Abstract
Patients undergoing metabolic surgery have factors ranging from anatomo-surgical, endocrine metabolic, eating patterns and physical activity, mental health and psychological factors. Some of the latter can explain the possible pathophysiological neuroendocrine, metabolic, and adaptive mechanisms that cause the high prevalence of weight regain in postbariatric patients. Even metabolic surgery has proven to be effective in reducing excess weight in patients with obesity; some of them regain weight after this intervention. In this vein, several studies have been conducted to search factors and mechanisms involved in weight regain, to stablish strategies to manage this complication by combining metabolic surgery with either lifestyle changes, behavioral therapies, pharmacotherapy, endoscopic interventions, or finally, surgical revision. The aim of this revision is to describe certain aspects and mechanisms behind weight regain after metabolic surgery, along with preventive and therapeutic strategies for this complication.
1. Introduction
The World Health Organization (WHO) defines overweight and obesity as an abnormal, excessive and harmful fat accumulation because it is a well-known independent risk factor for morbid conditions like diabetes mellitus (DM), dyslipidaemia, cardiovascular diseases, and cancer [1]. Over the last four decades, the prevalence of obesity has increased at an alarming rate in countries with Westernized lifestyles, becoming one of the major health concerns as a consequence of morbidity, mortality and the economic burden on national healthcare systems worldwide [2,3,4,5]. In fact, since 1975, obesity prevalence has shown a three-fold increase in adults and a five-fold increase in children and adolescents. Furthermore, according to the latest regional and national projections by the 2023 World Obesity Atlas report on obesity, the majority of the global population (51%, or over 4 billion individuals) will be suffering from being overweight or obesity, defined as a Body Mass Index (BMI) ≥ 25 kg/m2 and BMI ≥ 30 kg/m2. If current trends continue, the global economic impact of excess weight could reach $4.32 trillion annually, equivalent to 3% of the global GDP [6,7].
Despite multiple therapeutic strategies for weight loss (WL), combining several nutritional schemes, physical activity, cognitive-behavioral therapy, and pharmacologic intervention, medical obesity management is a challenging endeavor, often yielding limited success, as lifestyle-based interventions alone prove insufficient in achieving significant long-term weight loss in some patients, occasionally leading to a rebound effect where individuals regain more weight than initially present at the beginning [8,9].
On the other hand, the drugs approved for obesity are orlistat, phentermine/topiramate, naltrexone/bupropion, and the Glucagon-like peptide receptor agonists, like Liraglutide or Semaglutide, although these treatments can be expensive and may have adverse effects. Thus, it is important to carefully consider the potential benefits and risks of drug therapy before starting treatment in individuals with obesity [10]. It is important to mention that extensive pharmacological advances have been made, which will be discussed in greater depth below.
Given this challenge, along with the need for an effective and long-lasting treatment, metabolic surgery (MS) has proved its efficacy in losing massive amounts of both subcutaneous and visceral fat [11], which involves different techniques designed to correct or control obesity, aiming to improve quality of life by achieving adequate and long-lasting WL with minimal complications [12].
In 2022, the American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) states that MS is recommended in case of: BMI ≥ 35 kg/m2 (regardless of presence, absence, or severity of co-morbidities), patients with T2D and BMI ≥ 30 kg/m2, individuals with BMI of 30–34.9 kg/m2 who do not achieve weight loss or co-morbidity improvement using nonsurgical methods. Also, it is important to consider geographic factors; for example, obesity in Asiatic people is recognized as BMI > 25–27.5 kg/m2, so MS could be performed in these cases. On the other hand, age is not an exclusion or inclusion criteria for MS, and could be performed in Children and adolescents with BMI > 120% of the 95th percentile and a major co-morbidity, or a BMI > 140% of the 95th percentile [13,14,15,16].
MS procedures had traditionally been divided into restrictive, malabsorptive and mixed categories; however, it is now known that MS can cause weight loss not only through these mechanisms but also through appetite control, alterations of hormones of the gut brain axis, alterations in bile acid physiology and intestinal microbiota [17,18,19,20]. Even though MS successfully manages to decrease a significant percentage of body weight, not all patients can maintain the weight loss achieved and could surprisingly regain the lost weight [21]; this undesired scenario could affect the patient’s physical and mental health. Recently, this weight regain (WR) has been contemplated as a complication of multifactorial etiology; therefore, the current review will describe the main features and mechanisms behind WR together with preventive and therapeutic strategies [12,22].
2. Metabolic Surgery Complications
As previously mentioned, MS is a low-complication rate procedure with a minimum margin of risk. Overall, the combination of improved surgical techniques, surgeon expertise, patient selection, perioperative care, post-operative management, and advancements in technology and research have resulted in a notable decrease in the incidence of complications linked to bariatric surgery. Nonetheless, it is not exempt from complications, and MS has been associated with adverse surgical complications, including high-mortality complications [22]. Furthermore, Pallati et al. [23], in a systematic review and meta-analysis of 160,000 bariatric patients, reported a post-operative complications rate between 10–17% and a 7% reoperation rate; favorably, the mortality rate remained low (0.08–0.35%).
The perioperative or short-term complications can be divided into minor and major; the most common minor complications are usually at the surgical site (port bleeding or hematoma, skin infections, and post-operative neuropathic pain), hydro electrolyte imbalance, and urinary tract infections. Major complications involve anastomotic leaks, intra-abdominal bleeding, small bowel perforation, myocardial infarction, and pulmonary embolism <30 days after MS. Typically, these early complications frequency is below 1.6%, and the mortality rate is <0.7% [22]. Post-surgical leaks can arise from the gastrojejunal anastomosis of the RYGB (1.68–2.05%); in vertical sleeve gastrectomy, they emerge from the staple line (2.2%). Hemorrhages often begin in the staple line but can also come from anastomotic or gastric remnant ulcers [24].
Moreover, although mid- and long-term complications have been well described, establishing their exact incidence is difficult due to the increasingly significant number of patients who miss their follow-up visits as time goes by. These complications are stenosis, bowel obstruction, marginal ulcers, ventral hernia, fistula, gastroesophageal reflux disease, and metabolic complications like nephrolithiasis and hypoglycemia [25]. In this context, gastrojejunal stenosis is a common complication, with an incidence rate ranging from 4% to 27%, similar to gastroesophageal reflux disease, which occurs in 12% of cases. Meanwhile, gastric stenosis is uncommon, only occurring in 1% of the patients. Internal hernias generally cause small bowel obstructions after gastric bypass or rarely by intraperitoneal adhesions in 2–3% of the patients [25].
Several kinds of ulcers, most marginal, arise within the first 12 months after gastric bypass; their estimated incidence is around 16%, contrasting the much lower incidence of fistulas (1.2%). Unfortunately, fistulas can appear in almost any location of the digestive tract following surgery; gastro-gastric fistulas are an especially alarming RYGB complication. Another rare complication is hernias, which have a frequency of less than 1% in laparoscopic procedures; however, the frequency increases to around 8% for open procedures [24].
Additionally, vitamin and mineral deficiencies are also described as possible complications. Regarding nutritional deficiencies, biliopancreatic diversion leads to a more significant decrease in liposoluble vitamins, copper, and zinc compared to gastric bypass; contrarily, vitamin B12 deficiency, due to decreased levels of its intrinsic factors, is more frequently caused by gastric bypass in comparison to any other procedure [26].
In contrast to these low surgical complications, 10–20% of the patients are expected to regain a significant weight proportion in the long term. Likewise, it has been reported that 20–25% of the lost weight can be regained in a ten-year course, starting nearly 24 months after the surgery; thus, the mean weight regained after the surgery is 10 kg, ranging from 0.5 to 60 kg [27]. In this context, WR is considered clinically significant when WR is greater than or equal to 15% of the lowest weight reached while maintaining this increase for at least six months [28].
3. Risk Factors for Weight Regain after MS: Is It All about the Surgery?
The multifactorial nature behind WR after MS has hampered the comprehension of its mechanisms, and thus, the therapeutic approach. Psychological, behavioral, endocrine metabolic, genetic, and anatomical factors have been associated with WR [29,30].
3.1. Anatomic and Surgical Factors
The leading anatomical abnormality associated with WR is the enlargement of the gastric pouch (>6 cm long or >5 cm wide) and gastrojejunal stoma (diameter > 2 cm), and gastro-gastric fistula (GGF), which are mainly sequelae of procedures such as RYGB and vertical sleeve gastrectomy (VSG) [31,32,33]. A GGF is an abnormal communication between the proximal gastric pouch and the distal gastric remnant. Consequently, food detours to the “previous route” instead of the duodenum, increasing the available gastric volume and food’s absorption surface impairing the properties of MS [34,35,36].
By contrast, gastrojejunal stoma dilation leads to accelerated gastric pouch emptying and, therefore, a lack of satiety, instead accommodating larger amounts of food within the gastric pouch. Heneghan et al. [32] assessed the potential causes of WR by gastroscopy in patients submitted to RYGB (n = 380), reporting that only 28.8% of those who had WR (n = 205) had a normal-sized stoma, contrasting to 63.4% in patients who had successful weight loss (n = 175). Simultaneously, univariate statistical analysis demonstrates that the length and dilation of the stoma are the most influencing factors of WR; interestingly, the multivariate analysis only found the latter to be an independent factor for WR. Similarly, a retrospective study carried out by Yimcharoen et al. [33] reported that out of 205 patients with WR after RYGB, 58.9% had dilation of the gastrojejunal stoma, 28% had enlargement of the gastric pouch, and 12.3% had both of these findings.
Similarly, a study in people submitted to RYGB reported that limb length does not influence post-MS weight changes [37]. Still, this could be attributed to the methodology, study sample size, and confounding variables that could induce WR and enlargement of the gastric pouch, such as patients’ lifestyles and psychological status.
3.2. Endocrine and Metabolic Factors
RYGB has been associated with episodes of hypoglycemia, a significant clinical component of the dumping syndrome (DS). In a study involving 36 RYGB patients, Roslin et al. [38] assessed their glucose levels six months after the procedure. They found that 11 patients had weight regain exceeding 10%, and six of these experienced hypoglycemia two hours after glucose load. Likewise, a study performed by Varma et al. [39] on 428 American patients who underwent MS determined that the odds of WR were significantly higher in those who had symptoms of hypoglycemia (OR: 1.66; 95% CI: 1.04–2.65). The authors have suggested that the causal relation could be due to metabolic changes produced by glucose homeostasis effects on appetite and gastrointestinal functioning [39]. Additionally, it has been proved that in the long term, post-MS patients with WR exhibit alterations in the levels of gastrointestinal and neuronal peptides related to appetite and satiety, which could indicate that changes in hormonal parameters contribute to WR [40].
3.3. Lifestyle: Eating Patterns and Physical Activity
Implementing lifestyle changes that counter the “obesogenic” environment before surgery is essential to achieve meaningful results in the WL after MS. In this context, it has been described how post-MS patients regain weight by eventually neglecting their lifestyle changes [41]. Furthermore, once the patients reach their target weight, some may increase their caloric intake, an expected eating behavior two years after the surgery [42,43].
Some of patients either fail to keep adequate control of their post-MS nutritional status or refuse entirely to follow the dietary patterns suggested by the weight management team, contrarily, maintain a high caloric intake attributed to a large intake of high-fat food, junk food, sweets, and high-sugar drinks, leading to suboptimal WL or even WR [41,43,44]. Bassan et al. [44,45] conducted a retrospective study that included 80 patients at least 24 months after MS and reported that 23.7% presented a WR greater than 10% of the lowest post-operative weight. Moreover, supported by multivariate analysis, a positive association between Healthy Eating Index and WR was observed (OR 0.95; p = 0.04), correlating to similar results found by other authors [44,46].
Furthermore, common maladaptive eating patterns among post-MS patients, such as binge eating and grazing, are considered risk factors for reduced WL [47]. Grazing can be defined as repeated episodes of consuming small quantities of food over a long time and is usually accompanied by feelings of guilt and loss of control [47,48]. A systematic review including 994 post-MS patients showed 16.6–46.6% engaged in grazing and 47% engaged in WR; an association was found between these two variables regardless of the type of MS and the author’s definition of grazing [49].
Another factor linked to WR is dysphagia, a frequent complication after RYGB [50]. In a prospective cohort study by Runge et al. [51] on 245 post-MS patients, a higher WR was observed in those with dysphagia (n = 49) in comparison to the control group (n = 196) (37% vs. 25%). These things considered, patients with dysphagia are more likely to incline their diet partially or even entirely towards soft or liquid food since they are absorbed faster and produce less satiety, favoring a higher caloric intake and thus a positive energy balance that could explain the WR [51,52].
Regarding the food preferences observed after MS, although patients reported a diminished explicit liking for sweet foods at 3 months post-surgery and a lower desire to consume them at both 3- and 12-months post-surgery, intake of high-sugar foods was maintained in another study [53]. In this regard, a meta-analysis showed that bariatric surgery could be effective on energy and fat intake; however, there was no effect on carbohydrate intake [54], being considered another risk factor for developing weight gain after MS.
Besides unhealthy dietary habits, sedentarism or lack of physical activity can also be risk factors for WR. According to Rosenberg et al. [55], only 10–24% of the patients who underwent MS had performed the minimum physical activity to maintain their health status. Moreover, in a study by Yanos et al. [56] on 97 patients submitted to RYGB, 26% exhibited WR, associated with nocturnal eating, alcohol consumption, and diet and physical activity modifications. Correspondingly, Freire et al. [44] reported a lower incidence of WR among post-MS patients who exercised than those who did not.
3.4. Psychological Factors and Mental Health
Neuropsychiatric and Psychological disorders have been linked to WR and can hinder adherence to dietary and behavioral intervention plans during and after MS [46]. Binge eating disorder (BED), defined by The Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (DSM-5) as the uncontrolled consumption of larger and more than usual food quantities within two hours, is one of the main predictors of WR after MS [57]. However, BED prevalence among post-MS patients varies significantly according to the criteria used by different authors, ranging from under 5% to almost 24% [58,59]. Still, it has been demonstrated that BED is related to reduced WL or even WR two years after the procedure, along with developing worse maladaptive eating behaviors than prior MS [47,60]. Similarly, other eating disorders, such as soft food and night eating, have been recognized as predictors of WR after MS [61].
Furthermore, psychiatric disorders increase the risk of WR during post-operative periods; for instance, Rutledge et al. [62] showed that those individuals presenting two or more psychiatric disorders were six times more likely to develop WR post-MS. Under this framework, depression stands out among the most common disorders in bariatric patients, and although the association between this and WR or failure in post-MS WL has been demonstrated, as well as its presence predisposes individuals to be more prone to develop eating disorders, the results of studies tend to contradict each other since some show that depression is diminished after MS or, failing that, no causal relationships are observed in their analyses [56,63,64,65,66]. Similarly, patients with WR have high clinical or borderline anxiety and stress levels; however, these were not associated with higher energy consumption [66,67].
Finally, drug use and alcoholism have been described as influential factors in WR, as post-MS patients may seek relief from other substances through “addiction transfer” to substitute the needs established by the brain reward system for excessive energy consumption prior to MS [60,68,69]. Odom et al. [65] followed up on 203 post-RYGB patients, showing that decreased post-MS well-being, increased need to eat, and preoccupation with drug or alcohol use (addictive behavior) were independent predictors of WR. Thus, it is clear that bariatric patients need pre- and post-MS psychological assessment to ensure expected outcomes in WL and avoid relapse in maladaptive habits related to WR [70].
3.5. Preoperative and Other Factors
Numerous studies have found preoperative factors that may predispose patients to WR. A prospective study of 782 bariatric patients showed that approximately 50% of them presented WR, with patients in the super-obese group having a higher percentage of surgical failure (18.8%) and WR. The authors concluded that individuals with higher BMI before surgery are more likely to WR [71]. In particular, Keith et al. [72], in their retrospective study, described that preoperative factors such as male sex (p = 0.020), white race (p < 0.001), and high socioeconomic level (p = 0.035) were associated with WR. Furthermore, when multivariate analysis was performed, it was observed that socioeconomically advantaged patients were more likely to have WR than the rest (OR: 1.82, CI 1.18 to 2.79). However, other authors have differed with this study since their analyses establish that female sex and black race could be considered risk factors for WR [37,73,74].
Although age has been highlighted as a possible preoperative factor related to WR, results among studies vary significantly, with both young and older adults (>60 years) reported to be prone to WR [73,75,76]. Moreover, time elapsed after surgery, iron deficiency [77], work activity related to eating, and comorbidities such as T2DM have been linked to WR [78].
Remarkably, medications for psychiatric disorders, including tricyclic antidepressants, valproic acid, lithium, and antipsychotics, as well as antidiabetic drugs, steroids, and contraceptives, have been associated with weight gain and positive appetite modulation [79]. In this regard, post-MS patients treated with any of these drugs could theoretically be at high risk for WR [80]. In addition, there are genetic factors related to the development of obesity and adipose tissue (AT) biology that could be implicated in post-MS WR. Among these, genetic polymorphisms of AT adrenoreceptors such as the ADRB2 gene (Gly16Arg and Gln27Glu), and those related to leptin, such as LEPR-gene (LEPR Lys109Arg, LEPR Gln223Arg, LEPR Lys656Asn), stand out [81].
4. Underlying Mechanism of Weight Regain after Metabolic Surgery: Neuroendocrine and Metabolic Perspectives
The mechanisms involved in post-MS WR remain poorly understood and are mainly based on possible hormonal changes and the body’s response to caloric restriction following MS. Hypothetical models on neuroendocrine and metabolic mechanisms influencing WR in post-MS patients are presented below (Figure 1). However, the theoretical models will be grounded on the results of preclinical and clinical studies whose results tend to vary and be controversial, possibly due to all methodological aspects involved.
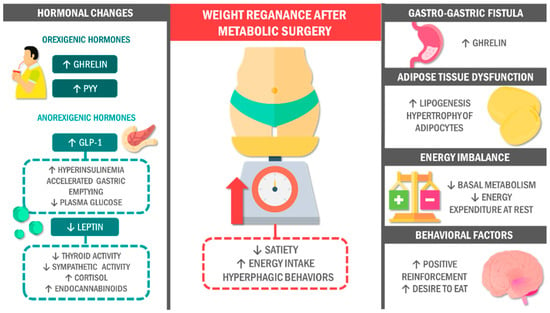
Figure 1.
Potential mechanisms associated with weight regain after metabolic surgery. However, the surgery results are not always as expected. On the contrary, patients exhibit altered levels of orexigenic hormones that promote appetite and an excess of certain anorectic hormones that could lead to metabolic alterations associated with weight regain. Likewise, post-surgical dietary changes can be associated with developing an energy gap that favors excessive food consumption. In addition, post-MS patients tend to have maladaptive eating patterns capable of eliciting ingesting amounts of food similar to those before surgery. Abbreviations: GLP-1: glucagon-like peptide 1; PYY: peptide YY; MS: metabolic surgery.
In this vein, gastrointestinal hormones and their interactions with the nervous system play a fundamental role in WR. The hypothalamus is the main energy regulator of physiology, appetite, and body weight. It maintains reciprocal communication with other central areas and peripheral organs through afferent and efferent fibers, neurotransmitters, hormones, and peptides [82,83]. In particular, the arcuate nucleus (ARC) has two neuronal populations with contrasting effects on appetite. In this respect, an orexigenic population promotes appetite by releasing neuropeptide Y (NPY) and agouti-related peptide (AgRP). On the other hand, anorexigenic neurons promote satiety by releasing pro-opiomelanocortin (POMC) metabolites such as melanocyte-stimulating hormone (α-MSH), together with cocaine and amphetamine-regulated transcript (CART) [84,85].
Other hypothalamic regions such as the paraventricular nucleus (PVN) and the lateral hypothalamic area (LHA) (where orexigenic peptides such as orexins 1 and 2 are released), as well as the nucleus of the solitary tract (NTS) and the postrema area (PA) of the brainstem, are part of the energy intake (EI) regulatory intercommunication network [86,87,88]. In addition, these areas are interconnected with the limbic system and regions of the cortex involved in reward control and stimulus perception, among other key functions for EI [89,90].
On the other hand, the gastrointestinal tract releases peptides according to nutrient availability; that is, under fasting or post-prandial conditions. In states of starvation or fasting, ghrelin is released by the X/A cells of the gastric fundus; this is a polypeptide of 28 amino acids that has its receptor (GHS-R) in several anatomic structures, including the vagus nerve and the hypothalamus. Ghrelin promotes appetite by activating NPY/AgRP and LHA orexigenic neurons [91,92,93,94] and possesses gastrointestinal prokinetic properties that promote gastric emptying. The latter mechanism enhanced appetite and EI by decreasing gastric distension and satiety signals [95,96].
Moreover, the mechanical and chemical stimuli produced by food intake, the formation of the food bolus, and its caloric content can lead to the release of anorexigenic gastrointestinal peptides involved in satiety, like peptide YY (PYY), cholecystokinin (CCK), glucagon-like peptide 1 (GLP-1), among others [83,97]. These peptides can counteract ghrelin activity by blocking its release, acting on anorexigenic POMC/CART neurons, and inhibiting NPY/AgRT signals in the hypothalamus. Therefore, anorexigenic hormones act either through direct action on receptors located in the hypothalamus (PYY binds directly to Y2 receptors) or by promoting peripheral mechanical satiety signals through vagal afferents that interact with areas such as the AP and NST (in the case of CCK) [98,99,100,101]. Moreover, these inhibitory signals by gastrointestinal peptides are reinforced by the activation of serotonergic 5-HT1b receptors in subcortical areas, which promotes the suppression of orexigenic pathways [102].
After RYGB, hormonal changes that promote WL are observed, mainly an increase in PYY levels and a decrease in ghrelin levels compared to obesity or pre-surgery subjects [103,104,105,106,107]. However, preclinical and clinical studies show that these hormone levels are affected in mice and individuals with post-RYGB and, curiously, post-VSG WR. In fact, ghrelin and PYY levels are similar to those prior to surgery or not high enough compared to individuals with long-term WL. Thus, there is a higher proportion of orexigenic peptides than anorexigenic peptides [40,108,109,110]. Accordingly, these changes could increase appetite and IE, correlating with maladaptive eating patterns in postbariatric patients [46]. This theory becomes relevant in patients with GGF and other post-MS anatomical alterations. GGF can increase ghrelin secretion by X/A cells in the sectioned stomach in these cases. Curiously, the levels of orexigenic hormones and the subjects’ weight are diminished after GGF correction and gastric stoma lengthening [111,112].
Another peptide that affects the metabolic status of post-RYGB patients is GLP-1. It has insulinotropic properties that contribute to the remission of metabolic comorbidities such as T2DM [113,114]. Studies have found that GLP-1 levels increase after RYGB [107,115], leading to a hyperinsulinemic response that may result in postprandial reactive hypoglycemia (a characteristic of DS), along with accelerated gastric emptying that may decrease satiety-related mechanical distention signals [38,116]. Since glucose is recognized as a modulator of appetite, low levels of this molecule following a hyperinsulinemic response may promote an increase in EI and WR [38,39].
Furthermore, post-MS caloric restriction is related to changes in the metabolic profile of AT, favoring adipogenesis and decreasing both adipocyte size and insulin sensitivity. These changes could augment glucose uptake by AT, which would generate increased lipogenesis and fat storage, contributing to adipocyte hypertrophy, a hallmark in patients with obesity [117,118,119,120]. In this sense, bariatric patients could maintain a dysfunctional AT, a cornerstone of deflective endocrine metabolic signals involved in WR-related hyperphagic states [118]. These mechanisms would support the hypothesis of the obesogenic memory of immune cells [121].
In this sense, dysfunctional AT is characterized by a proinflammatory secretory profile. One of the principal adipokines secreted by the dysfunctional AT is leptin, a hormone related to satiety control [122]. Leptin levels are proportional to the degree of energy storage; i.e., in patients with high levels of adiposity, leptin release is higher [123,124]. This increase leads to leptin resistance in the hypothalamus due to receptor downregulation [125,126]. Contrarily, lower leptin levels have been observed in mouse models under calorie-restricted diets and following RYGB compared to controls or cases where surgery was defective [127,128]. These results may reflect a compensatory mechanism to the energy deficit that promotes hunger for energy conservation [88,129,130].
Likewise, low leptin levels are associated with the production of endogenous endocannabinoids. These compounds bind to CB1 receptors in the hypothalamus and AT, triggering the secretion of orexigenic factors and lipogenic activity, respectively [131,132,133]. Similarly, other homeostatic mechanisms that seek to reestablish a state of energy balance have been described, including a reduction in thyroid and sympathetic activity due to low leptin levels. These homeostatic responses could promote lower resting energy expenditure, increasing appetite and energy replenishment [128,130,134,135]. In addition, these changes in energy and leptin levels can stimulate the PVN, favoring hypothalamic-pituitary-adrenal axis activity and thereby increasing cortisol release. High plasma cortisol concentrations are related to increased appetite and fat storage [136,137,138]. However, the effects of leptin on WR remain controversial [40,139,140].
Energy balance is essential for maintaining WL or, conversely, WR. Following MS, there is a depletion of EI that will eventually decrease basal metabolism and resting energy expenditure. These metabolic changes generate a negative energy imbalance. Consequently, our body seeks to respond to this deficit by promoting EI and WR [118,141,142,143,144]. This “energy gap” can be counteracted by physical activity, which is why it is always recommended to implement a physical exercise regimen along with the diet to achieve WL and its long-term maintenance [144,145].
In contrast, non-homeostatic WR-related mechanisms could be extrapolated to post-MS patients [146,147]. Brain areas of the mesocorticolimbic system play a key role in the reward system, eating behavior, and perception of sensory stimuli that may affect EI [89,90,148]. Studies using positron emission tomography and magnetic resonance imaging have described the activation of some of these regions in individuals who, after WL, responded positively to food stimuli that promoted motivation and desire to eat energy-rich foods, rectifying the role of hedonic pathways in WR [146,149,150]. At the same time, hormones such as ghrelin, leptin, and endogenous endocannabinoids constantly interact with the positive reinforcement of the reward system, so post-MS individuals likely relapse into previous high-calorie consumption patterns [151,152,153,154].
It should be emphasized that further research should be carried out despite the possible neuroendocrine, metabolic, and adaptive theories previously presented. Preclinical and clinical studies should be promoted to elucidate the exact mechanisms involved in WR and to establish an appropriate approach to combat the development of this complication of MS.
5. Strategies for Prevention and Management of Weight Regain after Metabolic Surgery
WR post-MS is a challenging and complex phenomenon that urges specialists to design an ideal plan to prevent and treat this condition. Due to the multifactorial component of WR, several studies have been conducted that consider various therapeutic perspectives to ameliorate WR. One is a specialized multidisciplinary care approach involving behavioral therapies, lifestyle changes, and weight management [155,156,157,158,159,160]. Other approaches under study include the use of appetite suppressants or anorexigenic pharmacotherapy [156,161] (Table 1), as well as endoscopic procedures and even revision surgery (Table 2) [162,163,164,165], considered as one of the last options due to its well-known complications [166].

Table 1.
Lifestyle changes and pharmacotherapy of weight regain after bariatric surgery.

Table 2.
Invasive strategies for prevention and management of weight regain after metabolic surgery.
5.1. Lifestyle Changes
First, according to several studies, assessing MS patients through a weight control program is a good approach to preventing WR [156]. In this regard, recognizing mild (0.2% to <0.5%), moderate (0.5% to 1.0%), and rapid (>1.0%) WR within 30 days is essential for early detection of this condition [60], finding that routine long-term clinical follow-up of the patient is necessary.
Recently, researchers have evidenced that in addition to having a weight control program, the establishment of healthy dietary and physical activity (PA) behaviors are good predictors of WL post-MS [167]. In a 2-year follow-up study, Nuijten et al. [157] found better body composition and quality of life in bariatric patients who achieved changes in their PA. Likewise, a prospective observational study linked higher WL to individuals who adhered to Mediterranean dietary patterns [158]. Because of the relationship between healthy behaviors and better post-operative outcomes, a multidisciplinary care program with weight control and lifestyle interventions is necessary to prevent and treat WR post-MS [159].
In another context, post-operative behavioral management was postulated as an emerging strategy for post-RYGB WR in a meta-analysis [168]. In this sense, a pilot study demonstrated the feasibility of the behavioral intervention based on acceptance and WL, since after ten weeks, WR managed to stop and reverse with an overall mean of 3.58 ± 3.02% of total body weight (TBW) loss among individuals who completed the treatment [180]. Similar findings were evidenced in post-BGYR WR patients, in which weight (mean 1.6 ± 2.38 kg), depressive symptoms (p ≤ 0.01), grazing patterns (p ≤ 0.01), and binge eating episodes (p ≤ 0.03) decreased significantly after behavioral intervention lasting six weeks [160].
Likewise, the support of a remotely applied behavioral therapy to help patients with WR was carried out by the Healthy Eating and Lifestyle Post-surgery (HELP) intervention [181]. This project demonstrated the feasibility and high acceptability of accompanying this therapy using e-learning software suite for 10-week sections. Nevertheless, the participants of this project presented a significant WL (5.1%) achieved in the first three months post-intervention, justifying the high potential of this supportive therapy intervention for post-MS WR patients. However, studies with larger samples and better-designed methodologies are required to give this approach more weight in the future.
5.2. Pharmacotherapy
However, different studies have found that, besides lifestyle changes, PA levels, and behavioral therapies, the addition of pharmacotherapy to decrease appetite and elicit satiety is an effective alternative to interrupt WR [161,182]. In the last decade, great progress has been made with obesity pharmacotherapy with impressive results in weight loss. Interestingly, recent findings have found a particular link between weight-loss medication (WLM) and patients with RYGB and gastric banding, as increased WL in response to pharmacotherapy and the metabolic surgical procedures previously mentioned have been evidenced [169,172].
In this setting, a multicenter study with 319 patients demonstrated a WL ranging from ≥5% to ≥15% of their TBW in subjects with inadequate WR or WL after RYGB or LSG [183]. To evaluate the efficacy of the therapy, Hanipah et al. [169] set out to determine the effectiveness of phentermine, extended-release phentermine/topiramate, lorcaserin, and slow-release naltrexone/bupropion. After a year of adjuvant therapy, more than one-third of patients lost >5% of total weight. In addition, the study found that with higher BMIs, the efficacy between BMI at baseline and total weight loss at one year increased (p = 0.025).
Similarly, topiramate could be a cost-effective adjuvant in those patients with adjustable gastric banding (AGB) and difficulty in WL [184]. Recent studies investigated the efficacy of this drug and found a statistically significant association with WL, as patients receiving topiramate lost up to 10% of TBW [183]. In addition, topiramate + phentermine has also been tested, as it has been postulated in studies to be one of the most effective WLMs among patients [185].
In this context, Istfan et al. [186] studied the impact of topiramate + phentermine on WR after RYGB, finding that both their individual use and combination could significantly reduce WR after RYGB. Similar findings were found in studies that attempted to evaluate the efficacy of such a pharmacological combination [185,187]. Moreover, there is evidence that phentermine and fenfluramine compounds could be used as adjunctive therapy to inhibit appetite in bariatric surgical patients after 12 weeks of treatment [188].
In the same way, another adjuvant and anorexigenic drug indicated to treat low WL or WR post-MS is liraglutide [189,190]. This long-acting GLP-1 analogue induces WL by slowing gastric emptying and augmenting insulin secretion and satiety control via stimulating POMC/AgRP neurons in the hypothalamus [191,192]. In this respect, Wharton et al. [170] observed a significant WL (±7.7 kg, p < 0.05) in patients with post-RYGB WR, banding, and gastric sleeve who took 3.0 mg of liraglutide for 7.6 ± 7.1 months. These findings seem to be in line with other similar results from studies that sought to determine the efficacy of liraglutide as a short-term alternative treatment [193] and at high doses (3.0 mg) in post-surgical WR patients [194].
Multiple studies have been conducted using liraglutide as well as some with semaglutide [189,195]. After undergoing bariatric surgery, participants in another study began semaglutide treatment around a year later. Despite regaining 12% of their body weight since the surgery, the participants saw a remarkable 6% reduction in weight after only three months of semaglutide treatment [195].
Meanwhile, metformin has also been added to the list of possible adjuvant medications for post-MS WR, following the results of an intervention in patients with post-MS WR, who presented improvements in body weight and metabolic profile. In this regard, Torbay et al. [171] demonstrated that after the administration of 1800 ± 350 mg/day of metformin added to an ad libitum, low-carbohydrate, non-ketogenic, or high-protein diet (LCNK), patients with WR post-MS were able to reduce the equivalent of 5% of their weight, as well as reduce fasting blood glucose, HBA1c, cholesterol, and HOMA-IR levels, improving the insulin resistance that the patients had at the beginning of the study.
However, it is important to stress that better research involving larger samples with longer follow-ups and better evidence quality is still needed. Thus, determining the efficacy of adjuvant pharmacotherapy after metabolic surgery could be scaled to a more optimal and safe level, adding anti-obesity drugs for those patients who require such an alternative to control WR post-MS [182].
5.3. Endoscopic Interventions
On the other hand, endoscopic interventions represent a different approach to post-MS WR [163,196]. In this regard, a systematic review and meta-analysis evaluated the safety and efficacy of transoral outlet reduction (TORe) to treat post-RYGB WR on 850 patients. Moreover, WL ranging from 6.14 (kg) to 10.15 (kg) over 3 to 12 months was evidenced [196]. Furthermore, these results were consistent with other studies evaluating the efficacy of TORe using endoluminal suturing [164,197,198,199], positioning it as a feasible and promising endoscopic approach for patients with post-RYGB WR as well as for other complications such as SD [200].
Suturing techniques to improve TORe have also been studied, including full-thickness suture plus argon plasma mucosal coagulation (APC-TORe) and argon plasma mucosal coagulation alone (APMC-TORe) [201]. Although APMC-TORe usually demands several endoscopic sessions, the study indicated that both suturing techniques provide significant WL for patients with WR post-MS [201]. Similar findings were found in other studies evaluating the effectiveness of APC-TORe and APMC-TORe [173,174,175,202,203]. In this concern, modified endoscopic submucosal dissection (ESD) for TORe (ESD-TORe) has also been closely studied [204]. Interestingly, the combination of ESD-TORe and suturing provided a higher WL for patients with post-BGYR WR, with the ESD-TORe group experiencing a WL of 12.1% ± 9.3% versus 7.5% ± 3.3% for the traditional APC-TORe group.
5.4. Revisional Surgery
As a final point, although there are not enough studies on the subject, post-MS WR in some cases, may ultimately require revision or conversion surgeries, which are becoming more frequent every year [176,205,206]. Recently, Kermansaravi et al. [207] conducted a systematic review and meta-analysis that found that most revision surgical methods following primary RYGB have been safe and effective for post-MS WR. However, it is important to note that either distal Roux-enY gastric bypass (DRGB), biliopancreatic diversion with duodenal switch (BPD-DS), or single anastomosis duodenum-ileal bypass with gastric sleeve (SADI-S) seem to be the most effective long-term alternatives.
On the other hand, a prospective study demonstrated that revision procedures offered higher WLs after failed primary sleeve gastrectomy (SG) [176]. In this regard, investigators found an initial BMI of 41.9 (11.7) kg/m2 and subsequently, at follow-up, 12 months later, resulted in RYGB 13.2 (11.3) kg, and re-sleeve 12.0 (11.9) kg; p = 0.023. This 6.3 (5.1) kg/m2 decrease in BMI at one year provides evidence on the one hand of higher WL with revision bariatric surgery and, on the other hand, the preference of using techniques such as bypass to re-sleeve surgeries according to studies [176,177]. Likewise, other studies emphasize that the placement of silicone rings again induces greater WL in patients than in patients who have had the band since the original operation [179].
Although the data suggest optimal results, more studies with longer follow-ups are needed, especially those regarding post-MS revision surgery since it has been shown that the longer the post-operative follow-up, the better the results regarding post-MS WL [179]. Therefore, future studies should involve better methodology to achieve better evidence from less invasive approaches, such as lifestyle changes and behavioral therapies, to more invasive procedures, such as endoscopic interventions and revision surgery [60,178,208].
6. Conclusions
Both obesity and overweight are worldwide public health problems. The long-term results of traditional weight loss therapies, e.g., diet, exercise, and medication, especially those above, have not yet been thoroughly explored. On the other hand, MS is an effective treatment that improves and remits many related comorbidities. In addition, it favors sustained weight loss and improved patient quality of life while reducing mortality. Currently, the procedures included are Adjustable Gastric Banding, Vertical Banded Gastroplasty, Jejunoileal Bypass, Jejuno-colic Bypass, Roux-enY Gastric Bypass and Biliopancreatic Diversion with Duodenal Switch.
In conclusion, although MS is associated with many benefits, it also has multiple complications. One of the most prevalent is post-MS weight regain [209], caused by several psychological, behavioral, genetic, and anatomical factors. In addition, studies have also identified neuroendocrine, metabolic, and adaptive mechanisms that could influence WR in post-MS patients. Due to its multifactorial component [209], various therapies have been proposed to ameliorate WR. These include specialized multidisciplinary care (i.e., a combination of behavioral therapies, lifestyle changes, and weight control), appetite suppressants or anorexigenic pharmacotherapy, endoscopic procedures, and even revision surgery. However, it is worth noting that this is a subject with limited scientific evidence; therefore, further research is needed to elucidate the exact mechanisms involved in WR to establish an approach to combat the development of this complication of DS. These types of approaches are necessary and should be susceptible to periodic study.
Author Contributions
Conceptualisation: J.S., H.P., P.D., V.B. and H.G.-P. Investigation: J.S., H.P., P.D., R.A., H.G.-P., M.C., N.V. and G.C.; Writing—original draft: B.G., M.H. and H.G.-P.; Writing—review and editing: C.C., R.A., M.C., G.C. and V.B.; Supervision: C.C., M.C. and V.B.; Visualisation: J.S., H.P., P.D., R.A., M.H. and M.C.; Funding acquisition: V.B. and H.G.-P. All authors have read and agreed to the published version of the manuscript.
Funding
Internal funds for research strengthening from Universidad Simon Bolívar. Vicerrectoría de Investigación, Extensión e Innovación, Barranquilla, Colombia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Abbreviations
| BMI | Body Mass Index |
| WHO | Health Organization |
| DM | diabetes mellitus |
| WL | weight loss |
| RYGB | roux-en-Y gastric bypass |
| WR | weight regain |
| MS | metabolic surgery |
| GGF | gastro-gastric fistula |
| LSG | laparoscopic sleeve gastrectomy |
| DS | dumping syndrome |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition |
| AT | adipose tissue |
| ARC | arcuate nucleus |
| NPY | neuropeptide Y |
| AgRP | agouti-related peptide |
| POMC | propiomelanocortin |
| CART | amphetamine-regulated transcript |
| PVN | paraventricular nucleus |
| LHA | lateral hypothalamic area |
| NTS | nuclei of the solitary tract |
| EI | energy intake |
| PYY | peptide YY |
| CCK | cholecystokinin |
| GLP-1 | glucagon-like peptide 1 |
| PA | physical activity |
| TBW | total body weight |
| HELP | Healthy Eating and Lifestyle Post-surgery |
| WLM | weight-loss medications |
| AGB | adjustable gastric banding |
| LCNK | ad-libitum, low-carbohydrate, non-ketogenic, or high-protein diet |
| TORe | transoral outlet reduction |
| APC-TORe | argon plasma mucosal coagulation |
| APMC-TORe | argon plasma mucosal coagulation alone |
| ESD | endoscopic submucosal dissection |
| DRGB | distal Roux-en-Y gastric bypass |
| SADI-S | single anastomosis duodeno-ileal bypass with gastric sleeve |
| SG | sleeve gastrectomy |
References
- Obesity and Overweight. Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 February 2020).
- GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef]
- The Global BMI Mortality Collaboration. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef]
- Lassale, C.; Tzoulaki, I.; Moons, K.G.M.; Sweeting, M.; Boer, J.; Johnson, L.; Huerta, J.M.; Agnoli, C.; Freisling, H.; Weiderpass, E.; et al. Separate and combined associations of obesity and metabolic health with coronary heart disease: A pan-European case-cohort analysis. Eur. Hear. J. 2018, 39, 397–406. [Google Scholar] [CrossRef]
- World Obesity Federation. World Obesity Atlas. 2023. Available online: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2023 (accessed on 20 August 2023).
- World Obesity Federation Global Obesity Observatory. World Obesity Day Atlases—Obesity Atlas 2023. Available online: https://data.worldobesity.org/publications/?cat=19 (accessed on 20 August 2023).
- Juchacz, K.; Kłos, P.; Dziedziejko, V.; Wójciak, R.W. The Effectiveness of Supportive Psychotherapy in Weight Loss in a Group of Young Overweight and Obese Women. Nutrients 2021, 13, 532. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.A. A review of current guidelines for the treatment of obesity. Am. J. Manag. Care 2022, 28, S288–S296. [Google Scholar] [PubMed]
- Tak, Y.J.; Lee, S.Y. Long-Term Efficacy and Safety of Anti-Obesity Treatment: Where Do We Stand? Curr. Obes. Rep. 2021, 10, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Kraschnewski, J.L.; Boan, J.; Esposito, J.; E Sherwood, N.; Lehman, E.B.; Kephart, D.K.; Sciamanna, C.N. Long-term weight loss maintenance in the United States. Int. J. Obes. 2010, 34, 1644–1654. [Google Scholar] [CrossRef]
- Mauro, M.F.F.; Papelbaum, M.; Brasil, M.A.A.; Carneiro, J.R.I.; Coutinho, E.S.F.; Coutinho, W.; Appolinario, J.C. Is weight regain after bariatric surgery associated with psychiatric comorbidity? A systematic review and meta-analysis. Obes. Rev. 2019, 20, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; De Luca, M.; Faria, S.L.; Goodpaster, K.P.; Haddad, A.; et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surg. Obes. Relat. Dis. 2022, 18, 1345–1356. [Google Scholar] [CrossRef]
- James, W.P.T.; Chunming, C.; Inoue, S. Appropriate Asian body mass indices? Obes. Rev. Off. J. Int. Assoc. Study Obes. 2002, 3, 139. [Google Scholar] [CrossRef]
- Pan, W.-H.; Yeh, W.-T. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: An extension of Asian-Pacific recommendations. Asia Pac. J. Clin. Nutr. 2008, 17, 370–374. [Google Scholar]
- World Health Organization, Regional Office for the Western Pacific. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia: Sydney, Australia, 2000; p. 55. Available online: https://apps.who.int/iris/handle/10665/206936 (accessed on 20 August 2023).
- Mulla, C.M.; Middelbeek, R.J.; Patti, M. Mechanisms of weight loss and improved metabolism following bariatric surgery. Ann. N. Y. Acad. Sci. 2017, 1411, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Yao, J.L.; Ji, G.; Qian, L.; Wang, J.; Zhang, G.S.; Tian, J.; Nie, Y.Z.; Zhang, Y.E.; et al. Obesity: Pathophysiology and Intervention. Nutrients 2014, 6, 5153–5183. [Google Scholar] [CrossRef]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric surgery: A systematic review and meta-analysis. JAMA 2004, 292, 1724–1737. [Google Scholar] [CrossRef]
- Buchwald, H.; Oien, D.M. Metabolic/Bariatric Surgery Worldwide 2011. Obes. Surg. 2013, 23, 427–436. [Google Scholar] [CrossRef]
- Samuel, N.; Jalal, Q.; Gupta, A.; Mazari, F.; Vasas, P.; Balachandra, S. Mid-term bariatric surgery outcomes for obese patients: Does weight matter? Ann. R. Coll. Surg. Engl. 2020, 102, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.M. Current Issues in Bariatric Surgery for Adolescents with Severe Obesity: Durability, Complications, and Timing of Intervention. J. Obes. Metab. Syndr. 2020, 29, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Pallati, P.K.; Shaligram, A.; Shostrom, V.K.; Oleynikov, D.; McBride, C.L.; Goede, M.R. Improvement in gastroesophageal reflux disease symptoms after various bariatric procedures: Review of the Bariatric Outcomes Longitudinal Database. Surg. Obes. Relat. Dis. 2014, 10, 502–507. [Google Scholar] [CrossRef]
- Hernández, J.; Boza, C. Novel treatments for complications after bariatric surgery. Ann. Surg. Innov. Res. 2016, 10, 3. [Google Scholar] [CrossRef]
- Puzziferri, N.; Roshek, T.B.; Mayo, H.G.; Gallagher, R.; Belle, S.H.; Livingston, E.H. Long-term follow-up after bariatric surgery: A systematic review. JAMA 2014, 312, 934–942. [Google Scholar] [CrossRef]
- Gletsu-Miller, N.; Wright, B.N. Mineral Malnutrition Following Bariatric Surgery. Adv. Nutr. Int. Rev. J. 2013, 4, 506–517. [Google Scholar] [CrossRef]
- Moizé, V.L.; Pi-Sunyer, X.; Mochari, H.; Vidal, J. Nutritional Pyramid for Post-gastric Bypass Patients. Obes. Surg. 2010, 20, 1133–1141. [Google Scholar] [CrossRef]
- Actualización del Consenso Argentino de Nutrición en Cirugía Bariátrica. Available online: https://docplayer.es/18417445-Actualizacion-del-consenso-argentino-de-nutricion-en-cirugia-bariatrica.html (accessed on 1 February 2022).
- Athanasiadis, D.I.; Martin, A.; Kapsampelis, P.; Monfared, S.; Stefanidis, D. Factors associated with weight regain post-bariatric surgery: A systematic review. Surg. Endosc. 2021, 35, 4069–4084. [Google Scholar] [CrossRef]
- El Ansari, W.; Elhag, W. Weight Regain and Insufficient Weight Loss After Bariatric Surgery: Definitions, Prevalence, Mechanisms, Predictors, Prevention and Management Strategies, and Knowledge Gaps—A Scoping Review. Obes. Surg. 2021, 31, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Weiner, R.A.; Weiner, S.; Pomhoff, I.; Jacobi, C.; Makarewicz, W.; Weigand, G. Laparoscopic Sleeve Gastrectomy—Influence of Sleeve Size and Resected Gastric Volume. Obes. Surg. 2007, 17, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, H.M.; Yimcharoen, P.; Brethauer, S.A.; Kroh, M.; Chand, B. Influence of pouch and stoma size on weight loss after gastric bypass. Surg. Obes. Relat. Dis. 2012, 8, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Yimcharoen, P.; Heneghan, H.M.; Singh, M.; Brethauer, S.; Schauer, P.; Rogula, T.; Kroh, M.; Chand, B. Endoscopic findings and outcomes of revisional procedures for patients with weight recidivism after gastric bypass. Surg. Endosc. 2011, 25, 3345–3352. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, S.G.D.; Pories, W.J.; MacDonald, K.G.; Morgan, E.J. Gastrogastric Fistulas A Complication of Divided Gastric Bypass Surgery. Ann. Surg. 1995, 221, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.J.B.; Kondo, W.; Nassif, L.S.; Garcia, M.J.; Tirapelle, R.D.A.; Dotti, C.M. Gastrogastric Fistula: A Possible Complication of Roux-En-Y Gastric Bypass. J. Soc. Laparoendosc. Surg. 2007, 10, 326–331. [Google Scholar]
- Carrodeguas, L.; Szomstein, S.; Soto, F.; Whipple, O.; Simpfendorfer, C.; Gonzalvo, J.P.; Villares, A.; Zundel, N.; Rosenthal, R. Management of gastrogastric fistulas after divided Roux-en-Y gastric bypass surgery for morbid obesity: Analysis of 1292 consecutive patients and review of literature. Surg. Obes. Relat. Dis. 2005, 1, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Christou, N.V.; Look, D.; MacLean, L.D. Weight Gain After Short- and Long-Limb Gastric Bypass in Patients Followed for Longer Than 10 Years. Ann. Surg. 2006, 244, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Roslin, M.; Damani, T.; Oren, J.; Andrews, R.; Yatco, E.; Shah, P. Abnormal glucose tolerance testing following gastric bypass demonstrates reactive hypoglycemia. Surg. Endosc. 2010, 25, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.; Clark, J.M.; Schweitzer, M.; Magnuson, T.; Brown, T.T.; Lee, C.J. Weight regain in patients with symptoms of post–bariatric surgery hypoglycemia. Surg. Obes. Relat. Dis. 2017, 13, 1728–1734. [Google Scholar] [CrossRef]
- Meguid, M.M.; Glade, M.J.; Middleton, F.A. Weight regain after Roux-en-Y: A significant 20% complication related to PYY. Nutrition 2008, 24, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Zalesin, K.C.; Franklin, B.A.; Miller, W.M.; Janosz, K.E.N.; Veri, S.; Odom, J.; McCullough, P.A. Preventing Weight Regain After Bariatric Surgery: An Overview of Lifestyle and Psychosocial Modulators. Am. J. Lifestyle Med. 2009, 4, 113–120. [Google Scholar] [CrossRef]
- Sjöström, L.; Lindroos, A.-K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjöström, C.D.; et al. Lifestyle, Diabetes, and Cardiovascular Risk Factors 10 Years after Bariatric Surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef]
- Rusch, M.D.; Andris, D.; Msn, A.D.A. Maladaptive Eating Patterns After Weight-Loss Surgery. Nutr. Clin. Pr. 2007, 22, 41–49. [Google Scholar] [CrossRef]
- Freire, R.H.; Borges, M.C.; Alvarez-Leite, J.I.; Correia, M.I.T.D. Food quality, physical activity, and nutritional follow-up as determinant of weight regain after Roux-en-Y gastric bypass. Nutrition 2012, 28, 53–58. [Google Scholar] [CrossRef]
- da Silva, F.B.L.; Gomes, D.L.; de Carvalho, K.M.B. Poor diet quality and postoperative time are independent risk factors for weight regain after Roux-en-Y gastric bypass. Nutrition 2016, 32, 1250–1253. [Google Scholar] [CrossRef]
- Kofman, M.D.; Lent, M.R.; Swencionis, C. Maladaptive Eating Patterns, Quality of Life, and Weight Outcomes Following Gastric Bypass: Results of an Internet Survey. Obesity 2010, 18, 1938–1943. [Google Scholar] [CrossRef]
- Colles, S.L.; Dixon, J.B.; O’Brien, P.E. Grazing and Loss of Control Related to Eating: Two High-risk Factors Following Bariatric Surgery. Obesity 2008, 16, 615–622. [Google Scholar] [CrossRef]
- Saunders, R. “Grazing”: A high-risk behavior. Obes. Surg. 2004, 14, 98–102. [Google Scholar] [CrossRef]
- Pizato, N.; Botelho, P.B.; Gonçalves, V.S.S.; Dutra, E.S.; De Carvalho, K.M.B. Effect of Grazing Behavior on Weight Regain Post-Bariatric Surgery: A Systematic Review. Nutrients 2017, 9, 1322. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.; Laws, H.L.; Gonzalez, Q.H.; Clements, R.H. Gastrointestinal symptomatic outcome after laparoscopic Roux-en-Y gastric bypass. J. Gastrointest. Surg. 2003, 7, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Runge, T.M.; Jirapinyo, P.; Chan, W.W.; Thompson, C.C. Dysphagia predicts greater weight regain after Roux-en-Y gastric bypass: A longitudinal case-matched study. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2019, 15, 2045–2051. [Google Scholar] [CrossRef]
- Garcia, J.M.; Chambers, E. Managing Dysphagia Through Diet Modifications. Am. J. Nurs. 2010, 110, 26–33. [Google Scholar] [CrossRef] [PubMed]
- E Livingstone, M.B.; Redpath, T.; Naseer, F.; Boyd, A.; Martin, M.; Finlayson, G.; Miras, A.D.; Bodnar, Z.; Kerrigan, D.; Pournaras, D.J.; et al. Food Intake Following Gastric Bypass Surgery: Patients Eat Less but Do Not Eat Differently. J. Nutr. 2022, 152, 2319–2332. [Google Scholar] [CrossRef]
- Janmohammadi, P.; Sajadi, F.; Alizadeh, S.; Daneshzad, E. Comparison of Energy and Food Intake Between Gastric Bypass and Sleeve Gastrectomy: A Meta-analysis and Systematic Review. Obes. Surg. 2019, 29, 1040–1048. [Google Scholar] [CrossRef]
- Rosenberger, P.H.; Henderson, K.E.; White, M.A.; Masheb, R.M.; Grilo, C.M. Physical Activity in Gastric Bypass Patients: Associations with Weight Loss and Psychosocial Functioning at 12-Month Follow-Up. Obes. Surg. 2010, 21, 1564–1569. [Google Scholar] [CrossRef]
- Yanos, B.R.; Saules, K.K.; Schuh, L.M.; Sogg, S. Predictors of Lowest Weight and Long-Term Weight Regain Among Roux-en-Y Gastric Bypass Patients. Obes. Surg. 2015, 25, 1364–1370. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013; Available online: https://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596 (accessed on 1 February 2022).
- Allison, K.; Wadden, T.; Sarwer, D.; Fabricatore, A.; Crerand, C.; Gibbons, L.; Stack, R.; Stunkard, A.; Williams, N. Night eating syndrome and binge eating disorder among persons seeking bariatric surgery: Prevalence and related features. Surg. Obes. Relat. Dis. 2006, 2, 153–158. [Google Scholar] [CrossRef]
- de Zwaan, M.; E Mitchell, J.; Howell, L.; Monson, N.; Swan-Kremeier, L.; Crosby, R.D.; Seim, H.C. Characteristics of morbidly obese patients before gastric bypass surgery. Compr. Psychiatry 2003, 44, 428–434. [Google Scholar] [CrossRef]
- Istfan, N.W.; Lipartia, M.; Anderson, W.A.; Hess, D.T.; Apovian, C.M. Approach to the Patient: Management of the Post–Bariatric Surgery Patient With Weight Regain. J. Clin. Endocrinol. Metab. 2021, 106, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Livhits, M.; Mercado, C.; Yermilov, I.; Parikh, J.A.; Dutson, E.; Mehran, A.; Ko, C.Y.; Gibbons, M.M. Patient behaviors associated with weight regain after laparoscopic gastric bypass. Obes. Res. Clin. Pr. 2011, 5, e258–e265. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, T.; Groesz, L.M.; Savu, M. Psychiatric Factors and Weight Loss Patterns Following Gastric Bypass Surgery in a Veteran Population. Obes. Surg. 2009, 21, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Taft, C.; Rydén, A.; Sjöström, L.; Sullivan, M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: The SOS intervention study. Int. J. Obes. 2007, 31, 1248–1261. [Google Scholar] [CrossRef]
- Sheets, C.S.; Peat, C.M.; Berg, K.C.; White, E.K.; Bocchieri-Ricciardi, L.; Chen, E.Y.; Mitchell, J.E. Post-operative Psychosocial Predictors of Outcome in Bariatric Surgery. Obes. Surg. 2014, 25, 330–345. [Google Scholar] [CrossRef]
- Odom, J.; Zalesin, K.C.; Washington, T.L.; Miller, W.W.; Hakmeh, B.; Zaremba, D.L.; Altattan, M.; Balasubramaniam, M.; Gibbs, D.S.; Krause, K.R.; et al. Behavioral Predictors of Weight Regain after Bariatric Surgery. Obes. Surg. 2009, 20, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, V.; Carrasco, F.; Cuevas, A.; Valenzuela, B.; Muñoz, G.; Ghiardo, D.; Burr, M.; Lehmann, Y.; Leiva, M.J.; Berry, M.; et al. Mechanisms of long-term weight regain in patients undergoing sleeve gastrectomy. Nutrition 2016, 32, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Elfhag, K.; Rössner, S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2005, 6, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.C.; Sedgmond, J.; Maizey, L.; Chambers, C.D.; Lawrence, N.S. Food Addiction: Implications for the Diagnosis and Treatment of Overeating. Nutrients 2019, 11, 2086. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-J.; Lindgren, E.; Gray, K.; Miller, G.; Tyler, R.; Wiers, C.E.; Volkow, N.D. Food addiction A common neurobiological mechanism with drug abuse. Front. Biosci. 2018, 23, 811–836. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.G. Psychological assessment of the patient undergoing bariatric surgery. Ochsner J. 2009, 9, 144–148. [Google Scholar]
- Magro, D.O.; Geloneze, B.; Delfini, R.; Pareja, B.C.; Callejas, F.; Pareja, J.C. Long-term Weight Regain after Gastric Bypass: A 5-year Prospective Study. Obes. Surg. 2008, 18, 648–651. [Google Scholar] [CrossRef]
- Keith, C.J.; Gullick, A.A.; Feng, K.; Richman, J.; Stahl, R.; Grams, J. Predictive factors of weight regain following laparoscopic Roux-en-Y gastric bypass. Surg. Endosc. 2017, 32, 2232–2238. [Google Scholar] [CrossRef]
- Shantavasinkul, P.C.; Omotosho, P.; Corsino, L.; Portenier, D.; Torquati, A. Predictors of weight regain in patients who underwent Roux-en-Y gastric bypass surgery. Surg. Obes. Relat. Dis. 2016, 12, 1640–1645. [Google Scholar] [CrossRef]
- Anderson, W.A.; Greene, G.W.; Forse, R.A.; Apovian, C.M.; Istfan, N.W. Weight Loss and Health Outcomes in African Americans and Whites After Gastric Bypass Surgery. Obesity 2007, 15, 1455–1463. [Google Scholar] [CrossRef]
- Al-Khyatt, W.; Ryall, R.; Leeder, P.; Ahmed, J.; Awad, S. Predictors of Inadequate Weight Loss After Laparoscopic Gastric Bypass for Morbid Obesity. Obes. Surg. 2016, 27, 1446–1452. [Google Scholar] [CrossRef]
- Paul, L.; Van Der Heiden, C.; Hoek, H.W. Cognitive behavioral therapy and predictors of weight loss in bariatric surgery patients. Curr. Opin. Psychiatry 2017, 30, 474–479. [Google Scholar] [CrossRef]
- Cambi, M.P.C.; Marchesini, S.D.; Baretta, G.A.P. Post-bariatric surgery weight regain: Evaluation of nutritional profile of candidate patients for endoscopic argon plasma coagulation. Arq. Bras. De Cir. Dig. 2015, 28, 40–43. [Google Scholar] [CrossRef]
- Bastos, E.C.L.; Barbosa, E.M.W.G.; Soriano, G.M.S.; dos Santos, E.A.; Vasconcelos, S.M.L. Determinants of weight regain after bariatric surgery. Arq. Bras. Cir. Dig. 2013, 26 (Suppl. S1), 26–32. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Lizcano, L.; Arenas-Villamizar, V.V.; Jaimes-Duarte, E.B.; García-Pacheco, H.; Paredes, C.S.; Bermúdez, V.; Rivera-Porras, D. Metabolic Adverse Effects of Psychotropic Drug Therapy: A Systematic Review. Eur. J. Investig. Health Psychol. Educ. 2023, 13, 1505–1520. [Google Scholar] [CrossRef] [PubMed]
- Hourneaux De Moura, D.T.; Thompson, C.C. Endoscopic management of weight regain following Roux-en-Y gastric bypass. Expert. Rev. Endocrinol. Metab. 2019, 14, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, C.T.M.; Hoebee, B.; Seidell, J.C.; Bouchard, C.; van Baak, M.A.; de Groot, C.P.G.M.L.; Chagnon, M.; De Graaf, C.; Saris, W.H.M. Genetic factors as predictors of weight gain in young adult Dutch men and women. Int. J. Obes. 2002, 26, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Perry, B.; Wang, Y. Appetite regulation and weight control: The role of gut hormones. Nutr. Diabetes 2012, 2, e26. [Google Scholar] [CrossRef]
- Murphy, K.G.; Dhillo, W.S.; Bloom, S.R. Gut Peptides in the Regulation of Food Intake and Energy Homeostasis. Endocr. Rev. 2006, 27, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Romanova, I.V.; Ramos, E.J.; Xu, Y.; Quinn, R.; Chen, C.; George, Z.M.; Inui, A.; Das, U.; Meguid, M.M. Neurobiologic changes in the hypothalamus associated with weight loss after gastric bypass. J. Am. Coll. Surg. 2004, 199, 887–895. [Google Scholar] [CrossRef] [PubMed]
- MacLean, P.S.; Bergouignan, A.; Cornier, M.-A.; Jackman, M.R.; Coutinho, S.R.; Rehfeld, J.F.; Holst, J.J.; Kulseng, B.; Martins, C.; Al-Najim, W.; et al. Biology’s response to dieting: The impetus for weight regain. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, R581–R600. [Google Scholar] [CrossRef]
- Yu, J.H.; Kim, M.-S. Molecular Mechanisms of Appetite Regulation. Diabetes Metab. J. 2012, 36, 391–398. [Google Scholar] [CrossRef]
- Sumithran, P.; Proietto, J. The defence of body weight: A physiological basis for weight regain after weight loss. Clin. Sci. 2012, 124, 231–241. [Google Scholar] [CrossRef]
- Ahima, R.S.; Qi, Y.; Singhal, N.S.; Jackson, M.B.; Scherer, P.E. Brain Adipocytokine Action and Metabolic Regulation. Diabetes 2006, 55 (Suppl. S2), S145–S154. [Google Scholar] [CrossRef]
- Kinasz, K.R.; Ross, D.A.; Cooper, J.J. Eat to Live or Live to Eat? The Neurobiology of Appetite Regulation. Biol. Psychiatry 2017, 81, e73–e75. [Google Scholar] [CrossRef] [PubMed]
- Alcaro, A.; Huber, R.; Panksepp, J. Behavioral functions of the mesolimbic dopaminergic system: An affective neuroethological perspective. Brain Res. Rev. 2007, 56, 283–321. [Google Scholar] [CrossRef]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, T.R.; Tong, J.; Datta, R.; Culler, M.D.; Tschöp, M.H. Ghrelin in the regulation of body weight and metabolism. Front. Neuroendocrinol. 2010, 31, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Currie, P.J.; Mirza, A.; Fuld, R.; Park, D.; Vasselli, J.R. Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. Am. J. Physiol. Integr. Comp. Physiol. 2005, 289, R353–R358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Date, Y.; Murakami, N.; Toshinai, K.; Matsukura, S.; Niijima, A.; Matsuo, H.; Kangawa, K.; Nakazato, M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 2002, 123, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.K.; Wu, J.C.-Y. Role of Ghrelin in the Pathophysiology of Gastrointestinal Disease. Gut Liver 2013, 7, 505–512. [Google Scholar] [CrossRef]
- Peeters, T.L. Ghrelin: A new player in the control of gastrointestinal functions. Gut 2005, 54, 1638–1649. [Google Scholar] [CrossRef]
- le Roux, C.W.; Aylwin, S.J.B.; Batterham, R.L.; Borg, C.M.; Coyle, F.; Prasad, V.; Shurey, S.; Ghatei, M.A.; Patel, A.G.; Bloom, S.R. Gut Hormone Profiles Following Bariatric Surgery Favor an Anorectic State, Facilitate Weight Loss, and Improve Metabolic Parameters. Ann. Surg. 2006, 243, 108–114. [Google Scholar] [CrossRef]
- Guijarro, A.; Kirchner, H.; Meguid, M.M. Catabolic effects of gastric bypass in a diet-induced obese rat model. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, G.H. Peptide YY(1-36) and Peptide YY(3-36): Part II. Changes after Gastrointestinal Surgery and Bariatric Surgery. Obes. Surg. 2006, 16, 795–803. [Google Scholar] [CrossRef]
- Owyang, C.; Heldsinger, A. Vagal Control of Satiety and Hormonal Regulation of Appetite. J. Neurogastroenterol. Motil. 2011, 17, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Appleyard, S.M.; Bailey, T.W.; Doyle, M.W.; Jin, Y.-H.; Smart, J.L.; Low, M.J.; Andresen, M.C. Proopiomelanocortin Neurons in Nucleus Tractus Solitarius Are Activated by Visceral Afferents: Regulation by Cholecystokinin and Opioids. J. Neurosci. 2005, 25, 3578–3585. [Google Scholar] [CrossRef] [PubMed]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Chan, J.L.; Mun, E.C.; Stoyneva, V.; Mantzoros, C.S.; Goldfine, A.B. Peptide YY Levels Are Elevated After Gastric Bypass Surgery. Obesity 2006, 14, 194–198. [Google Scholar] [CrossRef]
- Suzuki, S.; Ramos, E.J.; Goncalves, C.G.; Chen, C.; Meguid, M.M. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery 2005, 138, 283–290. [Google Scholar] [CrossRef]
- E Engström, B.; Öhrvall, M.; Sundbom, M.; Lind, L.; A Karlsson, F. Meal suppression of circulating ghrelin is normalized in obese individuals following gastric bypass surgery. Int. J. Obes. 2006, 31, 476–480. [Google Scholar] [CrossRef]
- Tsouristakis, A.I.; Febres, G.; McMahon, D.J.; Tchang, B.; Conwell, I.M.; Tsang, A.J.; Ahmed, L.; Bessler, M.; Korner, J. Long-Term Modulation of Appetitive Hormones and Sweet Cravings After Adjustable Gastric Banding and Roux-en-Y Gastric Bypass. Obes. Surg. 2019, 29, 3698–3705. [Google Scholar] [CrossRef]
- le Roux, C.W.; Welbourn, R.; Werling, M.; Osborne, A.; Kokkinos, A.; Laurenius, A.; Lönroth, H.; Fändriks, L.; Ghatei, M.A.; Bloom, S.R.; et al. Gut Hormones as Mediators of Appetite and Weight Loss After Roux-en-Y Gastric Bypass. Ann. Surg. 2007, 246, 780–785. [Google Scholar] [CrossRef]
- Bohdjalian, A.; Langer, F.B.; Shakeri-Leidenmühler, S.; Gfrerer, L.; Ludvik, B.; Zacherl, J.; Prager, G. Sleeve Gastrectomy as Sole and Definitive Bariatric Procedure: 5-Year Results for Weight Loss and Ghrelin. Obes. Surg. 2010, 20, 535–540. [Google Scholar] [CrossRef]
- Santo, M.A.; Riccioppo, D.; Pajecki, D.; Kawamoto, F.; de Cleva, R.; Antonangelo, L.; Marçal, L.; Cecconello, I. Weight Regain After Gastric Bypass: Influence of Gut Hormones. Obes. Surg. 2015, 26, 919–925. [Google Scholar] [CrossRef]
- Tamboli, R.A.; Breitman, I.; Marks-Shulman, P.A.; Jabbour, K.; Melvin, W.; Williams, B.; Clements, R.H.; Feurer, I.D.; Abumrad, N.N. Early weight regain after gastric bypass does not affect insulin sensitivity but is associated with elevated ghrelin. Obesity 2014, 22, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- O’brien, C.S.; Wang, G.; McGinty, J.; Agénor, K.K.; Dutia, R.; Colarusso, A.; Park, K.; Koshy, N.; Laferrère, B. Effects of gastrogastric fistula repair on weight loss and gut hormone levels. Obes. Surg. 2013, 23, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Ben Amor, I.; Petrucciani, N.; Kassir, R.; Malyshev, E.; Mazoyer, C.; Korkmaz, C.; Debs, T.; Gugenheim, J. Midterm Outcomes of Gastric Pouch Resizing for Weight Regain After Roux-en-Y Gastric Bypass. Obes. Surg. 2020, 30, 2723–2728. [Google Scholar] [CrossRef] [PubMed]
- Madsbad, S.; Holst, J.J. GLP-1 as a Mediator in the Remission of Type 2 Diabetes After Gastric Bypass and Sleeve Gastrectomy Surgery. Diabetes 2014, 63, 3172–3174. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hutch, C.R.; Sandoval, D. The Role of GLP-1 in the Metabolic Success of Bariatric Surgery. Endocrinology 2017, 158, 4139–4151. [Google Scholar] [CrossRef] [PubMed]
- Jirapinyo, P.; Jin, D.X.; Qazi, T.; Mishra, N.; Thompson, C.C. A Meta-Analysis of GLP-1 After Roux-En-Y Gastric Bypass: Impact of Surgical Technique and Measurement Strategy. Obes. Surg. 2018, 28, 615–626. [Google Scholar] [CrossRef]
- Salehi, M.; Prigeon, R.L.; D’alessio, D.A. Gastric Bypass Surgery Enhances Glucagon-Like Peptide 1–Stimulated Postprandial Insulin Secretion in Humans. Diabetes 2011, 60, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Rossmeislová, L.; Mališová, L.; Kračmerová, J.; Tencerová, M.; Kováčová, Z.; Koc, M.; Šiklová-Vítková, M.; Viquerie, N.; Langin, D.; Štich, V. Weight loss improves the adipogenic capacity of human preadipocytes and modulates their secretory profile. Diabetes 2013, 62, 1990–1995. [Google Scholar] [CrossRef]
- MacLean, P.S.; Higgins, J.A.; Giles, E.D.; Sherk, V.D.; Jackman, M.R. The role for adipose tissue in weight regain after weight loss. Obes. Rev. 2015, 16 (Suppl. S1), 45–54. [Google Scholar] [CrossRef]
- Escrivá, F.; Gavete, M.L.; Fermín, Y.; Pérez, C.; Gallardo, N.; Alvarez, C.; Andrés, A.; Ros, M.; Carrascosa, J.M. Effect of age and moderate food restriction on insulin sensitivity in Wistar rats: Role of adiposity. J. Endocrinol. 2007, 194, 131–141. [Google Scholar] [CrossRef]
- Jackman, M.R.; Steig, A.; Higgins, J.A.; Johnson, G.C.; Fleming-Elder, B.K.; Bessesen, D.H.; MacLean, P.S. Weight regain after sustained weight reduction is accompanied by suppressed oxidation of dietary fat and adipocyte hyperplasia. Am. J. Physiol. Integr. Comp. Physiol. 2008, 294, R1117–R1129. [Google Scholar] [CrossRef]
- Blaszczak, A.M.; Bernier, M.; Wright, V.P.; Gebhardt, G.; Anandani, K.; Liu, J.; Jalilvand, A.; Bergin, S.; Wysocki, V.; Somogyi, A.; et al. Obesogenic Memory Maintains Adipose Tissue Inflammation and Insulin Resistance. Immunometabolism 2020, 2, e200023. [Google Scholar] [CrossRef]
- Crowley, V.E.F. Overview of human obesity and central mechanisms regulating energy homeostasis. Ann. Clin. Biochem. 2008, 45 Pt 3, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Wang, Y.; Ricci, M.R.; Sullivan, S.; Russell, C.D.; Fried, S.K. Acute and chronic regulation of leptin synthesis, storage, and secretion by insulin and dexamethasone in human adipose tissue. Am. J. Physiol. Endocrin. Metab. 2007, 292, E858–E864. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Ahima, R.S. Leptin signaling. F1000prime Rep. 2014, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Morioka, T.; Mori, K.; Motoyama, K.; Emoto, M. Ectopic Fat Accumulation and Glucose Homeostasis: Role of Leptin in Glucose and Lipid Metabolism and Mass Maintenance in Skeletal Muscle. In Musculoskeletal Disease Associated with Diabetes Mellitus; Inaba, M., Ed.; Springer: Tokyo, Japan, 2016; pp. 201–213. Available online: http://link.springer.com/10.1007/978-4-431-55720-3_14 (accessed on 1 February 2022).
- Farr, O.M.; Gavrieli, A.; Mantzoros, C.S. Leptin applications in 2015: What have we learned about leptin and obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Guijarro, A.; Suzuki, S.; Chen, C.; Kirchner, H.; Middleton, F.A.; Nadtochiy, S.; Brookes, P.S.; Niijima, A.; Inui, A.; Meguid, M.M.; et al. Characterization of weight loss and weight regain mechanisms after Roux-en-Y gastric bypass in rats. Am. J. Physiol. Integr. Comp. Physiol. 2007, 293, R1474–R1489. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.S.; Harris, M.; Hollenberg, A.N. Leptin, nutrition, and the thyroid: The why, the wherefore, and the wiring. J. Clin. Invest. 2000, 105, 859–861. [Google Scholar] [CrossRef]
- Kissileff, H.R.; Thornton, J.C.; I Torres, M.; Pavlovich, K.; Mayer, L.S.; Kalari, V.; Leibel, R.L.; Rosenbaum, M. Leptin reverses declines in satiation in weight-reduced obese humans. Am. J. Clin. Nutr. 2012, 95, 309–317. [Google Scholar] [CrossRef]
- Korner, J.; Inabnet, W.; Febres, G.; Conwell, I.M.; McMahon, D.J.; Salas, R.; Taveras, C.; Schrope, B.; Bessler, M. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int. J. Obes. 2009, 33, 786–795. [Google Scholar] [CrossRef]
- Cota, D.; Marsicano, G.; Tschöp, M.; Grübler, Y.; Flachskamm, C.; Schubert, M.; Auer, D.; Yassouridis, A.; Thöne-Reineke, C.; Ortmann, S.; et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Invest. 2003, 112, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Matias, I.; Bisogno, T.; Di Marzo, V. Endogenous cannabinoids in the brain and peripheral tissues: Regulation of their levels and control of food intake. Int. J. Obes. 2006, 30 (Suppl. S1), S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-H.; Chen, Y.-J.J.; Chua, S.C., Jr.; Talmage, D.A.; Role, L.W. Integration of Endocannabinoid and Leptin Signaling in an Appetite-Related Neural Circuit. Neuron 2005, 48, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Hirsch, J.; Murphy, E.; Leibel, R.L. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am. J. Clin. Nutr. 2000, 71, 1421–1432. [Google Scholar] [CrossRef]
- Kozlowska, L.; Rosolowska-Huszcz, D. Leptin, Thyrotropin, and Thyroid Hormones in Obese/Overweight Women Before and After Two Levels of Energy Deficit. Endocrine 2004, 24, 147–154. [Google Scholar] [CrossRef]
- Johnstone, A.; Faber, P.; Andrew, R.; Gibney, E.; Elia, M.; Lobley, G.; Stubbs, R.; Walker, B. Influence of short-term dietary weight loss on cortisol secretion and metabolism in obese men. Eur. J. Endocrinol. 2004, 150, 185–194. [Google Scholar] [CrossRef]
- Tomiyama, A.J.; Mann, T.; Vinas, D.B.; Hunger, J.M.B.; DeJager, J.M.; Taylor, S.E. Low Calorie Dieting Increases Cortisol. Psychosom. Med. 2010, 72, 357–364. [Google Scholar] [CrossRef]
- Blomain, E.S.; Dirhan, D.A.; Valentino, M.A.; Kim, G.W.; Waldman, S.A. Mechanisms of Weight Regain following Weight Loss. ISRN Obes. 2013, 2013, 210524. [Google Scholar] [CrossRef]
- Guijarro, A.; Osei-Hyiaman, D.; Harvey-White, J.B.; Kunos, G.; Suzuki, S.; Nadtochiy, S.; Brookes, P.S.; Meguid, M.M.M. Sustained Weight Loss After Roux-en-Y Gastric Bypass Is Characterized by Down Regulation of Endocannabinoids and Mitochondrial Function. Ann. Surg. 2008, 247, 779–790. [Google Scholar] [CrossRef]
- Chan, J.L.; Williams, C.J.; Raciti, P.; Blakeman, J.; Kelesidis, T.; Kelesidis, I.; Johnson, M.L.; Thorner, M.O.; Mantzoros, C.S. Leptin Does Not Mediate Short-Term Fasting-Induced Changes in Growth Hormone Pulsatility but Increases IGF-I in Leptin Deficiency States. J. Clin. Endocrinol. Metab. 2008, 93, 2819–2827. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.L.; Kelly, E.; Faria, O.P. Energy Expenditure and Weight Regain in Patients Submitted to Roux-en-Y Gastric Bypass. Obes. Surg. 2009, 19, 856–859. [Google Scholar] [CrossRef] [PubMed]
- MacLean, P.S.; Higgins, J.A.; Jackman, M.R.; Johnson, G.C.; Fleming-Elder, B.K.; Wyatt, H.R.; Melanson, E.L.; Hill, J.O. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1577–R1588. [Google Scholar] [CrossRef] [PubMed]
- MacLean, P.S.; Higgins, J.A.; Johnson, G.C.; Fleming-Elder, B.K.; Donahoo, W.T.; Melanson, E.L.; Hill, J.O. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am. J. Physiol. Integr. Comp. Physiol. 2004, 287, R1306–R1315. [Google Scholar] [CrossRef]
- Hume, D.J.; Yokum, S.; Stice, E. Low energy intake plus low energy expenditure (low energy flux), not energy surfeit, predicts future body fat gain. Am. J. Clin. Nutr. 2016, 103, 1389–1396. [Google Scholar] [CrossRef]
- Blundell, J.E. Physical activity and appetite control: Can we close the energy gap? Nutr. Bull. 2011, 36, 356–366. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Sy, M.; Pavlovich, K.; Leibel, R.L.; Hirsch, J. Leptin reverses weight loss–induced changes in regional neural activity responses to visual food stimuli. J. Clin. Investig. 2008, 118, 2583–2591. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Morrison, C. The brain, appetite, and obesity. Ann. Rev. Psychol. 2008, 59, 55–92. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Yokum, S.; Orr, P.T.; Stice, E.; Corbin, W.R.; Brownell, K.D. Neural Correlates of Food Addiction. Arch. Gen. Psychiatry 2011, 68, 808–816. [Google Scholar] [CrossRef] [PubMed]
- DelParigi, A.; Chen, K.; Salbe, A.D.; O Hill, J.; Wing, R.R.; Reiman, E.M.; Tataranni, P.A. Persistence of abnormal neural responses to a meal in postobese individuals. Int. J. Obes. 2003, 28, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.-A.; Salzberg, A.K.; Endly, D.C.; Bessesen, D.H.; Rojas, D.C.; Tregellas, J.R. The Effects of Overfeeding on the Neuronal Response to Visual Food Cues in Thin and Reduced-Obese Individuals. PLoS ONE 2009, 4, e6310. [Google Scholar] [CrossRef] [PubMed]
- Fulton, S.; Woodside, B.; Shizgal, P. Modulation of Brain Reward Circuitry by Leptin. Science 2000, 287, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Naleid, A.M.; Grace, M.K.; Cummings, D.E.; Levine, A.S. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides 2005, 26, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Disse, E.; Bussier, A.-L.; Veyrat-Durebex, C.; Deblon, N.; Pfluger, P.T.; Tschöp, M.H.; Laville, M.; Rohner-Jeanrenaud, F. Peripheral ghrelin enhances sweet taste food consumption and preference, regardless of its caloric content. Physiol. Behav. 2010, 101, 277–281. [Google Scholar] [CrossRef]
- Koch, J.E.; Matthews, S.M. Delta9-tetrahydrocannabinol stimulates palatable food intake in Lewis rats: Effects of peripheral and central administration. Nutr. Neurosci. 2001, 4, 179–187. [Google Scholar] [CrossRef]
- Cambi, M.P.C.; Baretta, G.A.P.; Magro, D.D.O.; Boguszewski, C.L.; Ribeiro, I.B.; Jirapinyo, P.; de Moura, D.T.H. Multidisciplinary Approach for Weight Regain—How to Manage this Challenging Condition: An Expert Review. Obes. Surg. 2021, 31, 1290–1303. [Google Scholar] [CrossRef]
- Shah, M.; Almandoz, J.P.; Shukla, A.P. Editorial: Approaches to the management of weight regain after bariatric surgery. Front. Endocrinol. 2023, 14, 1267014. [Google Scholar] [CrossRef] [PubMed]
- Nuijten, M.A.H.; Tettero, O.M.; Wolf, R.J.; Bakker, E.A.; Eijsvogels, T.M.H.; Monpellier, V.M.; Hazebroek, E.J.; Janssen, I.M.C.; Hopman, M.T.E. Changes in Physical Activity in Relation to Body Composition, Fitness and Quality of Life after Primary Bariatric Surgery: A Two-Year Follow-Up Study. Obes. Surg. 2020, 31, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Gils Contreras, A.; Bonada Sanjaume, A.; Becerra-Tomás, N.; Salas-Salvadó, J. Adherence to Mediterranean Diet or Physical Activity After Bariatric Surgery and Its Effects on Weight Loss, Quality of Life, and Food Tolerance. Obes. Surg. 2020, 30, 687–696. [Google Scholar] [CrossRef]
- Tettero, O.M.; Aronson, T.; Wolf, R.J.; Nuijten, M.A.H.; Hopman, M.T.E.; Janssen, I.M.C. Increase in Physical Activity After Bariatric Surgery Demonstrates Improvement in Weight Loss and Cardiorespiratory Fitness. Obes. Surg. 2018, 28, 3950–3957. [Google Scholar] [CrossRef]
- Himes, S.M.; Grothe, K.B.; Clark, M.M.; Swain, J.M.; Collazo-Clavell, M.L.; Sarr, M.G. Stop Regain: A Pilot Psychological Intervention for Bariatric Patients Experiencing Weight Regain. Obes. Surg. 2015, 25, 922–927. [Google Scholar] [CrossRef]
- Wharton, S.; Kamran, E.; Muqeem, M.; Khan, A.; Christensen, R.A.G. The effectiveness and safety of pharmaceuticals to manage excess weight post-bariatric surgery: A systematic literature review. J. Drug Assess. 2019, 8, 184–191. [Google Scholar] [CrossRef]
- Velapati, S.R.; Shah, M.; Kuchkuntla, A.R.; Abu-Dayyeh, B.; Grothe, K.; Hurt, R.T.; Mundi, M.S. Weight Regain After Bariatric Surgery: Prevalence, Etiology, and Treatment. Curr. Nutr. Rep. 2018, 7, 329–334. [Google Scholar] [CrossRef]
- Storm, A.C.; Thompson, C.C. Endoscopic Treatments Following Bariatric Surgery. Gastrointest. Endosc. Clin. N. Am. 2017, 27, 233–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jirapinyo, P.; Kumar, N.; AlSamman, M.A.; Thompson, C.C. Five-year outcomes of transoral outlet reduction for the treatment of weight regain after Roux-en-Y gastric bypass. Gastrointest. Endosc. 2019, 91, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Patel, L.Y.; Lapin, B.; Brown, C.S.; Stringer, T.; Gitelis, M.E.; Linn, J.G.; Denham, W.E.; Farwell, E.; Haggerty, S.; Ujiki, M.B. Outcomes following 50 consecutive endoscopic gastrojejunal revisions for weight gain following Roux-en-Y gastric bypass: A comparison of endoscopic suturing techniques for stoma reduction. Surg. Endosc. 2016, 31, 2667–2677. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Szomstein, S.; Rosenthal, R.J. Reasons and Outcomes of Reoperative Bariatric Surgery for Failed and Complicated Procedures (Excluding Adjustable Gastric Banding). Obes. Surg. 2010, 21, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Gallé, F.; Marte, G.; Cirella, A.; Di Dio, M.; Miele, A.; Ricchiuti, R.; Liguori, F.; Maida, P.; Liguori, G. An exercise-based educational and motivational intervention after surgery can improve behaviors, physical fitness and quality of life in bariatric patients. PLoS ONE 2020, 15, e0241336. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.; Avenell, A. Behavioural Interventions for Severe Obesity Before and/or After Bariatric Surgery: A Systematic Review and Meta-analysis. Obes. Surg. 2015, 26, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Hanipah, Z.N.; Nasr, E.C.; Bucak, E.; Schauer, P.R.; Aminian, A.; Brethauer, S.A.; Cetin, D. Efficacy of adjuvant weight loss medication after bariatric surgery. Surg. Obes. Relat. Dis. 2018, 14, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Wharton, S.; Kuk, J.L.; Luszczynski, M.; Kamran, E.; Christensen, R.A.G. Liraglutide 3.0 mg for the management of insufficient weight loss or excessive weight regain post-bariatric surgery. Clin. Obes. 2019, 9, e12323. [Google Scholar] [CrossRef] [PubMed]
- Torbay, N.; Nawar, R. SAT-120 Weight Regain after Bariatric Surgery Rectified by Dietary Intervention and Metformin. J. Endocr. Soc. 2019, 3 (Suppl. S1), SAT-120. [Google Scholar] [CrossRef]
- Toth, A.T.; Gomez, G.; Shukla, A.P.; Pratt, J.S.; Cena, H.; Biino, G.; Aronne, L.J.; Stanford, F.C. Weight Loss Medications in Young Adults after Bariatric Surgery for Weight Regain or Inadequate Weight Loss: A Multi-Center Study. Children 2018, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Brunaldi, V.O.; Farias, G.F.A.; de Rezende, D.T.; Cairo-Nunes, G.; Riccioppo, D.; de Moura, D.T.H.; Santo, M.A.; de Moura, E.G.H. Argon plasma coagulation alone versus argon plasma coagulation plus full-thickness endoscopic suturing to treat weight regain after Roux-en-Y gastric bypass: A prospective randomized trial (with videos). Gastrointest. Endosc. 2020, 92, 97–107. [Google Scholar] [CrossRef] [PubMed]
- de Quadros, L.G.; Neto, M.G.; Marchesini, J.C.; Teixeira, A.; Grecco, E.; Junior, R.L.K.; Zundel, N.; Filho, I.J.Z.; de Souza, T.F.; Filho, A.C.; et al. Endoscopic Argon Plasma Coagulation vs. Multidisciplinary Evaluation in the Management of Weight Regain After Gastric Bypass Surgery: A Randomized Controlled Trial with SHAM Group. Obes. Surg. 2020, 30, 1904–1916. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.C.; Teixeira, A.F.; Neto, M.G.; Zundel, N.; Sander, B.Q.; Ramos, F.M.; Matz, F.; Baretta, G.A.; de Quadros, L.G.; Grecco, E.; et al. Efficacy of Utilizing Argon Plasma Coagulation for Weight Regain in Roux-en-Y Gastric Bypass Patients: A Multi-center Study. Obes. Surg. 2018, 28, 2737–2744. [Google Scholar] [CrossRef]
- Andalib, A.; Alamri, H.; Almuhanna, Y.; Bouchard, P.; Demyttenaere, S.; Court, O. Short-term outcomes of revisional surgery after sleeve gastrectomy: A comparative analysis of re-sleeve, Roux en-Y gastric bypass, duodenal switch (Roux en-Y and single-anastomosis). Surg. Endosc. 2020, 35, 4644–4652. [Google Scholar] [CrossRef]
- Shimon, O.; Keidar, A.; Orgad, R.; Yemini, R.; Carmeli, I. Long-Term Effectiveness of Laparoscopic Conversion of Sleeve Gastrectomy to a Biliopancreatic Diversion with a Duodenal Switch or a Roux-en-Y Gastric Bypass due to Weight Loss Failure. Obes. Surg. 2018, 28, 1724–1730. [Google Scholar] [CrossRef]
- Jabbour, G.; Salman, A. Bariatric Surgery in Adults with Obesity: The Impact on Performance, Metabolism, and Health Indices. Obes. Surg. 2021, 31, 1767–1789. [Google Scholar] [CrossRef]
- Ferraz, Á.A.B.; de Siqueira, L.T.; Filho, E.N.; de Araújo, J.G.C., Jr.; Campos, J.M.; de Barros-Correia, T.X.; Muniz, M.G.; Ferraz, E.M. Revision Surgery for Treatment of Weight Regain After Roux-En-Y Gastric Bypass. Obes. Surg. 2013, 24, 2–8. [Google Scholar] [CrossRef]
- Bradley, L.E.; Forman, E.M.; Kerrigan, S.G.; Butryn, M.L.; Herbert, J.D.; Sarwer, D.B. A Pilot Study of an Acceptance-Based Behavioral Intervention for Weight Regain After Bariatric Surgery. Obes. Surg. 2016, 26, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Bradley, L.E.; Forman, E.M.; Kerrigan, S.G.; Goldstein, S.P.; Butryn, M.L.; Thomas, J.G.; Herbert, J.D.; Sarwer, D.B. Project HELP: A Remotely Delivered Behavioral Intervention for Weight Regain after Bariatric Surgery. Obes. Surg. 2016, 27, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Gutt, S.; Schraier, S.; Bagnes, M.F.G.; Yu, M.; González, C.D.; Di Girolamo, G. Long-term pharmacotherapy of obesity in patients that have undergone bariatric surgery: Pharmacological prevention and management of body weight regain. Expert Opin. Pharmacother. 2019, 20, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Stanford, F.C.; Alfaris, N.; Gomez, G.; Ricks, E.T.; Shukla, A.P.; Corey, K.E.; Pratt, J.S.; Pomp, A.; Rubino, F.; Aronne, L.J. The utility of weight loss medications after bariatric surgery for weight regain or inadequate weight loss: A multi-center study. Surg. Obes. Relat. Dis. 2016, 13, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Zilberstein, B.; Pajecki, D.; de Brito, A.C.G.; Gallafrio, S.T.; Eshkenazy, R.; Andrade, C.G. Topiramate after Adjustable Gastric Banding in Patients with Binge Eating and Difficulty Losing Weight. Obes. Surg. 2004, 14, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, C.; Mehta, M.; Mou, D.; Dey, T.; Khaodhiar, L.; Tavakkoli, A. Patterns of Weight Loss Medication Utilization and Outcomes Following Bariatric Surgery. J. Gastrointest. Surg. 2021, 25, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Istfan, N.W.; Anderson, W.A.; Hess, D.T.; Yu, L.; Carmine, B.; Apovian, C.M. The Mitigating Effect of Phentermine and Topiramate on Weight Regain After Roux-en-Y Gastric Bypass Surgery. Obesity 2020, 28, 1023–1030. [Google Scholar] [CrossRef]
- Schwartz, J.; Suzo, A.; Wehr, A.M.; Foreman, K.S.; Mikami, D.J.; Needleman, B.J.; Noria, S.F. Pharmacotherapy in Conjunction with a Diet and Exercise Program for the Treatment of Weight Recidivism or Weight Loss Plateau Post-bariatric Surgery: A Retrospective Review. Obes. Surg. 2016, 26, 452–458. [Google Scholar] [CrossRef]
- Jester, L.; Wittgrove, A.C.; Clark, G.W. Adjunctive Use of Appetite Suppressant Medications for Improved Weight Management in Bariatric Surgical Patients. Obes. Surg. 1996, 6, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.B.; Renström, F.; Aczél, S.; Folie, P.; Biraima-Steinemann, M.; Beuschlein, F.; Bilz, S. Efficacy of the Glucagon-Like Peptide-1 Receptor Agonists Liraglutide and Semaglutide for the Treatment of Weight Regain After Bariatric surgery: A Retrospective Observational Study. Obes. Surg. 2023, 33, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, P.M.; Birkenfeld, A.L.; McGowan, B.; Mosenzon, O.; Pedersen, S.D.; Wharton, S.; Carson, C.G.; Jepsen, C.H.; Kabisch, M.; Wilding, J.P.H. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: A randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 2018, 392, 637–649. [Google Scholar] [CrossRef]
- Kaineder, K.; Birngruber, T.; Rauter, G.; Obermüller, B.; Eichler, J.; Münzker, J.; Al-Zoughbi, W.; I Mautner, S.; Torekov, S.S.; Hartmann, B.; et al. Chronic intrahypothalamic rather than subcutaneous liraglutide treatment reduces body weight gain and stimulates the melanocortin receptor system. Int. J. Obes. 2017, 41, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Al-Massadi, O.; Fernø, J.; Diéguez, C.; Nogueiras, R.; Quiñones, M. Glucagon Control on Food Intake and Energy Balance. Int. J. Mol. Sci. 2019, 20, 3905. [Google Scholar] [CrossRef] [PubMed]
- Pajecki, D.; Halpern, A.; Cercato, C.; Mancini, M.; de Cleva, R.; Santo, M.A. Tratamento de curto prazo com liraglutide no reganho de peso após cirurgia bariátrica. Rev. Colégio Bras. Cir. 2013, 40, 191–195. [Google Scholar] [CrossRef]
- Rye, P.; Modi, R.; Cawsey, S.; Sharma, A.M. Efficacy of High-Dose Liraglutide as an Adjunct for Weight Loss in Patients with Prior Bariatric Surgery. Obes. Surg. 2018, 28, 3553–3558. [Google Scholar] [CrossRef]
- Lautenbach, A.; Wernecke, M.; Huber, T.B.; Stoll, F.; Wagner, J.; Meyhöfer, S.M.; Meyhöfer, S.; Aberle, J. The Potential of Semaglutide Once-Weekly in Patients Without Type 2 Diabetes with Weight Regain or Insufficient Weight Loss After Bariatric Surgery—A Retrospective Analysis. Obes. Surg. 2022, 32, 3280–3288. [Google Scholar] [CrossRef]
- Dhindsa, B.S.; Saghir, S.M.; Naga, Y.; Dhaliwal, A.; Ramai, D.; Cross, C.; Singh, S.; Bhat, I.; Adler, D.G. Efficacy of transoral outlet reduction in Roux-en-Y gastric bypass patients to promote weight loss: A systematic review and meta-analysis. Endosc. Int. Open 2020, 08, E1332–E1340. [Google Scholar] [CrossRef]
- Vargas, E.J.; Bazerbachi, F.; Rizk, M.; Rustagi, T.; Acosta, A.; Wilson, E.B.; Wilson, T.; Neto, M.G.; Zundel, N.; Mundi, M.S.; et al. Transoral outlet reduction with full thickness endoscopic suturing for weight regain after gastric bypass: A large multicenter international experience and meta-analysis. Surg. Endosc. 2017, 32, 252–259. [Google Scholar] [CrossRef]
- Jirapinyo, P.; Kröner, P.T.; Thompson, C.C. Purse-string transoral outlet reduction (TORe) is effective at inducing weight loss and improvement in metabolic comorbidities after Roux-en-Y gastric bypass. Endoscopy 2017, 50, 371–377. [Google Scholar] [CrossRef]
- Thompson, C.C.; Chand, B.; Chen, Y.K.; DeMarco, D.C.; Miller, L.; Schweitzer, M.; Rothstein, R.I.; Lautz, D.B.; Slattery, J.; Ryan, M.B.; et al. Endoscopic Suturing for Transoral Outlet Reduction Increases Weight Loss After Roux-en-Y Gastric Bypass Surgery. Gastroenterology 2013, 145, 129–137.e3. [Google Scholar] [CrossRef]
- Relly, R.; Mati, S.; Aviv, C.N.; Fishman, S. Endoscopic trans-oral outlet reduction after bariatric surgery is safe and effective for dumping syndrome. Surg. Endosc. 2021, 35, 6846–6852. [Google Scholar] [CrossRef]
- Jaruvongvanich, V.; Vantanasiri, K.; Laoveeravat, P.; Matar, R.H.; Vargas, E.J.; Maselli, D.B.; Alkhatry, M.; Fayad, L.; Kumbhari, V.; Fittipaldi-Fernandez, R.J.; et al. Endoscopic full-thickness suturing plus argon plasma mucosal coagulation versus argon plasma mucosal coagulation alone for weight regain after gastric bypass: A systematic review and meta-analysis. Gastrointest. Endosc. 2020, 92, 1164–1175.e6. [Google Scholar] [CrossRef]
- Jirapinyo, P.; de Moura, D.T.; Dong, W.Y.; Farias, G.; Thompson, C.C. Dose response for argon plasma coagulation in the treatment of weight regain after Roux-en-Y gastric bypass. Gastrointest. Endosc. 2020, 91, 1078–1084. [Google Scholar] [CrossRef]
- Baretta, G.A.P.; Alhinho, H.C.A.W.; Matias, J.E.F.; Marchesini, J.B.; De Lima, J.H.F.; Empinotti, C.; Campos, J.M. Argon Plasma Coagulation of Gastrojejunal Anastomosis for Weight Regain After Gastric Bypass. Obes. Surg. 2014, 25, 72–79. [Google Scholar] [CrossRef]
- Jirapinyo, P.; de Moura, D.T.; Thompson, C.C. Endoscopic submucosal dissection with suturing for the treatment of weight regain after gastric bypass: Outcomes and comparison with traditional transoral outlet reduction (with video). Gastrointest. Endosc. 2020, 91, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Switzer, N.J.; Karmali, S.; Gill, R.S.; Sherman, V. Revisional Bariatric Surgery. Surg. Clin. N. Am. 2016, 96, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiao, S.; Zhang, S.; Zhou, J. Revisional Surgeries of Laparoscopic Sleeve Gastrectomy. Diabetes, Metab. Syndr. Obesity Targets Ther. 2021, 14, 575–588. [Google Scholar] [CrossRef]
- Kermansaravi, M.; Jazi, A.H.D.; Shahmiri, S.S.; Eghbali, F.; Valizadeh, R.; Rezvani, M. Revision procedures after initial Roux-en-Y gastric bypass, treatment of weight regain: A systematic review and meta-analysis. Updat. Surg. 2021, 73, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Noria, S.F.; Shelby, R.D.; Atkins, K.D.; Nguyen, N.T.; Gadde, K.M. Weight Regain After Bariatric Surgery: Scope of the Problem, Causes, Prevention, and Treatment. Curr. Diabetes Rep. 2023, 23, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.; Guimarães, L.F.; de Andrade Carvalho, L.; de Castro, C.T.; Lima Vieira, R.; Sernizon, N. Weight regain after bariatric surgery: A systematic review and meta-analysis of observational studies. Obes. Med. 2024, 45, 100528. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).