Deciphering Childhood Rosacea: A Comprehensive Review
Abstract
1. Introduction
2. Epidemiology
2.1. Pathophysiology
2.2. Clinical Features
2.3. Diagnosis of Childhood Rosacea
2.4. Differential Diagnosis of Childhood Rosacea
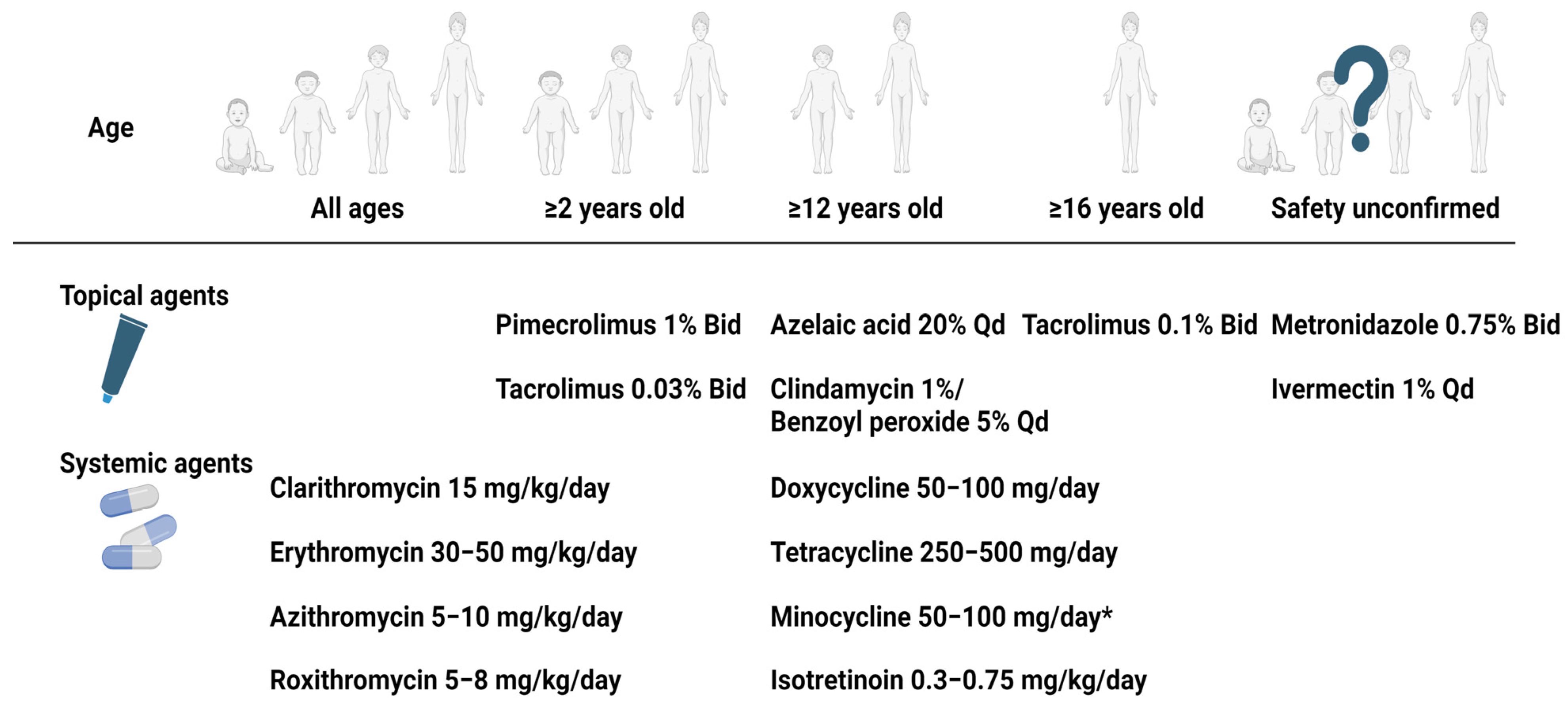
2.5. Treatment of Childhood Rosacea
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoepfner, A.; Marsela, E.; Clanner-Engelshofen, B.M.; Horvath, O.N.; Sardy, M.; French, L.E.; Reinholz, M. Rosacea and perioral dermatitis: A single-center retrospective analysis of the clinical presentation of 1032 patients. J. Dtsch. Dermatol. Ges. 2020, 18, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Rueda, L.J.; Motta, A.; Pabón, J.G.; Barona, M.I.; Meléndez, E.; Orozco, B.; Rojas, R.F. Epidemiology of rosacea in Colombia. Int. J. Dermatol. 2017, 56, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.S.; Huang, W.W. Rosacea Pathogenesis. Dermatol. Clin. 2018, 36, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Spoendlin, J.; Voegel, J.J.; Jick, S.S.; Meier, C.R. A study on the epidemiology of rosacea in the UK. Br. J. Dermatol. 2012, 167, 598–605. [Google Scholar] [CrossRef]
- Laude, T.A.; Salvemini, J.N. Perioral dermatitis in children. Semin. Cutan Med. Surg. 1999, 18, 206–209. [Google Scholar] [CrossRef]
- Chamaillard, M.; Mortemousque, B.; Boralevi, F.; Marques da Costa, C.; Aitali, F.; Taïeb, A.; Léauté-Labrèze, C. Cutaneous and ocular signs of childhood rosacea. Arch. Dermatol. 2008, 144, 167–171. [Google Scholar] [CrossRef]
- Tan, J.; Berg, M. Rosacea: Current state of epidemiology. J. Am. Acad. Dermatol. 2013, 69, S27–S35. [Google Scholar] [CrossRef]
- Noguera-Morel, L.; Hernández-Martín, A.; Torrelo, A. Childhood rosacea and related disorders. Clin. Exp. Dermatol. 2021, 46, 430–437. [Google Scholar] [CrossRef]
- Chosidow, O.; Cribier, B. Epidemiology of rosacea: Updated data. In Annales de Dermatologie et de Venereologie; Elsevier: Amsterdam, The Netherlands, 2011; pp. S179–S183. [Google Scholar]
- Baghad, B.; El Fatoiki, F.Z.; Benhsaien, I.; Bousfiha, A.A.; Puel, A.; Migaud, M.; Chiheb, S.; Ailal, F. Pediatric demodicosis associated with gain-of-function variant in STAT1 presenting as rosacea-type rash. J. Clin. Immunol. 2021, 41, 698–700. [Google Scholar] [CrossRef]
- Sáez-de-Ocariz, M.; Suárez-Gutiérrez, M.; Migaud, M.; O’Farrill-Romanillos, P.; Casanova, J.; Segura-Mendez, N.; Orozco-Covarrubias, L.; Espinosa-Padilla, S.; Puel, A.; Blancas-Galicia, L. Rosacea as a striking feature in family members with a STAT 1 gain-of-function mutation. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e265–e267. [Google Scholar] [CrossRef]
- Buhl, T.; Sulk, M.; Nowak, P.; Buddenkotte, J.; McDonald, I.; Aubert, J.; Carlavan, I.; Déret, S.; Reiniche, P.; Rivier, M. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J. Investig. Dermatol. 2015, 135, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Amir Ali, A.; Vender, R.; Vender, R. The role of IL-17 in papulopustular rosacea and future directions. J. Cutan. Med. Surg. 2019, 23, 635–641. [Google Scholar] [CrossRef]
- Erbagci, Z.; Özgöztaşi, O. The significance of Demodex folliculorum density in rosacea. Int. J. Dermatol. 1998, 37, 421–425. [Google Scholar] [CrossRef]
- Jarmuda, S.; O’Reilly, N.; Żaba, R.; Jakubowicz, O.; Szkaradkiewicz, A.; Kavanagh, K. Potential role of Demodex mites and bacteria in the induction of rosacea. J. Med. Microbiol. 2012, 61, 1504–1510. [Google Scholar] [CrossRef]
- Forton, F.; Seys, B. Density of Demodex folliculorum in rosacea: A case-control study using standardized skin-surface biopsy. Br. J. Dermatol. 1993, 128, 650–659. [Google Scholar] [CrossRef]
- Howard, R.; Tsuchiya, A. Adult skin disease in the pediatric patient. Dermatol. Clin. 1998, 16, 593–608. [Google Scholar] [CrossRef]
- Seo, J.-I.; Shin, M.K. Lupus Miliaris Disseminatus Faciei versus Granulomatous rosacea: A case report. Case Rep. Dermatol. 2021, 13, 321–329. [Google Scholar] [CrossRef]
- Tsai, Y.-W.; Hung, Y.-T.; Chen, W.-T. Clinical Characteristics of Adult-Onset Granulomatous Periorificial Dermatitis: A Case Series and Review of the Literature. Dermatitis® 2023, 35, 10-1097. [Google Scholar] [CrossRef]
- Fakih, A.; Makhoul, R.; Grozdev, I. Childhood granulomatous periorificial dermatitis: Case report and review of the literature. Dermatol. Online J. 2020, 26, 10. [Google Scholar] [CrossRef]
- Neri, I.; Raone, B.; Dondi, A.; Misciali, C.; Patrizi, A. Should idiopathic facial aseptic granuloma be considered granulomatous rosacea? Report of three pediatric cases. Pediatr. Dermatol. 2013, 30, 109–111. [Google Scholar] [CrossRef]
- Weir, S.A.; Amin, S.; Higgins, A.; Kelly, D.; Theos, A. Idiopathic facial aseptic granuloma: Case series and review of histological findings. Bayl. Univ. Med. Cent. Proc. 2023, 36, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Knöpfel, N.; Gómez-Zubiaur, A.; Noguera-Morel, L.; Torrelo, A.; Hernandez-Martin, A. Ultrasound findings in idiopathic facial aseptic granuloma: Case series and literature review. Pediatr. Dermatol. 2018, 35, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Salerni, G.; Peralta, R.; Bertaina, C.; Gorosito, M.; Fernández-Bussy, R. Dermoscopy of idiopathic facial aseptic granuloma. Clin. Exp. Dermatol. 2020, 45, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Plewig, G.; Jansen, T.; Kligman, A.M. Pyoderma faciale. A review and report of 20 additional cases: Is it rosacea? Arch. Dermatol. 1992, 128, 1611–1617. [Google Scholar] [CrossRef]
- Firooz, A.; Firoozabadi, M.R.; Dowlati, Y. Rosacea fulminans (pyoderma faciale): Successful treatment of a 3-year-old girl with oral isotretinoin. Int. J. Dermatol. 2001, 40, 203–205. [Google Scholar] [CrossRef]
- Çetinkaya, A.; Akova, Y.A. Pediatric ocular acne rosacea: Long-term treatment with systemic antibiotics. Am. J. Ophthalmol. 2006, 142, 816–821. [Google Scholar] [CrossRef]
- Donmez, O.; Akova, Y.A. Pediatric ocular acne Rosacea: Clinical features and long term follow-up of sixteen cases. Ocul. Immunol. Inflamm. 2021, 29, 57–65. [Google Scholar] [CrossRef]
- Tavassoli, S.; Wong, N.; Chan, E. Ocular manifestations of rosacea: A clinical review. Clin. Exp. Ophthalmol. 2021, 49, 104–117. [Google Scholar] [CrossRef]
- Borok, J.; Holmes, R.; Dohil, M. Idiopathic facial aseptic granuloma—A diagnostic challenge in pediatric dermatology. Pediatr. Dermatol. 2018, 35, 490–493. [Google Scholar] [CrossRef]
- Trave, I.; Micalizzi, C.; Gasparini, G.; Cozzani, E.; Parodi, A. Dermoscopy of papulopustular rosacea and comparison of dermoscopic features in patients with or without concomitant Demodex folliculorum. Clin. Exp. Dermatol. 2021, 46, 1434–1440. [Google Scholar] [CrossRef]
- Weston, W.L.; Morelli, J.G. Steroid rosacea in prepubertal children. Arch. Pediatr. Adolesc. Med. 2000, 154, 62–64. [Google Scholar] [PubMed]
- Fernandez-Faith, E.; McDonnell, J. Cutaneous sarcoidosis: Differential diagnosis. Clin. Dermatol. 2007, 25, 276–287. [Google Scholar] [CrossRef]
- Aggarwal, A.; Srivastava, P. Childhood onset systemic lupus erythematosus: How is it different from adult SLE? Int. J. Rheum. Dis. 2015, 18, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Wananukul, S.; Watana, D.; Pongprasit, P. Cutaneous manifestations of childhood systemic lupus erythematosus. Pediatr. Dermatol. 1998, 15, 342–346. [Google Scholar] [CrossRef]
- Park, K.Y. Management of Rosacea. J. Korean Acne Rosacea Soc. 2021, 9, 10–15. [Google Scholar]
- Breneman, D.L.; Stewart, D.; Hevia, O.; Hino, P.D.; Drake, L.A. A double-blind, multicenter clinical trial comparing efficacy of once-daily metronidazole 1 percent cream to vehicle in patients with rosacea. Cutis 1998, 61, 44–47. [Google Scholar]
- Bjerke, J.R.; Nyfors, A.; Austad, J.; Rajka, G.; Gjertsen, B.T.; Haavelsrud, O.; Volden, G.; Abrahamsen, A. Metronidazole (Elyzol) 1-percent cream v. placebo cream in the treatment of rosacea. Clin. Trials J. 1989, 26, 187–194. [Google Scholar]
- van Zuuren, E.J.; Fedorowicz, Z.; Tan, J.; Van Der Linden, M.M.; Arents, B.W.M.; Carter, B.; Charland, L. Interventions for rosacea based on the phenotype approach: An updated systematic review including GRADE assessments. Br. J. Dermatol. 2019, 181, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Orion, C.; Sfecci, A.; Tisseau, L.; Darrieux, L.; Safa, G. Idiopathic facial aseptic granuloma in a 13-year-old boy dramatically improved with oral doxycycline and topical metronidazole: Evidence for a link with childhood rosacea. Case Rep. Dermatol. 2016, 8, 197–201. [Google Scholar] [CrossRef]
- Eghlileb, A.M.; Finlay, A.Y. Granulomatous rosacea in Cornelia de Lange syndrome. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 74–75. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Ferreiro, A.; Marqués-Fernández, V.; Martín, Á.J.; González-Márquez, P.; Vega-Gutiérrez, J. Nódulos palpebrales en el granuloma aséptico facial idiopático. Arch. Soc. Española Oftalmol. 2021, 96, 556–560. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. Azelaic acid topical formulations: Differentiation of 15% gel and 15% foam. J. Clin. Aesthet. Dermatol. 2017, 10, 37–40. [Google Scholar]
- Clanner-Engelshofen, B.M.; Bernhard, D.; Dargatz, S.; Flaig, M.J.; Gieler, U.; Kinberger, M.; Klövekorn, W.; Kuna, A.C.; Läuchli, S.; Lehmann, P.; et al. S2k guideline: Rosacea. J. Dtsch Dermatol. Ges. 2022, 20, 1147–1165. [Google Scholar] [CrossRef]
- Bjerke, R.; Fyrand, O.; Graupe, K. Double-blind comparison of azelaic acid 20% cream and its vehicle in treatment of papulo-pustular rosacea. Acta Derm. Venereol. 1999, 79, 456–459. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Elewski, B.; Staedtler, G.; Havlickova, B. Azelaic acid foam 15% in the treatment of papulopustular rosacea: A randomized, double-blind, vehicle-controlled study. Cutis 2013, 92, 306–317. [Google Scholar] [PubMed]
- Goldman, D. Tacrolimus ointment for the treatment of steroid-induced rosacea: A preliminary report. J. Am. Acad. Dermatol. 2001, 44, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Koca, R.; Altinyazar, H.C.; Ankarali, H.; Muhtar, S.; Tekin, N.S.; Cinar, S. A comparison of metronidazole 1% cream and pimecrolimus 1% cream in the treatment of patients with papulopustular rosacea: A randomized open-label clinical trial. Clin. Exp. Dermatol. 2010, 35, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Sloan, L. Is Topical 1% Pimecrolimus Cream an Effective Treatment for Rosacea? Ph.D. Thesis, Philadelphia College of Osteopathic Medicine, Philadelphia, PA, USA, 2011. [Google Scholar]
- Stein, L.; Kircik, L.; Fowler, J.; Tan, J.; Draelos, Z.; Fleischer, A.; Appell, M.; Steinhoff, M.; Lynde, C.; Liu, H.; et al. Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: Results of two randomized, double-blind, vehicle-controlled pivotal studies. J. Drugs Dermatol. 2014, 13, 316–323. [Google Scholar]
- Noguera-Morel, L.; Gerlero, P.; Torrelo, A.; Hernández-Martín, Á. Ivermectin therapy for papulopustular rosacea and periorificial dermatitis in children: A series of 15 cases. J. Am. Acad. Dermatol. 2017, 76, 567–570. [Google Scholar] [CrossRef]
- Trave, I.; Micalizzi, C.; Cozzani, E.; Gasparini, G.; Parodi, A. Papulopustular Rosacea Treated with Ivermectin 1% Cream: Remission of the Demodex Mite Infestation over Time and Evaluation of Clinical Relapses. Dermatol. Pract. Concept 2022, 12, e2022201. [Google Scholar] [CrossRef]
- Garais, J.A.; Bonetto, V.N.; Frontino, L.; Salduna, M.D.; Ruiz Lascano, A. Idiopathic facial aseptic granuloma: A case report. Arch. Argent Pediatr. 2019, 117, e56–e58. [Google Scholar] [CrossRef]
- Léoni, S.; Mesplié, N.; Aitali, F.; Chamaillard, M.; Boralevi, F.; Marques da Costa, C.; Taïeb, A.; Léauté-Labrèze, C.; Colin, J.; Mortemousque, B. Metronidazole: Alternative treatment for ocular and cutaneous rosacea in the pediatric population. J. Fr. Ophtalmol. 2011, 34, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.D.S.; Lafargue, O.; Dompmartin, A. Idiopathic facial aseptic granuloma in children: Management and long-term follow-up. In Annales de Dermatologie et de Vénéréologie; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Miconi, F.; Principi, N.; Cassiani, L.; Celi, F.; Crispoldi, R.; Russo, A.; Esposito, S.; Papini, M. A cheek nodule in a child: Be aware of idiopathic facial aseptic granuloma and its differential diagnosis. Int. J. Environ. Res. Public Health 2019, 16, 2471. [Google Scholar] [CrossRef]
- González Rodríguez, A.; Jordá Cuevas, E. Idiopathic facial aseptic granuloma. Clin. Exp. Dermatol. 2015, 40, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Lenders, D.; Lenders, M.M.; Jäger, M.; Schaller, M. Treatment of aseptic facial granuloma as a manifestation of pediatric rosacea with oral macrolides. Pediatr. Dermatol. 2023, 40, 1064–1067. [Google Scholar] [CrossRef]
- Vieira, A.C.C.; Höfling-Lima, A.L.; Mannis, M.J. Ocular rosacea—A review. Arq. Bras. Oftalmol. 2012, 75, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Zanella, A.; Dresco, F.; Aubin, F.; Puzenat, E. Lower Eyelid Nodule: Chalazion or Idiopathic Facial Aseptic Granuloma? A Case Series. Acta Derm.-Venereol. 2021, 101, 687. [Google Scholar] [CrossRef] [PubMed]
- Doan, S.; Gabison, E.; Chiambaretta, F.; Touati, M.; Cochereau, I. Efficacy of azithromycin 1.5% eye drops in childhood ocular rosacea with phlyctenular blepharokeratoconjunctivitis. J. Ophthalmic Inflamm. Infect. 2013, 3, 38. [Google Scholar] [CrossRef]
- Donaldson, K.E.; Karp, C.L.; Dunbar, M.T. Evaluation and treatment of children with ocular rosacea. Cornea 2007, 26, 42–46. [Google Scholar] [CrossRef]
- Gonser, L.; Gonser, C.; Deuter, C.; Heister, M.; Zierhut, M.; Schaller, M. Systemic therapy of ocular and cutaneous rosacea in children. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1732–1738. [Google Scholar] [CrossRef]
- Cantarutti, N.; Claps, A.; Angelino, G.; Chessa, L.; Callea, F.; El Hachem, M.; Diociaiuti, A.; Finocchi, A. Multi-drugs resistant acne rosacea in a child affected by Ataxia-Telangiectasia: Successful treatment with Isotretinoin. Ital. J. Pediatr. 2015, 41, 23. [Google Scholar] [CrossRef]
- Lee, G.; Fischer, G. Isotretinoin therapy for idiopathic aseptic facial granuloma. Australas. J. Dermatol. 2020, 61, 283–285. [Google Scholar] [CrossRef]
- Sanchez-Espino, L.F.; Sibbald, C. Idiopathic facial aseptic granuloma: Report of successful treatment with low-dose isotretinoin in a pediatric patient with trisomy 21. JAAD Case Rep. 2022, 24, 88–90. [Google Scholar] [CrossRef]
- Brown, M.; Hernández-Martín, A.; Clement, A.; Colmenero, I.; Torrelo, A. Severe Demodexfolliculorum–Associated Oculocutaneous Rosacea in a Girl Successfully Treated with Ivermectin. JAMA Dermatol. 2014, 150, 61–63. [Google Scholar] [CrossRef]
- Chabchoub, I.; Litaiem, N.; Zeglaoui, F. Pediatric rosacea in a patient with a dark phototype: Clinical and dermoscopic features. Clin. Case Rep. 2020, 8, 3256–3258. [Google Scholar] [CrossRef]
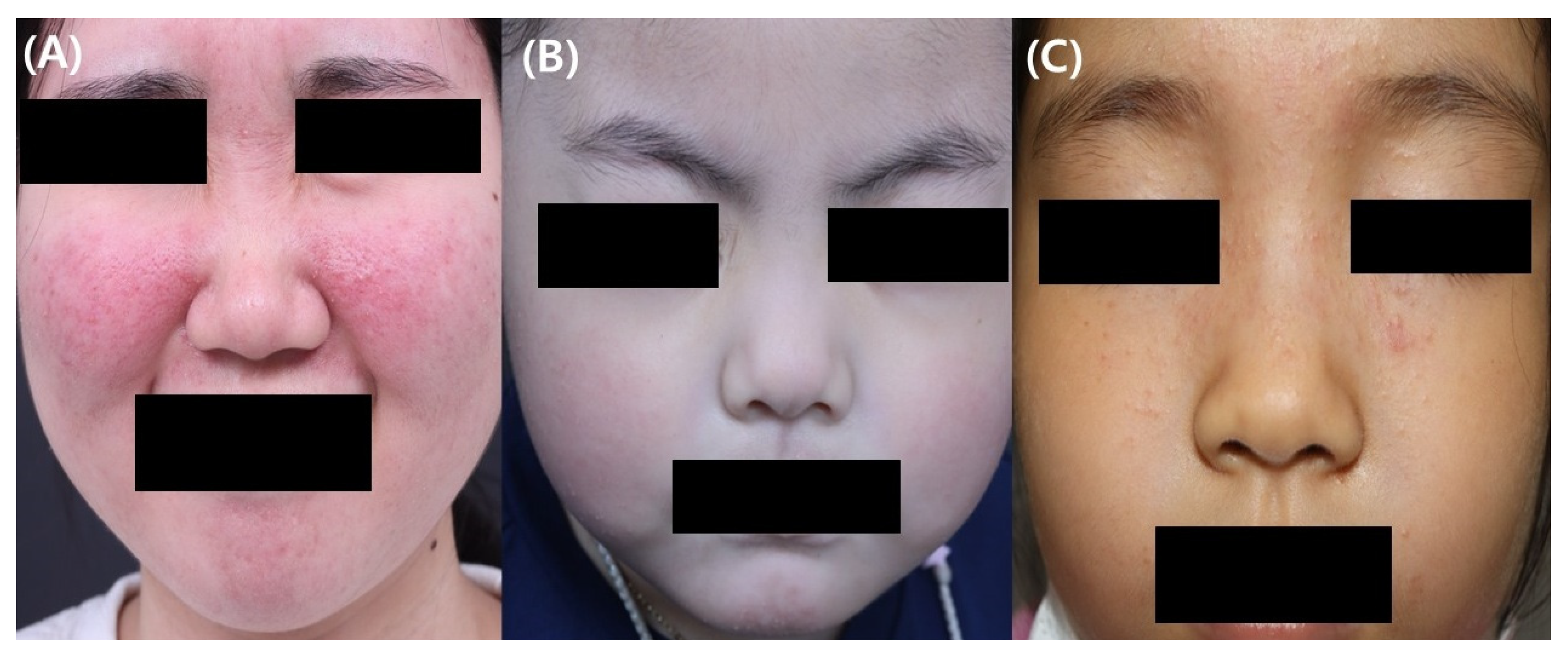

| Clinical Manifestation | Characteristics |
|---|---|
| Papulopustular | Crops of papules and pustules with or without facial erythema or flushing |
| Telangiectatic | Persistent erythema with or without flushing |
| Granulomatous | Firm erythematous papules and pustules on a background of a normal-appearing skin |
| Idiopathic facial aseptic granuloma | Non-tender solitary or multiple red to violaceous nodules on the cheeks |
| Ocular rosacea | Occurs with or without cutaneous manifestations ofBlepharoconjuctivitis, meibomitis, recurrent chalazion, episcleritis, iritis, corneal vascularization, keratitis, corneal ulcer and scarring, lid margin telangiectasia, conjunctival hyperemia with or without inferior corneal vascularization |
| Reference | Age (Number of Patients) | Subtype | Doses and Duration | Clinical Response |
|---|---|---|---|---|
| Azithromycin alone | ||||
| Zanella et al. [60] | 3 y (n = 1) | IFAG | 1.5% 3 days in a row every 15 days for 3 months | Favorable outcome |
| Doan et al. [61] | 4–16 y (n = 16) | O | 1.5% Bid for 3 days every 10 days | Effective in 15 of 16 patients |
| Clarithromycin alone | ||||
| Borok et al. [30] | 1 y (n = 1) | IFAG | 15 mg/kg BID for 4 months | Complete resolution |
| Neri et al. [21] | 4 y (n = 1) | G | 15 mg/kg BID for 2 months | Complete resolution |
| Doxycycline alone | ||||
| Donaldson et al. [62] | Mean 9.2 y (n = 2) | O | 50 mg or 100 mg BID | Well tolerated |
| Leoni et al. [54] | 14 y (n = 1) | G | 100 mg daily for 2 months | Complete remission |
| Leoni et al. [54] | 12 y (n = 2) | O | 100 mg daily | Complete remission |
| Erythromycin alone | ||||
| Gonser et al. [63] | 2 y (n = 1) | PP and O | 300 mg BID for 10 months | Complete remission |
| Neri et al. [21] | 2 y (n = 1) | G | 50 mg/kg TID for 2 months | Almost complete remission |
| Isotretinoin alone | ||||
| Cantarutti et al. [64] | 10 y (n = 1) | G | 0.5 mg/kg daily for 6 months | Almost complete disappearance, worsened after tapering |
| Lee and Fischer [65] | 2–7 y (n = 4) | IFAG | 0.25 mg/kg daily for 6–9 months | Successful treatment, minimal side effects |
| Sanchez-Espino and Sibbald [66] | 7 y (n = 1) | IFAG | 1 mg/kg twice weekly | Clear resolution |
| Ivermectin alone | ||||
| Brown et al. [67] | 12 y (n = 1) | PP and O | A single dose (250 μg/kg) | Significant improvement at 1 month |
| Metronidazole alone | ||||
| Borok et al. [30] | 2 y (n = 1) | IFAG | 0.75% BID for 4 months | Complete resolution at follow-up |
| Eghlileb and Finlay [41] | 16 y (n = 1) | G | 0.75% BID for 2 months | Some improvement |
| Galindo-Ferreiro et al. [42] | 3–10 y (n = 1) | IFAG | 0.75% BID | Partial improvement |
| Garais et al. [53] | 1 y (n = 1) | IFAG | 20 mg/kg daily for 2 months | Complete resolution |
| Leoni et al. [54] | 1–5 y (n = 10) | PP; PP and O; ETR, PP and O; PP and O; and O | 20–30 mg/kg per day for at least 3 months | Alternative treatment for ocular and cutaneous rosacea |
| Feature | Childhood Rosacea | Adult Rosacea |
|---|---|---|
| Age of onset | 4–5 years old | 35 to 45 years in women 45 to 55 years in men |
| Sex | Similarly observed both in boys and girls | Predominance in women |
| Clinical presentation | -Papulopustular rosacea -Telangiectatic rosacea -Granulomatous rosacea -IFAG: pediatric specific subtype -Ocular rosacea: more common and more frequently preceding cutaneous features in children -Lack of consensus on the classification for childhood rosacea | -Major subtypes Papulopustular Erythematotelangiectatic Phymatous Ocular rosacea -Other subtypes Granulomatous rosacea Neurogenic rosacea |
| Treatment | -Lack of consensus, refer to the adults’ treatments | -General skin care, topical therapy, systemic therapy, laser therapy or IPL or surgical intervention can be conducted based on the patient’s symptom. |
| Prognosis | -Having rosacea during childhood may increase the risk of developing rosacea as an adult -IFAG: resolve spontaneously, often within an average of 12 months | Chronic conditions with fluctuating course |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, Y.R.; Kim, H.S. Deciphering Childhood Rosacea: A Comprehensive Review. J. Clin. Med. 2024, 13, 1126. https://doi.org/10.3390/jcm13041126
Woo YR, Kim HS. Deciphering Childhood Rosacea: A Comprehensive Review. Journal of Clinical Medicine. 2024; 13(4):1126. https://doi.org/10.3390/jcm13041126
Chicago/Turabian StyleWoo, Yu Ri, and Hei Sung Kim. 2024. "Deciphering Childhood Rosacea: A Comprehensive Review" Journal of Clinical Medicine 13, no. 4: 1126. https://doi.org/10.3390/jcm13041126
APA StyleWoo, Y. R., & Kim, H. S. (2024). Deciphering Childhood Rosacea: A Comprehensive Review. Journal of Clinical Medicine, 13(4), 1126. https://doi.org/10.3390/jcm13041126






