Complex Regional Pain Syndrome after Distal Radius Fracture—Case Report and Mini Literature Review
Abstract
1. Introduction
1.1. Distal Radius Fractures: Presentation and Epidemiology
1.2. Distal Radius Fractures: Treatment and Complications
1.2.1. Complex Regional Pain Syndrome: Natural History and Presentation
1.2.2. Complex Regional Pain Syndrome: Diagnosis and Epidemiology
1.2.3. Complex Regional Pain Syndrome: Phases of CRPS
1.2.4. Complex Regional Pain Syndrome: Prevention
1.2.5. Complex Regional Pain Syndrome: Treatment
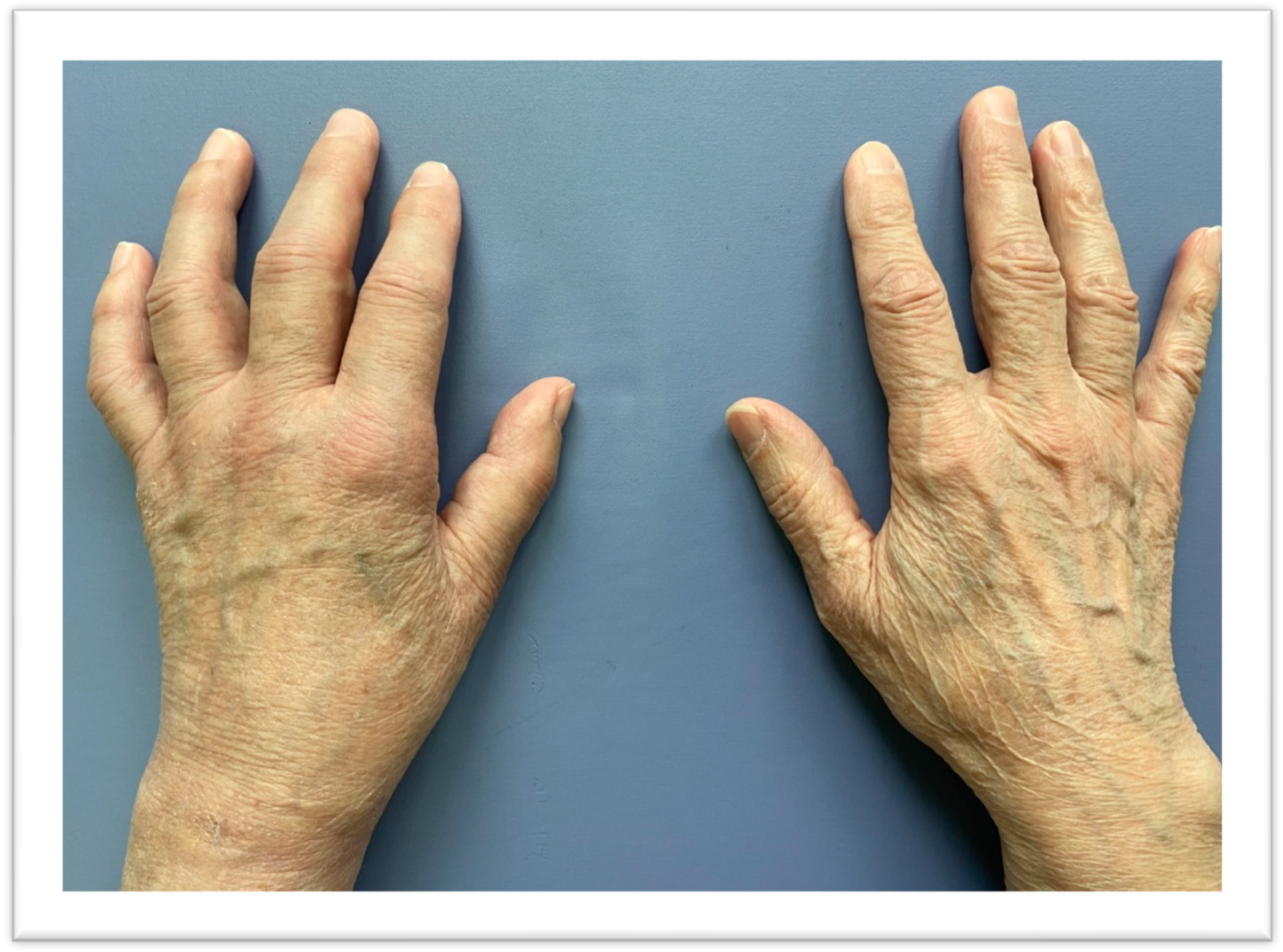
2. Case Report
2.1. Patient
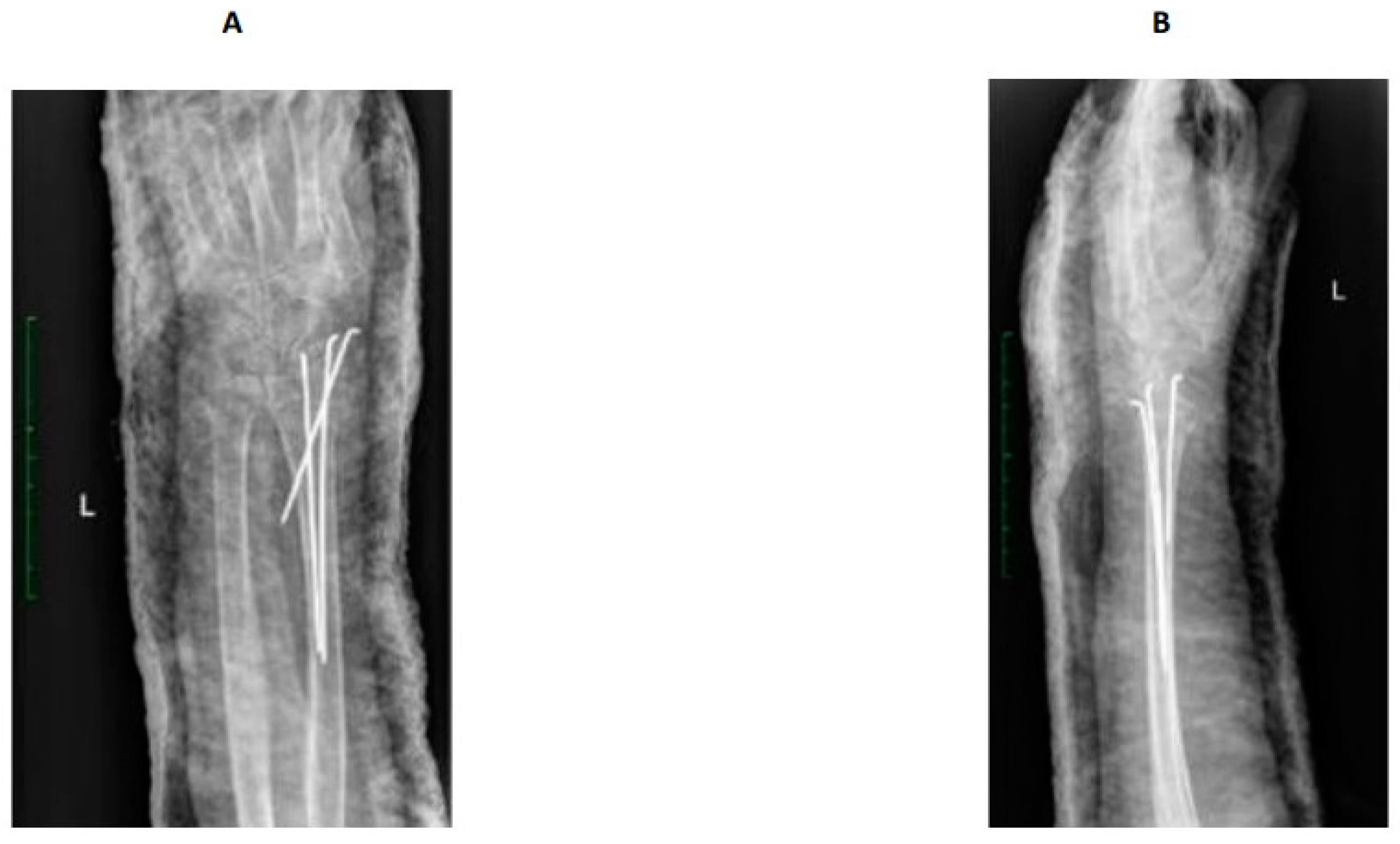
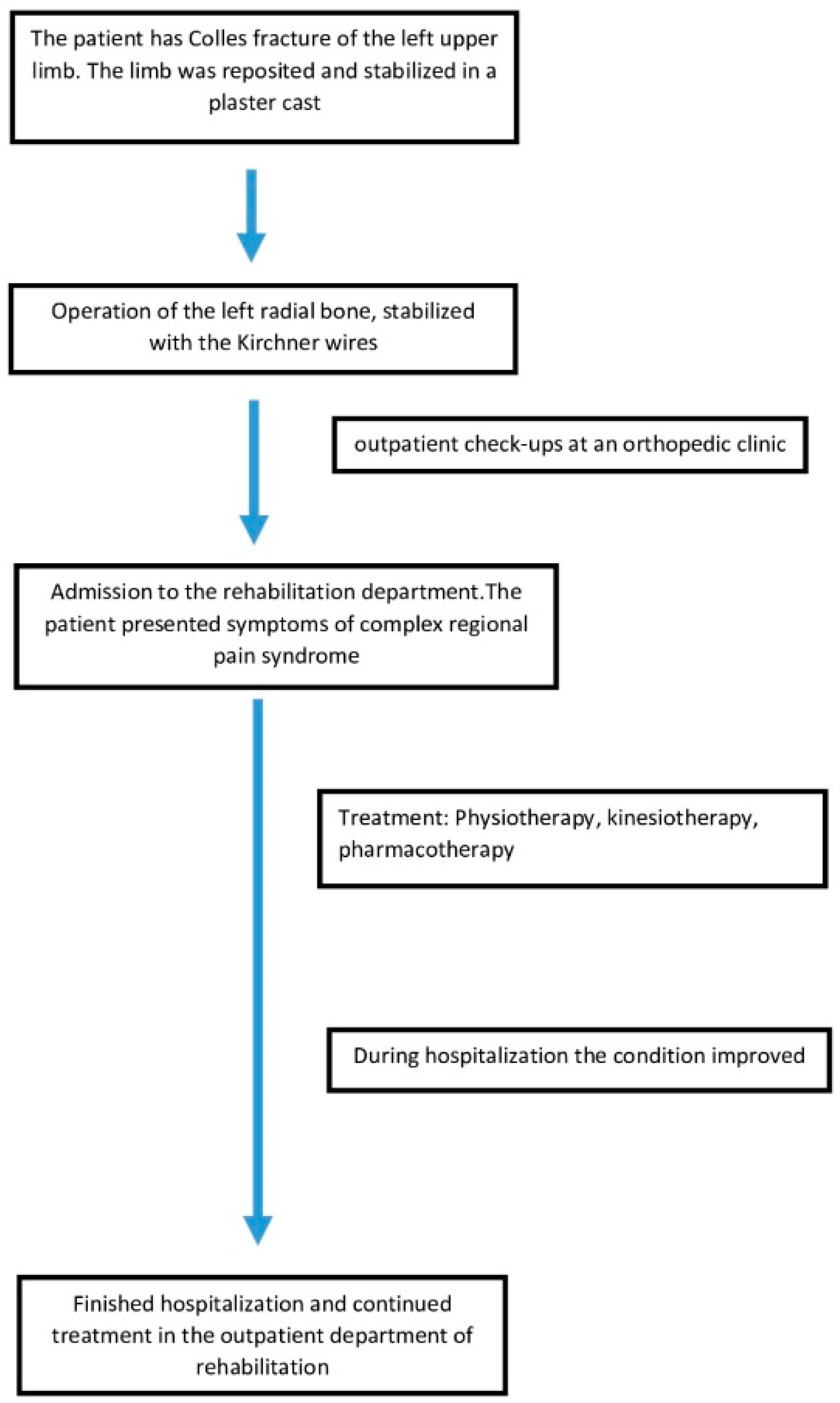
2.2. Fracture and Initial Treatment
2.3. After Admission to the Rehabilitation Department
2.3.1. Patient Examination
2.3.2. Pharmacology and Rehabilitation Treatment
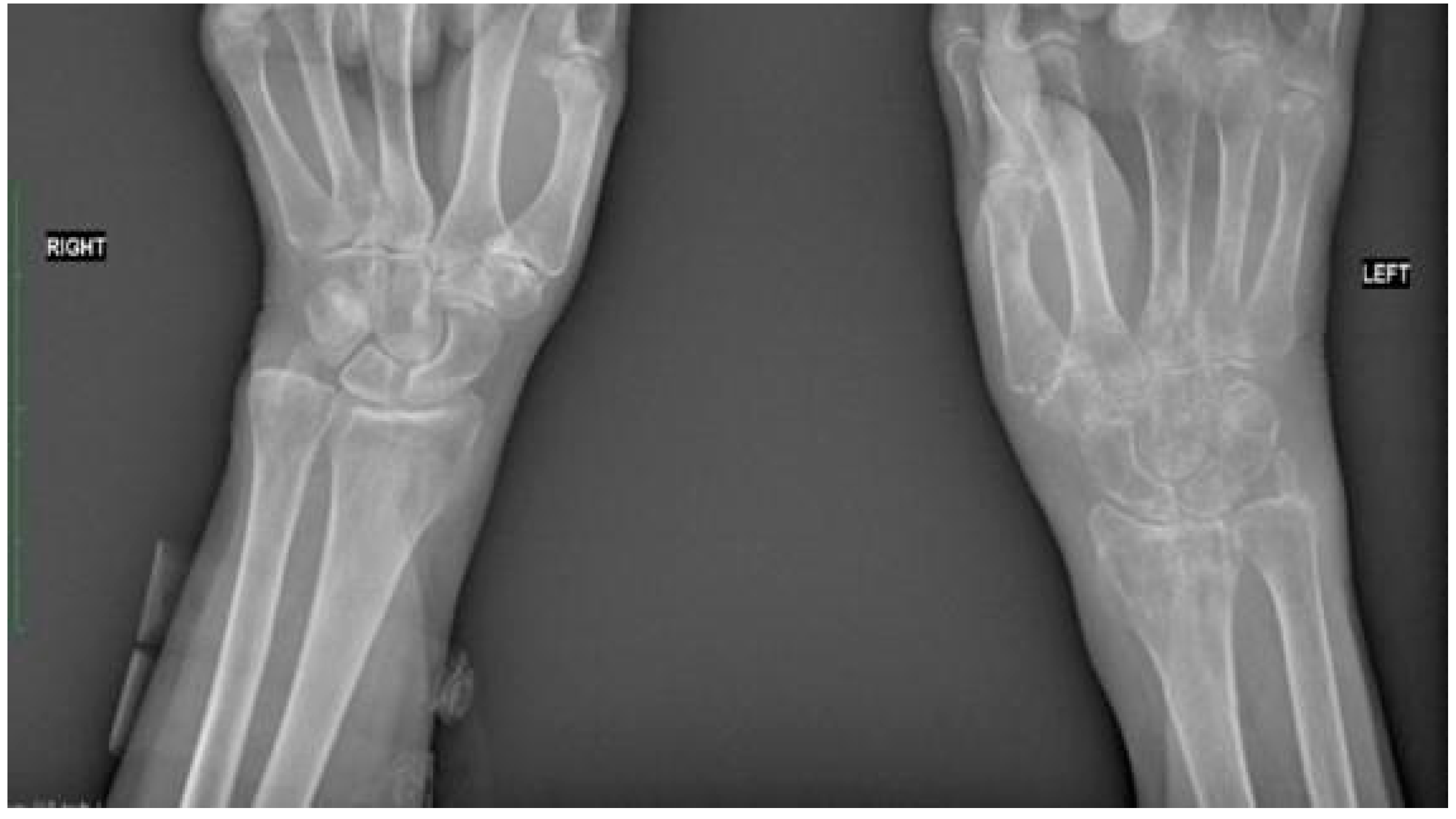
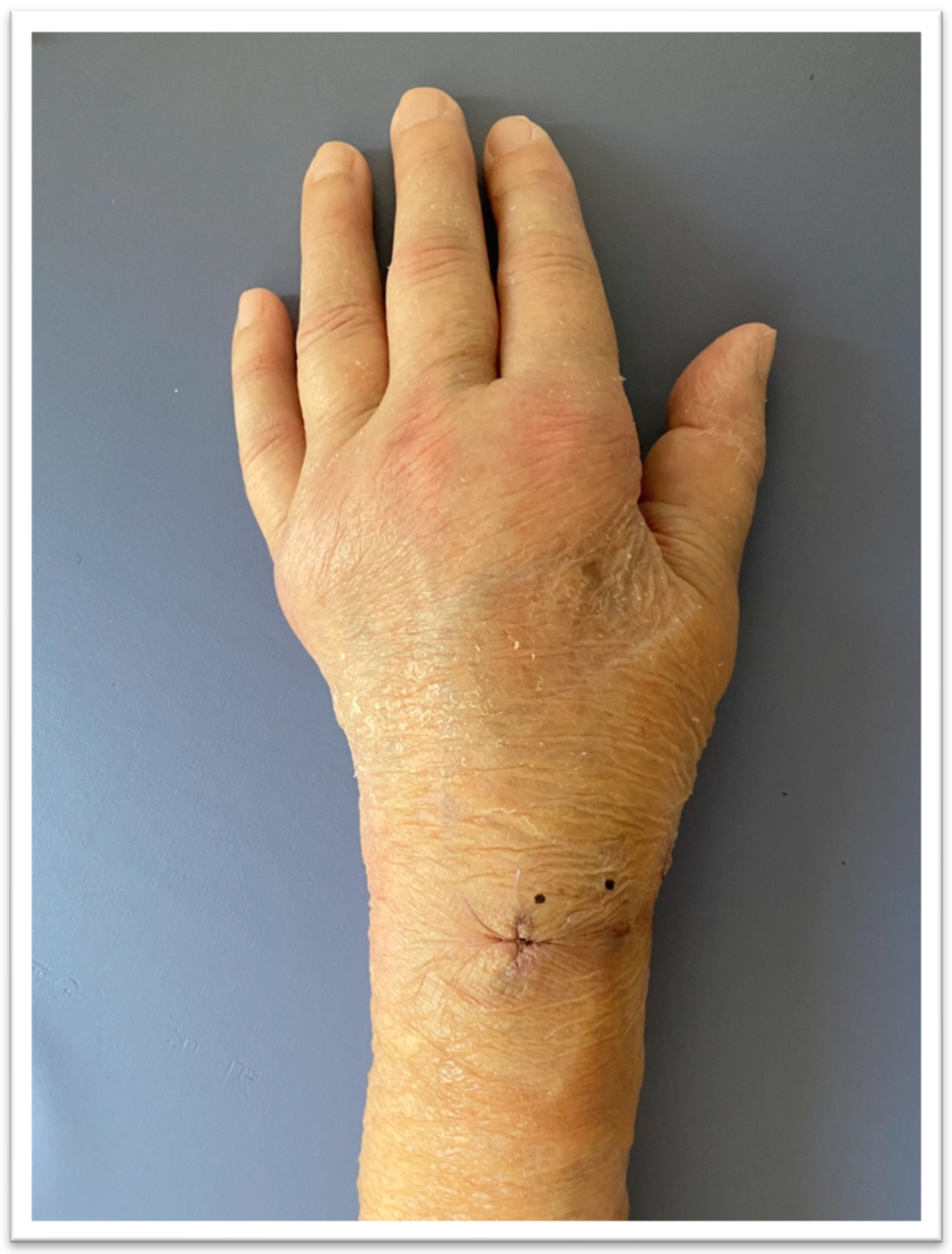
2.3.3. Follow-Up Outcome
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MacIntyre, N.J.; Dewan, N. Epidemiology of distal radius fractures and factors predicting risk and prognosis. J. Hand Ther. 2016, 29, 136–145. [Google Scholar] [CrossRef]
- Mauck, B.M.; Swigler, C.W. Evidence-Based Review of Distal Radius Fractures. Orthop. Clin. N. Am. 2018, 49, 211–222. [Google Scholar] [CrossRef]
- Summers, K.; Mabrouk, A.; Fowles, S.M. Colles Fracture. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Rundgren, J.; Bojan, A.; Mellstrand Navarro, C.; Enocson, A. Epidemiology, classification, treatment and mortality of distal radius fractures in adults: An observational study of 23,394 fractures from the national Swedish fracture register. BMC Musculoskelet. Disord. 2020, 21, 88. [Google Scholar] [CrossRef]
- Ju, J.H.; Jin, G.Z.; Li, G.X.; Hu, H.Y.; Hou, R.X. Comparison of treatment outcomes between nonsurgical and surgical treatment of distal radius fracture in elderly: A systematic review and meta-analysis. Langenbecks Arch. Surg. 2015, 400, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Mathews, A.L.; Chung, K.C. Management of complications of distal radius fractures. Hand Clin. 2015, 31, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Espinoza, H.; Araya-Quintanilla, F.; Olguín-Huerta, C.; Gutiérrez-Monclus, R.; Valenzuela-Fuenzalida, J.; Román-Veas, J.; Campos-Jara, C. Effectiveness of surgical versus conservative treatment of distal radius fractures in elderly patients: A systematic review and meta-analysis. Orthop. Traumatol. Surg. Res. 2022, 108, 103323. [Google Scholar] [CrossRef]
- Shen, O.; Chen, C.T.; Jupiter, J.B.; Chen, N.C.; Liu, W.C. Functional outcomes and complications after treatment of distal radius fracture in patients sixty years and over: A systematic review and network meta-analysis. Injury 2023, 54, 110767. [Google Scholar] [CrossRef]
- Turner, R.G.; Faber, K.J.; Athwal, G.S. Complications of distal radius fractures. Orthop. Clin. N. Am. 2007, 38, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.S.; Noor, N.; Urits, I.; Paladini, A.; Sadhu, M.S.; Gibb, C.; Carlson, T.; Myrcik, D.; Varrassi, G.; Viswanath, O. Complex Regional Pain Syndrome: A Comprehensive Review. Pain Ther. 2021, 10, 875–892. [Google Scholar] [CrossRef]
- Ott, S.; Maihöfner, C. Signs and Symptoms in 1043 Patients with Complex Regional Pain Syndrome. J. Pain 2018, 19, 599–611. [Google Scholar] [CrossRef]
- Eldufani, J.; Elahmer, N.; Blaise, G. A medical mystery of complex regional pain syndrome. Heliyon 2020, 6, e03329. [Google Scholar] [CrossRef]
- Michels, T. Peripheral Neuropathic Pain and Pain Related to Complex Regional Pain Syndrome with and without Fixed Dystonia-Efficient Therapeutic Approach with Local Anesthetics. Local Reg. Anesth. 2020, 13, 11–16. [Google Scholar] [CrossRef]
- Jo, Y.H.; Kim, K.; Lee, B.G.; Kim, J.H.; Lee, C.H.; Lee, K.H. Incidence of and Risk Factors for Complex Regional Pain Syndrome Type 1 after Surgery for Distal Radius Fractures: A Population-based Study. Sci. Rep. 2019, 9, 4871. [Google Scholar] [CrossRef]
- Mangnus, T.J.P.; Dirckx, M.; Huygen, F.J. Different Types of Pain in Complex Regional Pain Syndrome Require a Personalized Treatment Strategy. J. Pain Res. 2023, 16, 4379–4391. [Google Scholar] [CrossRef]
- Misidou, C.; Papagoras, C. Complex Regional Pain Syndrome: An update. Mediterr. J. Rheumatol. 2019, 30, 16–25. [Google Scholar] [CrossRef]
- Marinus, J.; Moseley, G.L.; Birklein, F.; Baron, R.; Maihöfner, C.; Kingery, W.S.; van Hilten, J.J. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol. 2011, 10, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.; Rose, J.; Halle, S.; Shekane, P. Complex regional pain syndrome: A narrative review for the practising clinician. Br. J. Anaesth. 2019, 123, e424–e433. [Google Scholar] [CrossRef] [PubMed]
- Harnik, M.A.; Kesselring, P.; Ott, A.; Urman, R.D.; Luedi, M.M. Complex Regional Pain Syndrome (CRPS) and the Value of Early Detection. Curr. Pain Headache Rep. 2023, 27, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Goebel, A.; Barker, C.; Birklein, F.; Brunner, F.; Casale, R.; Eccleston, C.; Eisenberg, E.; McCabe, C.S.; Moseley, G.L.; Perez, R.; et al. Standards for the diagnosis and management of complex regional pain syndrome: Results of a European Pain Federation task force. Eur. J. Pain 2019, 23, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Harden, N.R.; Bruehl, S.; Perez, R.S.G.M.; Birklein, F.; Marinus, J.; Maihofner, C.; Lubenow, T.; Buvanendran, A.; Mackey, S.; Graciosa, J.; et al. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for Complex Regional Pain Syndrome. Pain 2010, 150, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Yoo, M.; Calisoff, R. Complex regional pain syndrome: An updated comprehensive review. NeuroRehabilitation 2020, 47, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Wertli, M.M.; Brunner, F.; Steurer, J.; Held, U. Usefulness of bone scintigraphy for the diagnosis of Complex Regional Pain Syndrome 1: A systematic review and Bayesian meta-analysis. PLoS ONE 2017, 12, e0173688. [Google Scholar] [CrossRef] [PubMed]
- Beerthuizen, A.; Stronks, D.L.; Huygen, F.J.; Passchier, J.; Klein, J.; Spijker, A.V. The association between psychological factors and the development of complex regional pain syndrome type 1 (CRPS1)—A prospective multicenter study. Eur. J. Pain 2011, 15, 971–975. [Google Scholar] [CrossRef] [PubMed]
- de Mos, M.; de Bruijn, A.G.; Huygen, F.J.; Dieleman, J.P.; Stricker, B.H.; Sturkenboom, M.C. The incidence of complex regional pain syndrome: A population-based study. Pain 2007, 129, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, G.; de Sire, A.; Moretti, A.; Gimigliano, F. Complex regional pain syndrome (CRPS) type I: Historical perspective and critical issues. Clin. Cases Min. Bone Metab. 2015, 12 (Suppl. 1), 4–10. [Google Scholar] [CrossRef] [PubMed]
- Marczyński, W.J. Traumatologia Narządu Ruchu: Biologia i Biomechanika Leczenia; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2017; pp. 324–330. [Google Scholar]
- Besse, J.L.; Gadeyne, S.; Galand-Desmé, S.; Lerat, J.L.; Moyen, B. Effect of vitamin C on prevention of complex regional pain syndrome type I in foot and ankle surgery. Foot Ankle Surg. 2009, 15, 179–182. [Google Scholar] [CrossRef]
- Meena, S.; Sharma, P.; Gangary, S.K.; Chowdhury, B. Role of vitamin C in prevention of complex regional pain syndrome after distal radius fractures: A meta-analysis. Eur. J. Orthop. Surg. Traumatol. 2015, 25, 637–641. [Google Scholar] [CrossRef]
- Harden, R.N.; McCabe, C.S.; Goebel, A.; Massey, M.; Suvar, T.; Grieve, S.; Bruehl, S. Complex Regional Pain Syndrome: Practical Diagnostic and Treatment Guidelines, 5th Edition. Pain Med. 2022, 23, S1–S53. [Google Scholar] [CrossRef]
- Lloyd, E.C.O.; Dempsey, B.; Romero, L. Complex Regional Pain Syndrome. Am. Fam. Physician 2021, 104, 49–55. [Google Scholar]
- Gungor, S.; Aiyer, R.; Baykoca, B. Sympathetic blocks for the treatment of complex regional pain syndrome: A case series. Medicine 2018, 97, e0705. [Google Scholar] [CrossRef]
- Halicka, M.; Vittersø, A.D.; Proulx, M.J.; Bultitude, J.H. Pain reduction by inducing sensory-motor adaptation in Complex Regional Pain Syndrome (CRPS PRISMA): Protocol for a double-blind randomized controlled trial. BMC Neurol. 2020, 20, 62. [Google Scholar] [CrossRef]
- McGee, C.; Skye, J.; van Heest, A. Graded motor imagery for women at risk for developing type I CRPS following closed treatment of distal radius fractures: A randomized comparative effectiveness trial protocol. BMC Musculoskelet. Disord. 2018, 19, 202. [Google Scholar] [CrossRef]
- Simonyan, A.S.; Tyurnikov, V.M.; Simonyan, A.D.; Gushcha, A.O. Epidural unilateral stimulation with “adaptive stim” option in treatment of type II CRPS. Clin. Case Rep. 2022, 10, e05305. [Google Scholar] [CrossRef] [PubMed]
- Mirone, G.; Natale, M.; Rotondo, M. Peripheral median nerve stimulation for the treatment of iatrogenic complex regional pain syndrome (CRPS) type II after carpal tunnel surgery. J. Clin. Neurosci. 2009, 16, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.B.; Mikkelsen, K.L.; Lauritzen, J.B.; Krogsgaard, M.R. Risk Factors for Post-Treatment Complex Regional Pain Syndrome (CRPS): An Analysis of 647 Cases of CRPS from the Danish Patient Compensation Association. Pain Pract. 2018, 18, 341–349. [Google Scholar] [CrossRef]
- Pons, T.; Shipton, E.A.; Williman, J.; Mulder, R.T. Potential risk factors for the onset of complex regional pain syndrome type 1: A systematic literature review. Anesthesiol. Res. Pract. 2015, 2015, 956539. [Google Scholar] [CrossRef] [PubMed]
- Limerick, G.; Christo, D.K.; Tram, J.; Moheimani, R.; Manor, J.; Chakravarthy, K.; Karri, J.; Christo, P.J. Complex Regional Pain Syndrome: Evidence-Based Advances in Concepts and Treatments. Curr. Pain Headache Rep. 2023, 27, 269–298. [Google Scholar] [CrossRef] [PubMed]
- Smart, K.M.; Ferraro, M.C.; Wand, B.M.; O’Connell, N.E. Physiotherapy for pain and disability in adults with complex regional pain syndrome (CRPS) types I and II. Cochrane Database Syst. Rev. 2022, 5, CD010853. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Rebolledo, G.; Gatica-Rojas, V.; Torres-Cueco, R.; Albornoz-Verdugo, M.; Guzmán-Muñoz, E. Update on the effects of graded motor imagery and mirror therapy on complex regional pain syndrome type 1: A systematic review. J. Back Musculoskelet. Rehabil. 2017, 30, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Körbler, C.; Pfau, M.; Becker, F.; Koester, U.; Werdin, F. Die Handtherapie in der Behandlung des CRPS [Hand Therapy in the Treatment of Patients with CRPS]. Handchir. Mikrochir. Plast. Chir. 2015, 47, 182–189. [Google Scholar] [CrossRef]
- Breuer, B.; Pappagallo, M.; Ongseng, F.; Chen, C.I.; Goldfarb, R. An open-label pilot trial of ibandronate for complex regional pain syndrome. Clin. J. Pain 2008, 24, 685–689. [Google Scholar] [CrossRef]
- Lee, S.U.; Na, K.T.; Lee, Y.M.; Park, J.H.; Joo, S.Y. Low vitamin D levels in post-menopausal women are associated with complex regional pain syndrome type I in surgically treated distal radius fractures. J. Orthop. Surg. Res. 2020, 15, 328. [Google Scholar] [CrossRef]
- Benedetti, M.G.; Cavazzuti, L.; Mosca, M.; Fusaro, I.; Zati, A. Bio-Electro-Magnetic-Energy-Regulation (BEMER) for the treatment of type I complex regional pain syndrome: A pilot study. Physiother. Theory Pract. 2020, 36, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Kreska, Z.; Mátrai, P.; Nemeth, B.; Ajtay, B.; Kiss, I.; Hejjel, L.; Ajtay, Z. Physical Vascular Therapy (BEMER) Affects Heart Rate Asymmetry in Patients With Coronary Heart Disease. Vivo 2022, 36, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Bohn, W.; Hess, L.; Burger, R. The effects of the “physical BEMER® vascular therapy”, a method for the physical stimulation of the vasomotion of precapillary microvessels in case of impaired microcirculation, on sleep, pain and quality of life of patients with different clinical pictures on the basis of three scientifically validated scales. J. Complement. Integr. Med. 2013, 10, S5–S13. [Google Scholar] [CrossRef] [PubMed]
- Piatkowski, J.; Kern, S.; Ziemssen, T. Effect of BEMER magnetic field therapy on the level of fatigue in patients with multiple sclerosis: A randomized, double-blind controlled trial. J. Altern. Complement. Med. 2009, 15, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Gyulai, F.; Rába, K.; Baranyai, I.; Berkes, E.; Bender, T. BEMER Therapy Combined with Physiotherapy in Patients with Musculoskeletal Diseases: A Randomised, Controlled Double Blind Follow-Up Pilot Study. Evid.-Based Complement. Altern. Med. 2015, 2015, 245742. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Martínez, F.; Angulo-Díaz-Parreño, S.; Feijóo-Rubio, X.; Fernández-Solís, M.M.; León-Hernández, J.V.; La Touche, R.; Suso-Martí, L. Motor effects of movement representation techniques and cross-education: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2022, 58, 94–107. [Google Scholar] [CrossRef]
- Tofani, M.; Santecchia, L.; Conte, A.; Berardi, A.; Galeoto, G.; Sogos, C.; Petrarca, M.; Panuccio, F.; Castelli, E. Effects of Mirror Neurons-Based Rehabilitation Techniques in Hand Injuries: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 5526. [Google Scholar] [CrossRef]
- Wittkopf, P.G.; Johnson, M.I. Mirror therapy: A potential intervention for pain management. Rev. Assoc. Med. Bras. 2017, 63, 1000–1005. [Google Scholar] [CrossRef]
- Domerchie, P.N.; Dijkstra, P.U.; Geertzen, J.H.B. Long-standing complex regional pain syndrome-type i: Perspectives of patients not amputated. J. Rehabil. Med.-Clin. Commun. 2023, 6, 7789. [Google Scholar] [CrossRef] [PubMed]
- Baygutalp, F.; Kul, A. Effect of Early Orthopedic Rehabilitation on Development of Complex Regional Pain Syndrome Type 1. Eurasian J. Med. 2020, 52, 110–114. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, G.A.J.; Perez, R.S.G.M.; van Gestel, M.A.; Huygen, F.J.; van Kleef, M.; van Eijs, F.; Dahan, A.; van Hilten, J.J.; Marinus, J. Health-related quality of life in 975 patients with complex regional pain syndrome type 1. Pain 2014, 155, 629–634. [Google Scholar] [CrossRef] [PubMed]
| Categories | Objective Signs | Subjective Symptoms |
|---|---|---|
| hyperesthesia and/or allodynia | evidence of hyperalgesia (to pinprick) and/or allodynia (to light touch and/or deep somatic pressure and/or joint movement) |
| temperature asymmetry and/or skin color changes and/or skin color asymmetry | evidence of temperature asymmetry >1 °C and/or skin color changes and/or asymmetry |
| edema or sweating changes or sweating asymmetry | evidence of edema and/or sweating changes and/or sweating asymmetry |
| decreased range of motion and/or motor dysfunction (weakness, tremor, dystonia) and/or trophic changes (hair, nail, skin) | evidence of decreased range of motion and/or motor dysfunction (weakness, tremor, dystonia) and/or trophic changes (hair, nail, skin) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świta, M.; Szymonek, P.; Talarek, K.; Tomczyk-Warunek, A.; Turżańska, K.; Posturzyńska, A.; Winiarska-Mieczan, A. Complex Regional Pain Syndrome after Distal Radius Fracture—Case Report and Mini Literature Review. J. Clin. Med. 2024, 13, 1122. https://doi.org/10.3390/jcm13041122
Świta M, Szymonek P, Talarek K, Tomczyk-Warunek A, Turżańska K, Posturzyńska A, Winiarska-Mieczan A. Complex Regional Pain Syndrome after Distal Radius Fracture—Case Report and Mini Literature Review. Journal of Clinical Medicine. 2024; 13(4):1122. https://doi.org/10.3390/jcm13041122
Chicago/Turabian StyleŚwita, Michał, Paweł Szymonek, Konrad Talarek, Agnieszka Tomczyk-Warunek, Karolina Turżańska, Agnieszka Posturzyńska, and Anna Winiarska-Mieczan. 2024. "Complex Regional Pain Syndrome after Distal Radius Fracture—Case Report and Mini Literature Review" Journal of Clinical Medicine 13, no. 4: 1122. https://doi.org/10.3390/jcm13041122
APA StyleŚwita, M., Szymonek, P., Talarek, K., Tomczyk-Warunek, A., Turżańska, K., Posturzyńska, A., & Winiarska-Mieczan, A. (2024). Complex Regional Pain Syndrome after Distal Radius Fracture—Case Report and Mini Literature Review. Journal of Clinical Medicine, 13(4), 1122. https://doi.org/10.3390/jcm13041122







