Behavioral Disorders of Spatial Cognition in Patients with Mild Cognitive Impairment due to Alzheimer’s Disease: Preliminary Findings from the BDSC-MCI Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Technological Apparatus: Sensory Smart Textile and Digital Health Applications
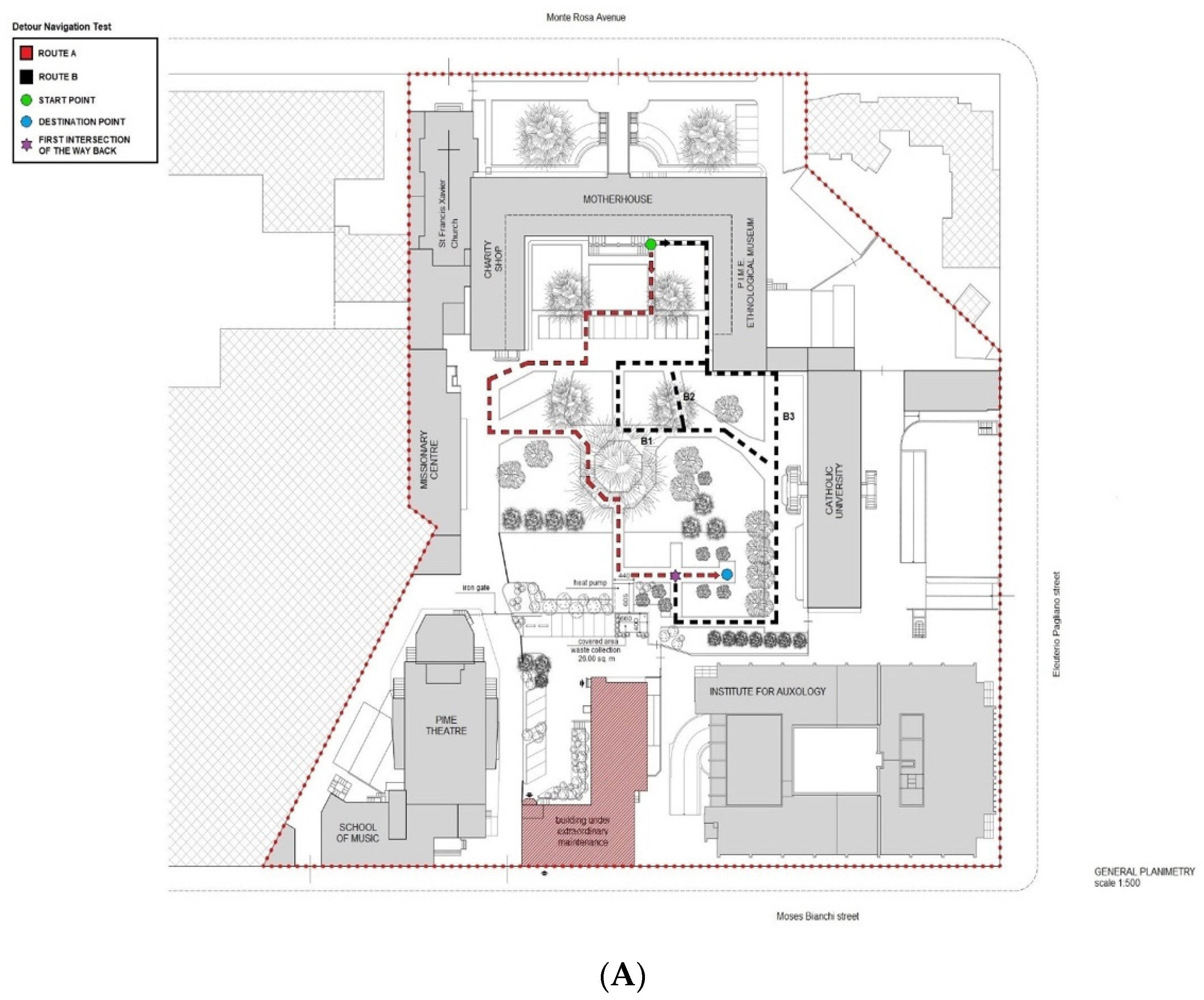
2.3. Real-Space Navigation Testing
2.4. Statistical Analysis
3. Results
3.1. Demographics
3.2. Naturalistic Test Findings and Related Measures
3.3. Physiological Parameters and Gait Data Collected via the Digital Apps
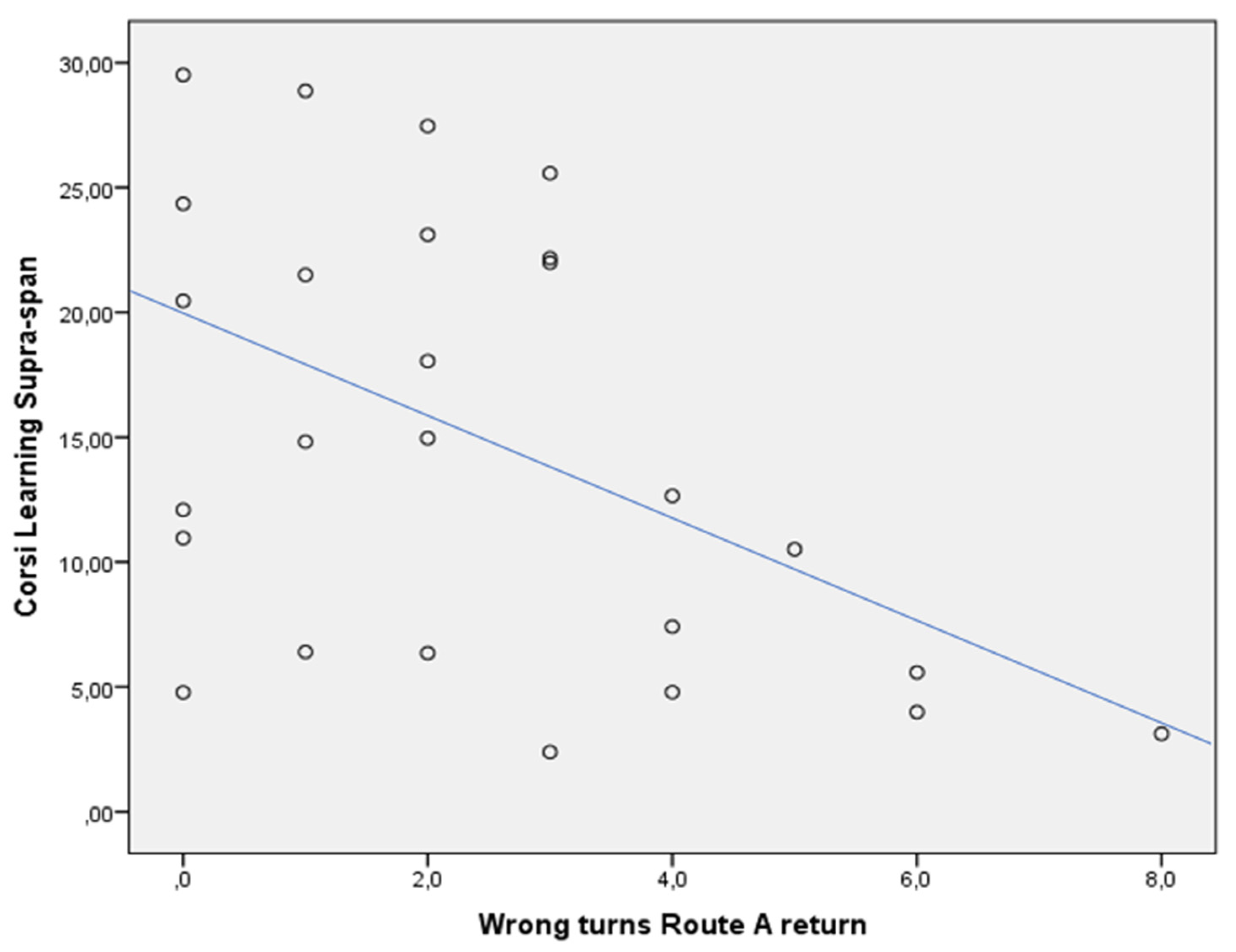
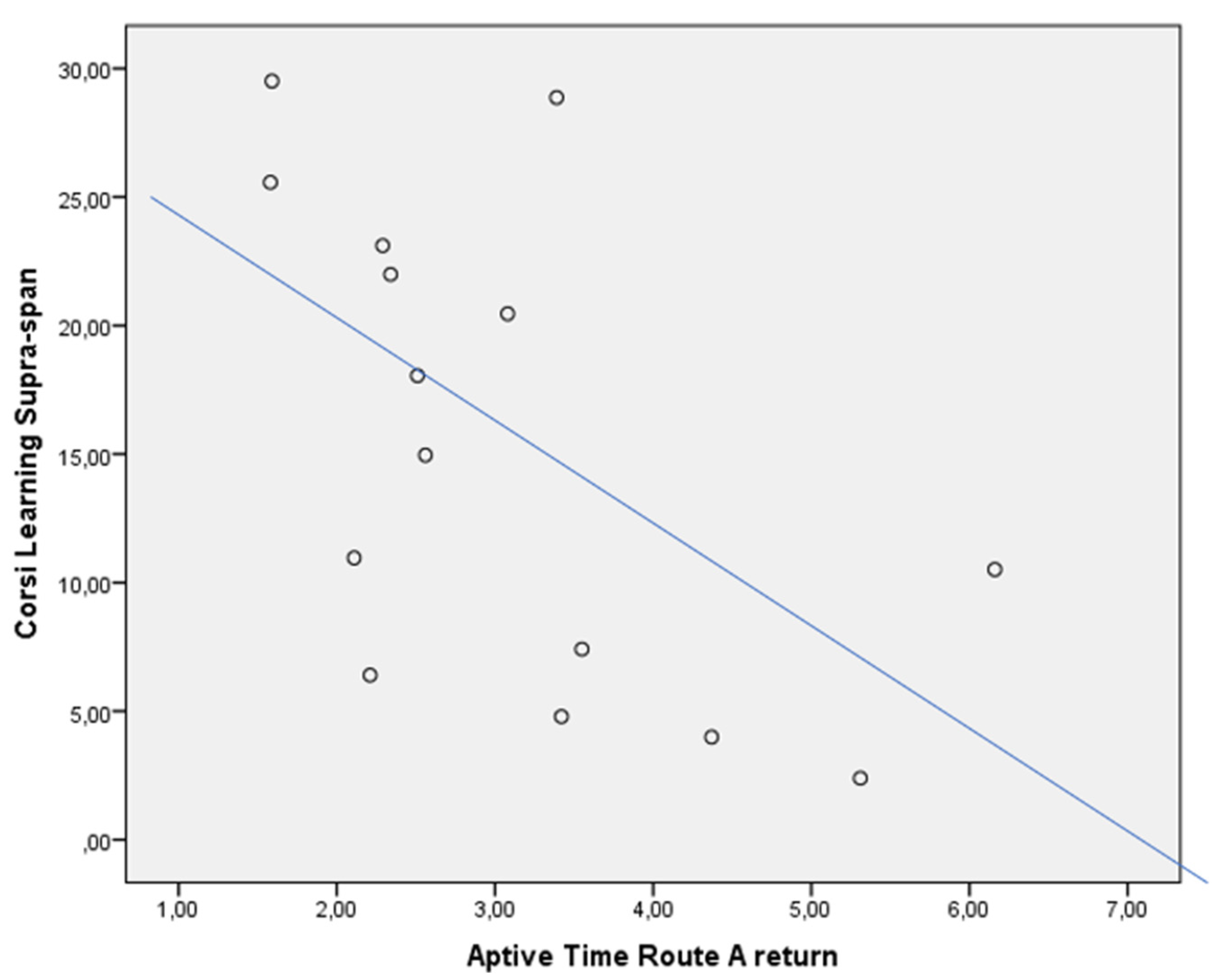
3.4. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coughlan, G.; Laczó, J.; Hort, J.; Minihane, A.M.; Hornberger, M. Spatial navigation deficits-overlooked cognitive marker for preclinical Alzheimer disease? Nat. Rev. Neurol. 2018, 14, 496–506. [Google Scholar] [CrossRef]
- Plácido, J.; de Almeida, C.A.; Ferreira, J.V.; de Oliveira Silva, F.; Monteiro-Junior, R.S.; Tangen, G.G.; Laks, J.; Deslandes, A.C. Spatial navigation in older adults with mild cognitive impairment and dementia: A systematic review and meta-analysis. Exp. Gerontol. 2022, 165, 111852. [Google Scholar] [CrossRef]
- Tuena, C.; Mancuso, V.; Stramba-Badiale, C.; Pedroli, E.; Stramba-Badiale, M.; Riva, G.; Repetto, C. Egocentric and allocentric spatial memory in mild cognitive impairment with real-world and virtual navigation tasks: A systematic review. J. Alzheimer’s Dis. 2021, 79, 95–116. [Google Scholar] [CrossRef]
- Bierbrauer, A.; Kunz, L.; Gomes, C.A.; Luhmann, M.; Deuker, L.; Getzmann, S.; Wascher, E.; Gajewski, P.D.; Hengstler, J.G.; Fernandez-Alvarez, M.; et al. Unmasking selective path integration deficits in Alzheimer’s disease risk carriers. Sci. Adv. 2020, 6, eaba1394. [Google Scholar] [CrossRef]
- Iglói, K.; Zaoui, M.; Berthoz, A.; Rondi-Reig, L. Sequential egocentric strategy is acquired as early as allocentric strategy: Parallel acquisition of these two navigation strategies. Hippocampus 2009, 19, 1199–1211. [Google Scholar] [CrossRef]
- Rondi-Reig, L.; Petit, G.H.; Tobin, C.; Tonegawa, S.; Mariani, J.; Berthoz, A. Impaired sequential egocentric and allocentric memories in forebrain-specific-NMDA receptor knock-out mice during a new task dissociating strategies of navigation. J. Neurosci. 2006, 26, 4071–4081. [Google Scholar] [CrossRef]
- Doeller, C.F.; Barry, C.; Burgess, N. Evidence for grid cells in human memory network. Nature 2010, 463, 657–661. [Google Scholar] [CrossRef]
- Squire, L.R. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992, 99, 195–231. [Google Scholar] [CrossRef]
- Cooper, R.A.; Ritchey, M. Progression from feature-specific brain activity to hippocampal binding during episodic encoding. J. Neurosci. 2020, 40, 1701–1709. [Google Scholar] [CrossRef]
- Newcomer, J.W.; Krystal, J.H. NMDA receptor regulation of memory and behavior in humans. Hippocampus 2001, 11, 529–542. [Google Scholar] [CrossRef]
- Kunz, L.; Schröder, T.N.; Lee, H.; Montag, C.; Lachmann, B.; Sariyska, R.; Reuter, M.; Strirberg, R.; Stöcker, T.; Messing-Floter, P.C.; et al. Reduced grid-cell–like representations in adults at genetic risk for Alzheimer’s disease. Science 2015, 350, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Lithfous, S.; Dufour, A.; Després, O. Spatial navigation in normal aging and the prodromal stage of Alzheimer’s disease: Insights from imaging and behavioral studies. Ageing Res. Rev. 2013, 12, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.L.; Fagan, A.M.; Morris, J.C.; Head, D. Spatial navigation in preclinical Alzheimer’s disease. J. Alzheimer’s Dis. 2016, 52, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Lipton, R.; Ayers, E. Spatial navigation and risk of cognitive impairment: A prospective cohort study. Alzheimers Dement. 2017, 13, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Boccia, M.; Di Vita, A.; Diana, S.; Margiotta, R.; Imbriano, L.; Rendace, L.; Campanelli, A.; D’Antonio, F.; Trebbastoni, A.; de Lena, C.; et al. Is losing one’s way a sign of cognitive decay? Topographical memory deficit as an early marker of pathological aging. J. Alzheimer’s Dis. 2019, 68, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Gazova, I.; Laczó, J.; Rubinova, E.; Mokrisova, I.; Hyncicova, E.; Andel, R.; Vyhnalek, M.; Sheardova, K.; Coulson, E.J.; Hort, J. Spatial navigation in young versus older adults. Front. Aging Neurosci. 2013, 19, 5–94. [Google Scholar] [CrossRef] [PubMed]
- Schöberl, F.; Pradhan, C.; Irving, S.; Buerger, K.; Xiong, G.; Kugler, G.; Kohlbecher, D.I.; Engmann, J.; Werner, P.; Brendel, M.; et al. Real-space navigation testing differentiates between amyloid-positive and-negative aMCI. Neurology 2020, 94, e861–e873. [Google Scholar] [CrossRef]
- Montero-Odasso, M. Gait as a biomarker of cognitive impairment and dementia syndromes. Eur. J. Neurol. 2016, 23, 437–438. [Google Scholar] [CrossRef]
- McCarthy, I.; Suzuki, T.; Holloway, C.; Poole, T.; Frost, C.; Carton, A.; Tyler, N.; Crutch, S.; Yong, K. Detection and localization of hesitant steps in people with Alzheimer’s disease navigating routes of varying complexity. Healthc. Technol. Lett. 2019, 6, 42–47. [Google Scholar] [CrossRef]
- Nicolini, P.; Lucchi, T.; Abbate, C.; Inglese, S.; Tomasini, E.; Mari, D.; Rossi, P.D.; Vicenzi, M. Autonomic function predicts cognitive decline in mild cognitive impairment: Evidence from power spectral analysis of heart rate variability in a longitudinal study. Front. Aging Neurosci. 2022, 14, 886023. [Google Scholar] [CrossRef]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Cummings, J.L.; Dekosky, S.T.; Barberger-Gateau, P.; Delacourte, A.; Frisoni, G.; Fox, N.C.; Galasko, D.; et al. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol. 2010, 9, 1118–1127. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, G.; Siciliano, M.; Pedone, R.; Vitale, C.; Falco, F.; Bisogno, R.; Siano, P.; Barone, P.; Grossi, D.; Santangelo, F.; et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol. Sci. 2015, 36, 585–591. [Google Scholar] [CrossRef]
- Puthusseryppady, V.; Morrissey, S.; Spiers, H.; Patel, M.; Hornberger, M. Predicting real world spatial disorientation in Alzheimer’s disease patients using virtual reality navigation tests. Sci. Rep. 2022, 12, 13397. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Puthusseryppady, V.; Chan, D.; Mascolo, C.; Hornberger, M. Machine learning detects altered spatial navigation features in outdoor behaviour of Alzheimer’s disease patients. Sci. Rep. 2022, 12, 3160. [Google Scholar] [CrossRef]
- Spinnler, H.; Tognoni, G. Italian Group on the Neuropsychological Study of Ageing: Italian standardization and classification of neuropsychological tests. Ital. J. Neurol. Sci. 1987, 6, 1–20. [Google Scholar]
- Cerman, J.; Ross, A.; Laczo, J.; Martin, V.; Zuzana, N.; Ivana, M.; Katerina, S.; Jakub, H. Subjective Spatial navigation complaints-a frequent symptom reported by patients with subjective cognitive decline, mild cognitive impairment and Alzheimer’s disease. Curr. Alzheimer Res. 2018, 15, 219–228. [Google Scholar] [CrossRef]
- Yesavage, J.A. Geriatric depression scale. Psychopharmacol. Bull. 1988, 24, 709–711. [Google Scholar]
- Parizkova, M.; Andel, R.; Lerch, O.; Marková, H.; Gažová, I.; Vyhnálek, M.; Hort, J.; Laczó, J. Homocysteine and real-space navigation performance among non-demented older adults. J. Alzheimer’s Dis. 2017, 55, 951–964. [Google Scholar] [CrossRef]
- Laczó, J.; Andel, R.; Nedelska, Z.; Vyhnalek, M.; Vlcek, K.; Crutch, S.; Harrison, J.; Hort, J. Exploring the contribution of spatial navigation to cognitive functioning in older adults. Neurobiol. Aging 2017, 51, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Cushman, L.A.; Stein, K.; Duffy, C.J. Detecting navigational deficits in cognitive aging and Alzheimer disease using virtual reality. Neurology 2008, 71, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Benke, T.; Karner, E.; Petermichl, S.; Pranters, V.; Kemmler, G. Neuropsychological deficit associated with route learning in Alzheimer’s disease, MCI, and normal aging. Alzheimer Dis. Assoc. Disord. 2014, 28, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Brunec, I.K.; Ozubko, J.D.; Ander, T.; Guo, R.; Moscovitch, M.; Barense, M.D. Turns during navigation act as boundaries that enhance spatial memory and expand time estimation. Neuropsychologia 2020, 141, 107437. [Google Scholar] [CrossRef] [PubMed]
- Schapiro, A.C.; Turk-Browne, N.B.; Norman, K.A.; Botvinick, M.M. Statistical learning of temporal community structure in the hippocampus. Hippocampus 2016, 26, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Moodley, K.; Minati, L.; Contarino, V.; Prioni, S.; Wood, R.; Cooper, R.; D’Incerti, L.; Tgliavini, F.; Chan, D. Diagnostic differentiation of mild cognitive impairment due to Alzheimer’s disease using a hippocampus-dependent test of spatial memory. Hippocampus 2015, 25, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Schaat, S.; Koldrack, P.; Yordanova, K.; Kirste, T.; Teipel, S. Real-time detection of spatial disorientation in persons with mild cognitive impairment and dementia. Gerontology 2020, 66, 85–94. [Google Scholar] [CrossRef]
- Nedelska, Z.; Andel, R.; Laczó, J.; Vlcek, K.; Horinek, D.; Lisy, J.; Sheardova, K.; Bures, J.; Hort, J. Spatial navigation impairment is proportional to right hippocampal volume. Proc. Natl. Acad. Sci. USA 2012, 109, 2590–2594. [Google Scholar] [CrossRef]
- Iachini, T.; Iavarone, A.; Senese, V.P.; Ruotolo, F.; Ruggiero, G. Visuospatial memory in healthy elderly, AD and MCI: A review. Curr. Aging Sci. 2009, 2, 43–59. [Google Scholar] [CrossRef]
- Mitolo, M.; Gardini, S.; Fasano, F.; Crisi, G.; Pelosi, A.; Pazzaglia, F.; Caffarra, P. Visuospatial memory and neuroimaging correlates in mild cognitive impairment. J. Alzheimer’s Dis. 2013, 35, 75–90. [Google Scholar] [CrossRef]
- Markova, H.; Andel, R.; Stepankova, H.; Kopecek, M.; Nikolai, T.; Hort, J.; Thomas-Antérion, C.; Vyhnalek, M. Subjective cognitive complaints in cognitively healthy older adults and their relationship to cognitive performance and depressive symptoms. J. Alzheimer’s Dis. 2017, 59, 871–881. [Google Scholar] [CrossRef]
- Plummer-D’Amato, P.; Brancato, B.; Dantowitz, M.; Birken, S.; Bonke, C.; Furey, E. Effects of gait and cognitive task difficulty on cognitive-motor interference in aging. J. Aging Res. 2012, 2012, 583894. [Google Scholar] [CrossRef]
- Delpolvi, A.R.; Rankin, K.P.; Mucke, L.; Miller, B.L.; Gorno-Tempini, M.L. Spatial cognition and the human navigation network in AD and MCI. Neurology 2007, 69, 986–997. [Google Scholar]
- Electrophysiology, Task Force of the European Society of Cardiology the North American Society of Pacing. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Vlček, K.; Laczó, J. Neural correlates of spatial navigation changes in mild cognitive impairment and Alzheimer’s disease. Front. Behav. Neurosci. 2014, 8, 89. [Google Scholar] [PubMed]
- Bellassen, V.; Iglói, K.; De Souza, L.C.; Dubois, B.; Rondi-Reig, L. Temporal order memory assessed during spatiotemporal navigation as a behavioral cognitive marker for differential Alzheimer’s disease diagnosis. J. Neurosci. 2012, 32, 1942–1952. [Google Scholar] [CrossRef] [PubMed]
- Coutrot, A.; Silva, R.; Manley, E.; de Cothi, W.; Sami, S.; Bohbot, V.; Wiener, J.M.; Hölscher, C.; Dalton, R.C.; Hornberger, M.; et al. Global determinants of navigation ability. Curr. Biol. 2018, 28, 2861–2866. [Google Scholar] [CrossRef]
- Kirova, A.M.; Bays, R.B.; Lagalwar, S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. BioMed Res. Int. 2015, 2015, 748212. [Google Scholar] [CrossRef] [PubMed]
- Serino, S.; Pedroli, E.; Tuena, C.; De Leo, G.; Stramba-Badiale, M.; Goulene, K.; Mariotti, N.G.; Riva, G. A Novel Virtual Reality-Based Training Protocol for the Enhancement of the “Mental Frame Syncing” in Individuals with Alzheimer’s Disease: A Development-of-Concept Trial. Front. Aging Neurosci. 2017, 27, 240. [Google Scholar] [CrossRef]
- Mosca, I.E.; Salvadori, E.; Gerli, F.; Fabbri, L.; Pancani, S.; Lucidi, G.; Lombardi, G.; Bocchi, L.; Pazzi, S.; Baglio, F.; et al. Analysis of feasibility, adherence, and appreciation of a newly developed tele-rehabilitation program for people with MCI and VCI. Front. Neurol. 2020, 11, 583368. [Google Scholar] [CrossRef]
- Utoomprurkporn, N.; Limkitisupasin, P.; Tundiew, N.; Magauina, G.; Tunvirachaisakul, C.; Tangwongchai, S. Acceptability of a Balance Rehabilitation Program Delivered via a Tele-Remote Platform Among a Thai MCI Cohort. Alzheimers Dement. 2023, 19, e077961. [Google Scholar] [CrossRef]


| Demographic Variables | MCI due to AD (n. 20) | HCs (n. 20) | Significance |
|---|---|---|---|
| Age (yrs.) | 70.98 ± 4.75 | 72.26 ± 5.99 | p = n.s. |
| Education (yrs.) | 9.50 | 13.00 | p = n.s. |
| Gender | M = 75%; F = 15% | M = 40%; F = 60% | p = 0.025 |
| MoCA score | 21.79 | 24.52 | p = 0.015 |
| DMT-mv and Related Measures | MCI due to AD (n. 20) | HCs (n. 20) | Significance |
|---|---|---|---|
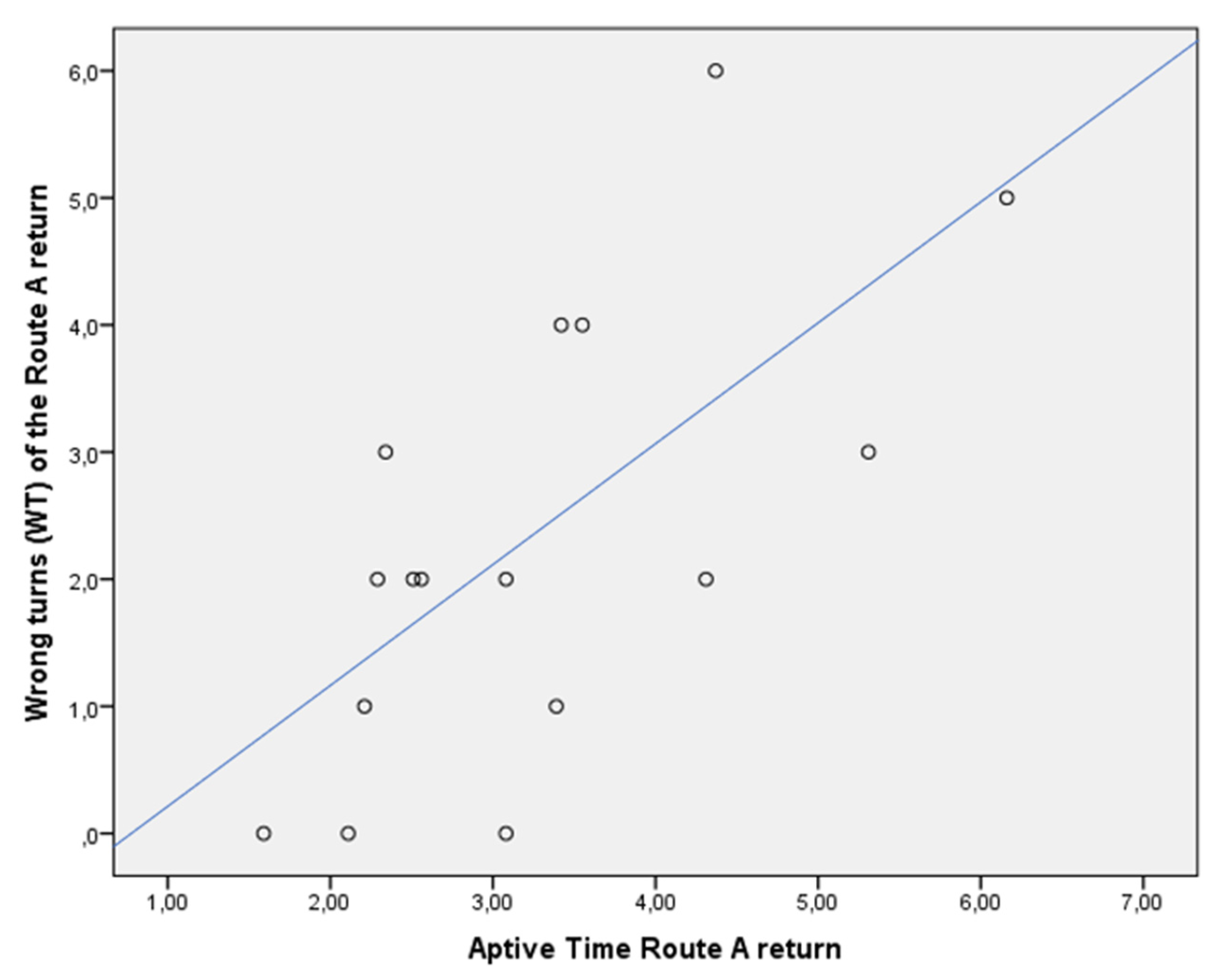
| Route A return, WTs (n.) | 4 | 1 | p = 0.001 |
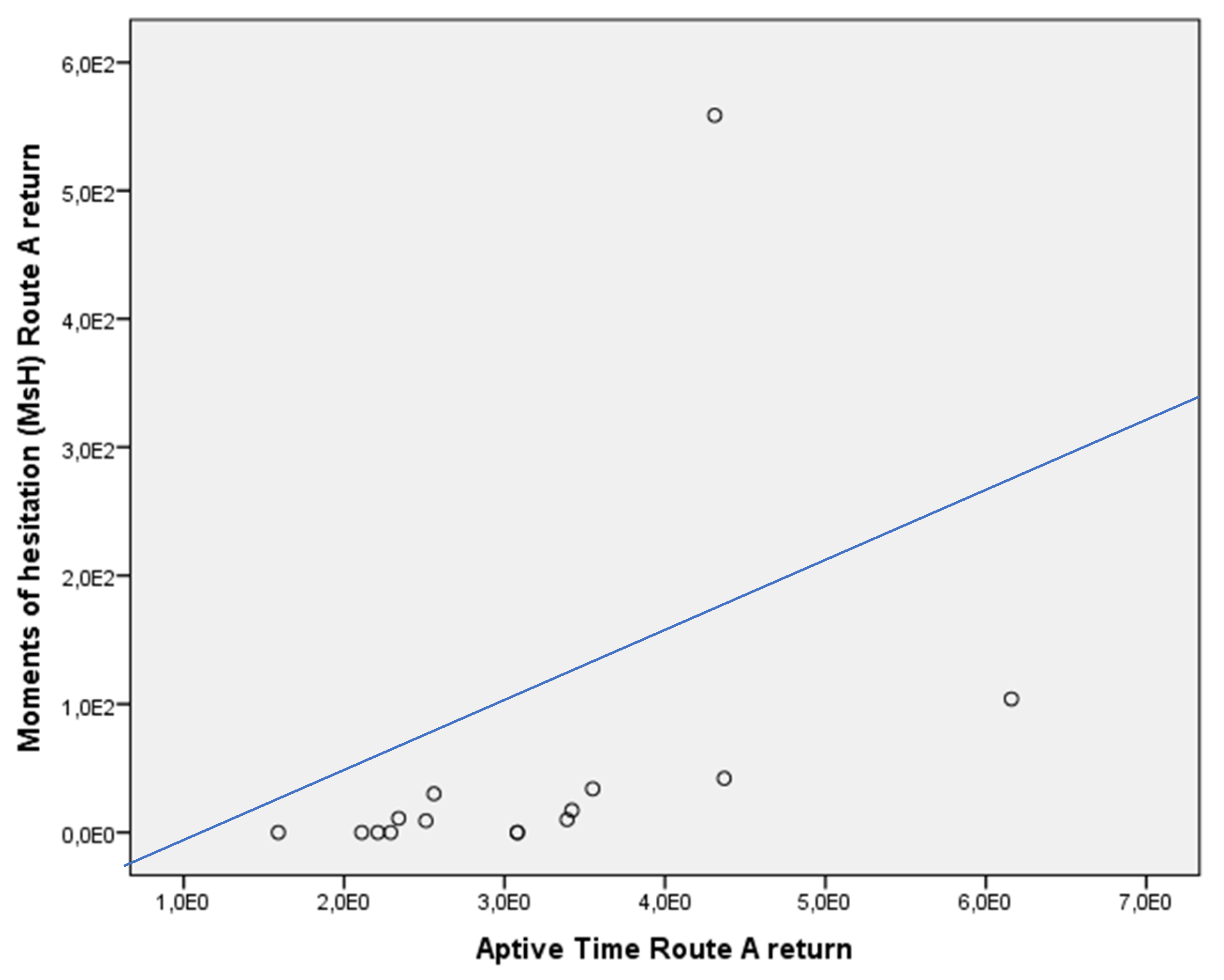
| Route A return, MsH (s) | 44 | 0 | p = 0.001 |
| Route B return, WTs (n.) | 1 | 0 | p = 0.009 |
| Route B return, MsH (s) | 14 | 0 | p = 0.004 |
| CLSS score | 5.99 | 21.98 | p = 0.004 |
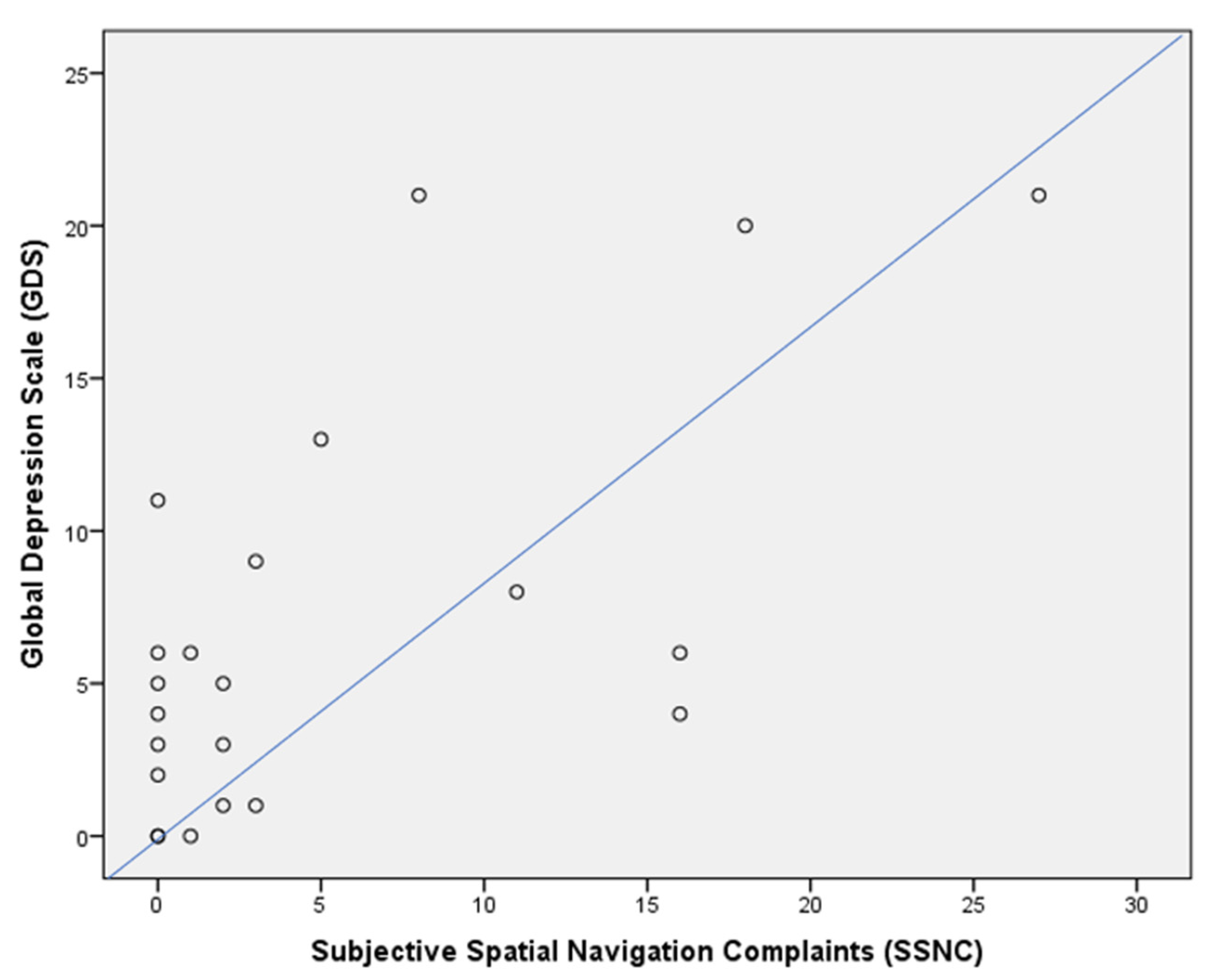
| SSNC score | 8 | 1 | p = 0.006 |
| GDS score | 3.5 | 7.5 | p = n.s. |
| Route A outward, landmarks recognition score | 14.4 | 2 | p = n.s. |
| Route B returns, alternative paths choice | B1 = 0; B2 = 13; B3 = 3 | B1 = 3; B2 = 10; B3 = 2 | p = n.s. |
| Route A return, overlap = 4 | Route A, return overlap = 5 |
| Physiological Parameters | MCI Due to AD (n. 20) | HCs (n. 20) | Significance |
|---|---|---|---|
| Rest state | |||
| Cardiac functions | |||
| BSI output | 9.88 | 6.50 | p = n.s. |
| LF/HF (Hz) | 2.24 | 2,.21 | p = n.s. |
| Respiratory functions | |||
| agvBR (b/m) | 23.40 | 20.60 | p = n.s. |
| SDBR (SD b/m) | 20.36 | 5.84 | p = n.s. |
| Route A, outward | |||
| Cardiac functions | |||
| BSI output | 88.63 | 85.11 | p = n.s. |
| LF/HF (Hz) | 2.28 | 0.66 | p = n.s. |
| Respiratory functions | |||
| agvBR (b/m) | 26.30 | 24.96 | p = n.s. |
| SDBR (SD b/m) | 34.44 | 4.98 | p = n.s. |
| Route A, return | |||
| Cardiac functions | |||
| BSI output | 87.66 | 93.88 | p = n.s. |
| LF/HF (Hz) | 2.47 | 0.82 | p = n.s. |
| Respiratory functions | |||
| agvBR (b/m) | 28.44 | 26.29 | p = n.s. |
| SDBR (SD b/m) | 22.97 | 6.19 | p = n.s. |
| Route B, outward | |||
| Cardiac functions | |||
| BSI output | 105.46 | 109.31 | p = n.s. |
| LF/HF (Hz) | 3.71 | 1.05 | p = n.s. |
| Respiratory functions | |||
| agvBR (b/m) | 27.49 | 26.56 | p = n.s. |
| SDBR (SD b/m) | 26.08 | 5.68 | p = n.s. |
| Route B, return | |||
| Cardiac functions | |||
| BSI output | 99.61 | 94.97 | p = n.s. |
| LF/HF (Hz) | 1.66 | 0.85 | p = n.s. |
| Respiratory functions | |||
| agvBR (b/m) | 23.55 | 27.24 | p = n.s. |
| SDBR (SD b/m) | 33.20 | 5.43 | p = n.s. |
| Gait Data | MCI Due to AD (n. 20) | HCs (n. 20) | Significance |
|---|---|---|---|
| Route A, outward | |||
| Aptive Time (min) | 2.45 | 2.19 | p = n.s. |
| Aptive Speed (m/s) | 0.50 | 0.61 | p = n.s. |
| Aptive Direction (degrees) | 177.90 | 199.39 | p = n.s. |
| Route A, return | |||
| Aptive Time (min) | 3.55 | 2.51 | p = n.s. |
| Aptive Speed (m/s) | 0.43 | 0.60 | p = n.s. |
| Aptive Direction (degrees) | 149.68 | 142.58 | p = n.s. |
| Route B, outward | |||
| Aptive Time (min) | 2.14 | 2.04 | p = n.s. |
| Aptive Speed (m/s) | 0.80 | 0.70 | p = n.s. |
| Aptive Direction (degrees) | 193.50 | 195.08 | p = n.s. |
| Route B, return | |||
| Aptive Time (min) | 2.07 | 1.42 | p = n.s. |
| Aptive Speed (m/s) | 0.57 | 0.71 | p = n.s. |
| Aptive Direction (degrees) | 139.40 | 116.07 | p = n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cammisuli, D.M.; Isella, V.; Verde, F.; Silani, V.; Ticozzi, N.; Pomati, S.; Bellocchio, V.; Granese, V.; Vignati, B.; Marchesi, G.; et al. Behavioral Disorders of Spatial Cognition in Patients with Mild Cognitive Impairment due to Alzheimer’s Disease: Preliminary Findings from the BDSC-MCI Project. J. Clin. Med. 2024, 13, 1178. https://doi.org/10.3390/jcm13041178
Cammisuli DM, Isella V, Verde F, Silani V, Ticozzi N, Pomati S, Bellocchio V, Granese V, Vignati B, Marchesi G, et al. Behavioral Disorders of Spatial Cognition in Patients with Mild Cognitive Impairment due to Alzheimer’s Disease: Preliminary Findings from the BDSC-MCI Project. Journal of Clinical Medicine. 2024; 13(4):1178. https://doi.org/10.3390/jcm13041178
Chicago/Turabian StyleCammisuli, Davide Maria, Valeria Isella, Federico Verde, Vincenzo Silani, Nicola Ticozzi, Simone Pomati, Virginia Bellocchio, Valentina Granese, Benedetta Vignati, Gloria Marchesi, and et al. 2024. "Behavioral Disorders of Spatial Cognition in Patients with Mild Cognitive Impairment due to Alzheimer’s Disease: Preliminary Findings from the BDSC-MCI Project" Journal of Clinical Medicine 13, no. 4: 1178. https://doi.org/10.3390/jcm13041178
APA StyleCammisuli, D. M., Isella, V., Verde, F., Silani, V., Ticozzi, N., Pomati, S., Bellocchio, V., Granese, V., Vignati, B., Marchesi, G., Prete, L. A., Pavanello, G., & Castelnuovo, G. (2024). Behavioral Disorders of Spatial Cognition in Patients with Mild Cognitive Impairment due to Alzheimer’s Disease: Preliminary Findings from the BDSC-MCI Project. Journal of Clinical Medicine, 13(4), 1178. https://doi.org/10.3390/jcm13041178








