short-Term Outcome in Crohn’s Disease and Ulcerative Colitis? Inflamm. Bowel Dis. 2022, 28, 1658–1666. [Google Scholar] [CrossRef]
- Feng, W.; Zhu, L.; Liu, Y.; Xu, L.; Shen, H. C-reactive protein/albumin ratio and IL-6 are associated with disease activity in patients with ulcerative colitis. J. Clin. Lab. Anal. 2023, 37, e24843. [Google Scholar] [CrossRef]
- Ghoneima, A.S.; Flashman, K.; Dawe, V.; Baldwin, E.; Celentano, V. High risk of septic complications following surgery for Crohn’s disease in patients with preoperative anaemia, hypoalbuminemia and high CRP. Int. J. Color. Dis. 2019, 34, 2185–2188. [Google Scholar] [CrossRef] [PubMed]
- Ziade, F.; Rungoe, C.; Kallemose, T.; Paerregaard, A.; Wewer, A.V.; Jakobsen, C. Biochemical Markers, Genotype, and Inflammation in Pediatric Inflammatory Bowel Disease: A Danish Population-Based Study. Dig. Dis. 2019, 37, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Yerushalmy-Feler, A.; Singer, D.; Berkovitch, G.; Lubetzky, R.; Dotan, I.; Ziv-Baran, T.; Cohen, S. Predictors for poor outcome of hospitalized children with inflammatory bowel disease. Eur. J. Pediatr. 2019, 179, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.J.; Hartery, K.; Doherty, J.; Nolan, J.; Keegan, D.; Byrne, K.; Martin, S.T.; Buckley, M.; Sheridan, J.; Horgan, G.; et al. CRP/Albumin Ratio: An Early Predictor of Steroid Responsiveness in Acute Severe Ulcerative Colitis. J. Clin. Gastroenterol. 2018, 52, e48–e52. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.T.; Vande Casteele, N.; Vermeire, S.; de Buck van Overstraeten, A.; Billiet, T.; Baert, F.; Wolthuis, A.; Van Assche, G.; Noman, M.; Hoffman, I.; et al. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2015, 13, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Rodrigues-Pinto, E.; Santos-Antunes, J.; Vilas-Boas, F.; Lopes, S.; Nunes, A.; Camila-Dias, C.; Macedo, G. High C-reactive protein in Crohn’s disease patients predicts nonresponse to infliximab treatment. J. Crohn’s Colitis 2014, 8, 129–136. [Google Scholar] [CrossRef]
- Jürgens, M.; Mahachie John, J.M.; Cleynen, I.; Schnitzler, F.; Fidder, H.; van Moerkercke, W.; Ballet, V.; Noman, M.; Hoffman, I.; van Assche, G.; et al. Levels of C-reactive protein are associated with response to infliximab therapy in patients with Crohn’s disease. Clin. Gastroenterol. Hepatol. 2011, 9, 421–427.e1. [Google Scholar] [CrossRef]
- Schnitzler, F.; Fidder, H.; Ferrante, M.; Noman, M.; Arijs, I.; Van Assche, G.; Hoffman, I.; Van Steen, K.; Vermeire, S.; Rutgeerts, P. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: Results from a single-centre cohort. Gut 2009, 58, 492–500. [Google Scholar] [CrossRef]
- Iwasa, R.; Yamada, A.; Sono, K.; Furukawa, R.; Takeuchi, K.; Suzuki, Y. C-reactive protein level at 2 weeks following initiation of infliximab induction therapy predicts outcomes in patients with ulcerative colitis: A 3 year follow-up study. BMC Gastroenterol. 2015, 15, 103. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.W.; Heys, D.; Ho, G.T.; Noble, C.L.; Shand, A.G.; Mowat, C.; Boulton-Jones, R.; Williams, A.; Church, N.; Satsangi, J.; et al. A retrospective analysis of the efficacy and safety of infliximab as rescue therapy in acute severe ulcerative colitis. Aliment. Pharmacol. Ther. 2007, 26, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.W.; Lewis, S.; Barton, J.R.; Corbett, S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm. Bowel Dis. 2006, 12, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Travis, S.P.; Farrant, J.M.; Ricketts, C.; Nolan, D.J.; Mortensen, N.M.; Kettlewell, M.G.; Jewell, D.P. Predicting outcome in severe ulcerative colitis. Gut 1996, 38, 905–910. [Google Scholar] [CrossRef]
- Buckell, N.A.; Lennard-Jones, J.E.; Hernandez, M.A.; Kohn, J.; Riches, P.G.; Wadsworth, J. Measurement of serum proteins during attacks of ulcerative colitis as a guide to patient management. Gut 1979, 20, 22–27. [Google Scholar] [CrossRef]
- De Dombal, F.T. Prognostic value of the serum proteins during severe attacks of ulcerative colitis. Gut 1968, 9, 144–149. [Google Scholar] [CrossRef][Green Version]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
| Characteristic | All Patients (n = 2883) | Crohn’s Disease (n = 1626) | Ulcerative Colitis (n = 1257) |
|---|---|---|---|
| Gender | |||
| Females | 1487 (51.6) | 853 (52.5) | 634 (50.4) |
| Males | 1396 (48.4) | 773 (47.5) | 623 (49.6) |
| Age, years 1 | |||
| Range | 1.1–89.3 | 1.1–82.3 | 3.7–89.3 |
| Median (IQR) | 34.0 (23.0–47.6) | 30.9 (21.6–46.2) | 36.9 (25.5–50.0) |
| Body mass index 1 | |||
| <18.5 | 52 (1.8) | 38 (2.3) | 14 (1.1) |
| ≥18.5, <25 | 544 (18.9) | 310 (19.1) | 234 (18.6) |
| ≥25, <30 | 329 (11.4) | 177 (10.9) | 152 (12.1) |
| ≥30, <35 | 142 (4.9) | 84 (5.2) | 58 (4.6) |
| ≥35 | 62 (2.2) | 30 (1.9) | 32 (2.6) |
| Missing | 1754 (60.8) | 987 (60.7) | 767 (61.0) |
| C-reactive protein, measured 2 | |||
| Patients | 2817 (97.7) | 1594 (98.0) | 1223 (97.3) |
| Number of values | |||
| All | 12,730 | 6208 | 6522 |
| Per patient | |||
| Range | 1–51 | 1–51 | 1–45 |
| Median (IQR) | 3 (2–5) | 3 (2–4) | 4 (3–6) |
| Serum albumin, measured 2 | |||
| Patients | 2709 (94.0) | 1543 (94.9) | 1166 (92.8) |
| Number of values | |||
| All | 12,040 | 5876 | 6164 |
| Per patient | |||
| Range | 1–52 | 1–52 | 1–37 |
| Median (IQR) | 3 (2–5) | 3 (2–4) | 4 (3–6) |
| Hemoglobin, measured 2 | |||
| Patients | 2880 (99.9) | 1624 (99.9) | 1256 (99.9) |
| Number of values | |||
| All | 13,538 | 6594 | 6944 |
| Per patient | |||
| Range | 1–49 | 1–49 | 1–46 |
| Median (IQR) | 4 (3–5) | 2 (3–5) | 4 (3–7) |
| Gastrointestinal surgery 3 | 18 (0.6) | 8 (0.5) | 10 (0.8) |
| Shift to another biological drug 4 | 284 (9.9) | 140 (8.6) | 144 (11.5) |
| Ceasing biological treatment 4 | 139 (4.8) | 56 (3.4) | 83 (6.6) |
| Model | OR (95% CI) | AUROC 1 |
|---|---|---|
| log10 of CRP 1, earliest | 1.12 (0.94–1.34) | 0.523 |
| log10 of CRP, latest | 1.72 (1.39–2.13) | 0.568 |
| Serum albumin, earliest | 0.98 (0.96–1.00) | 0.529 |
| Serum albumin, latest | 0.95 (0.93–0.97) | 0.570 |
| Hemoglobin, earliest | 0.90 (0.81–1.00) | 0.528 |
| Hemoglobin, latest | 0.74 (0.67–0.83) | 0.575 |
| log10 of CRP, 0–25 percentile change | 1 (ref.) | 0.572 |
| log10 of CRP, 25–75 percentile change | 0.92 (0.68–1.26) | |
| log10 of CRP, 75–100 percentile change | 0.90 (0.68–1.21) | |
| log10 of CRP, latest | 1.76 (1.41–2.20) | |
| Serum albumin, 0–25 percentile change | 1 (ref.) | 0.576 |
| Serum albumin, 25–75 percentile change | 0.76 (0.57–1.02) | |
| Serum albumin, 75–100 percentile change | 0.73 (0.52–1.02) | |
| Serum albumin, latest | 0.95 (0.93–0.98) | |
| Hemoglobin, 0–25 percentile change | 1 (ref.) | 0.594 |
| Hemoglobin, 25–75 percentile change | 0.74 (0.57–0.96) | |
| Hemoglobin, 75–100 percentile change | 0.60 (0.44–0.82) | |
| Hemoglobin, latest | 0.78 (0.70–0.88) |
| Cofactor | Model A (0.595) 1 | Model B (0.616) | Model C (0.614) | Model D (0.610) | Model E (0.623) |
|---|---|---|---|---|---|
| log10 of CRP, latest | 1.43 (1.12–1.82) | 1.41 (1.09–1.82) | 1.39 (1.09–1.77) | 1.48 (1.19–1.85) | 1.49 (1.18–1.87) |
| Serum albumin, latest | 0.98 (0.95–1.01) | 0.98 (0.95–1.01) | 0.98 (0.95–1.01) | ||
| Hemoglobin, latest | 0.80 (0.70–0.91) | 0.85 (0.74–0.98) | 0.84 (0.73–0.96) | 0.83 (0.73–0.94) | 0.81 (0.71–0.93) |
| log10 of CRP, 0–25 percentile difference | 1 (reference) | ||||
| log10 of CRP, 25–75 percentile difference | 0.95 (0.67–1.35) | ||||
| log10 of 75–100 percentile difference | 0.93 (0.65–1.32) | ||||
| Serum albumin, 0–25 percentile difference | 1 (reference) | ||||
| Serum albumin, 25–75 percentile difference | 0.85 (0.62–1.19) | ||||
| Serum albumin, 75–100 percentile difference | 0.98 (0.65–1.47) | ||||
| Hemoglobin, 0–25 percentile difference | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | |
| Hemoglobin, 25–75 percentile difference | 0.74 (0.55–0.99) | 0.73 (0.55–0.97) | 0.71 (0.54–0.93) | 0.70 (0.53–0.93) | |
| Hemoglobin, 75–100 percentile difference | 0.54 (0.37–0.80) | 0.56 (0.39–0.80) | 0.56 (0.40–0.78) | 0.57 (0.41–0.80) | |
| Males | 0.96 (0.75–1.23) | ||||
| Age, 0–16 years | 1 (reference) | ||||
| Age, 17–39 years | 1.00 (0.57–1.76) | ||||
| Age, 40–59 years | 1.48 (0.82–2.68) | ||||
| Age, ≥60 years | 1.33 (0.69–2.55) | ||||
| Body mass index, <18.5 | 0.55 (0.19–1.62) | ||||
| Body mass index, ≥18.5 and <25 | 1 (reference) | ||||
| Body mass index, ≥25 and <30 | 0.82 (0.54–1.25) | ||||
| Body mass index, ≥30 and <35 | 0.98 (0.57–1.67) | ||||
| Body mass index, ≥35 | 0.59 (0.25–1.38) | ||||
| Body mass index, missing | 0.78 (0.58–1.05) | ||||
| Time, diag-biol 2, 0–187 days | 1 (reference) | ||||
| Time, diag-biol, 188–808 days | 1.15 (0.83–1.59) | ||||
| Time, diag-biol, 809–3053 days | 1.07 (0.76–1.50) | ||||
| Time, diag-biol, 3054–14,652 days | 1.00 (0.70–1.42) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gradel, K.O.; Nørgård, B.M.; Friedman, S.; Kjeldsen, J.; Larsen, M.D. Longitudinal Measurements of Blood Biomarkers in Patients with Crohn’s Disease or Ulcerative Colitis Treated with Infliximab: Only the Latest Values in the Induction Period Predict Treatment Failure. J. Clin. Med. 2024, 13, 926. https://doi.org/10.3390/jcm13040926
Gradel KO, Nørgård BM, Friedman S, Kjeldsen J, Larsen MD. Longitudinal Measurements of Blood Biomarkers in Patients with Crohn’s Disease or Ulcerative Colitis Treated with Infliximab: Only the Latest Values in the Induction Period Predict Treatment Failure. Journal of Clinical Medicine. 2024; 13(4):926. https://doi.org/10.3390/jcm13040926
Chicago/Turabian StyleGradel, Kim Oren, Bente Mertz Nørgård, Sonia Friedman, Jens Kjeldsen, and Michael Due Larsen. 2024. "Longitudinal Measurements of Blood Biomarkers in Patients with Crohn’s Disease or Ulcerative Colitis Treated with Infliximab: Only the Latest Values in the Induction Period Predict Treatment Failure" Journal of Clinical Medicine 13, no. 4: 926. https://doi.org/10.3390/jcm13040926
APA StyleGradel, K. O., Nørgård, B. M., Friedman, S., Kjeldsen, J., & Larsen, M. D. (2024). Longitudinal Measurements of Blood Biomarkers in Patients with Crohn’s Disease or Ulcerative Colitis Treated with Infliximab: Only the Latest Values in the Induction Period Predict Treatment Failure. Journal of Clinical Medicine, 13(4), 926. https://doi.org/10.3390/jcm13040926








