Advancing Prone-Transpsoas Spine Surgery: A Narrative Review and Evolution of Indications with Representative Cases
Abstract
1. Historical Review
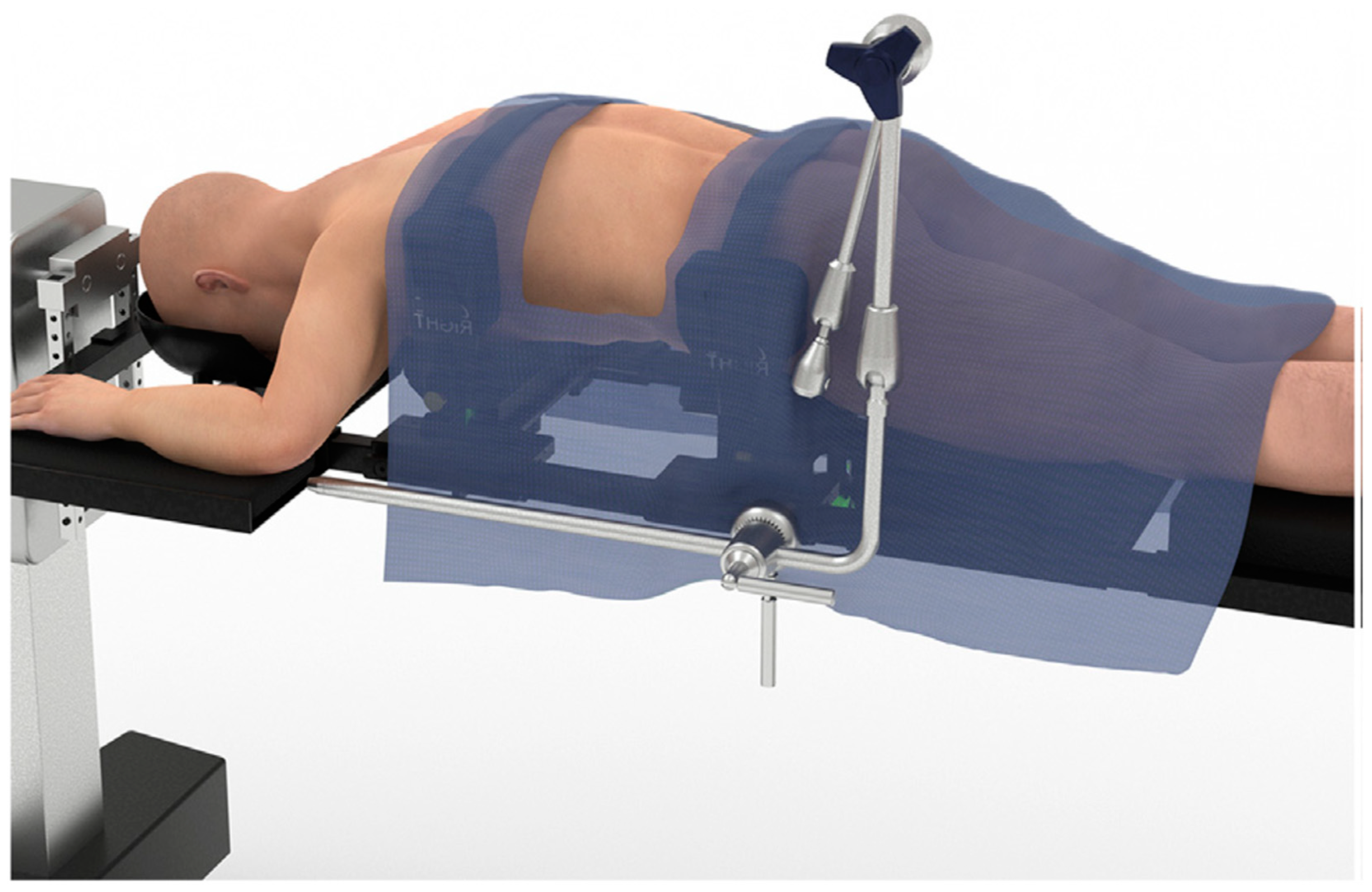
2. Techniques
2.1. CT Navigation and Robotic Assistance
2.2. Fluoroscopic—Instrument Tracking
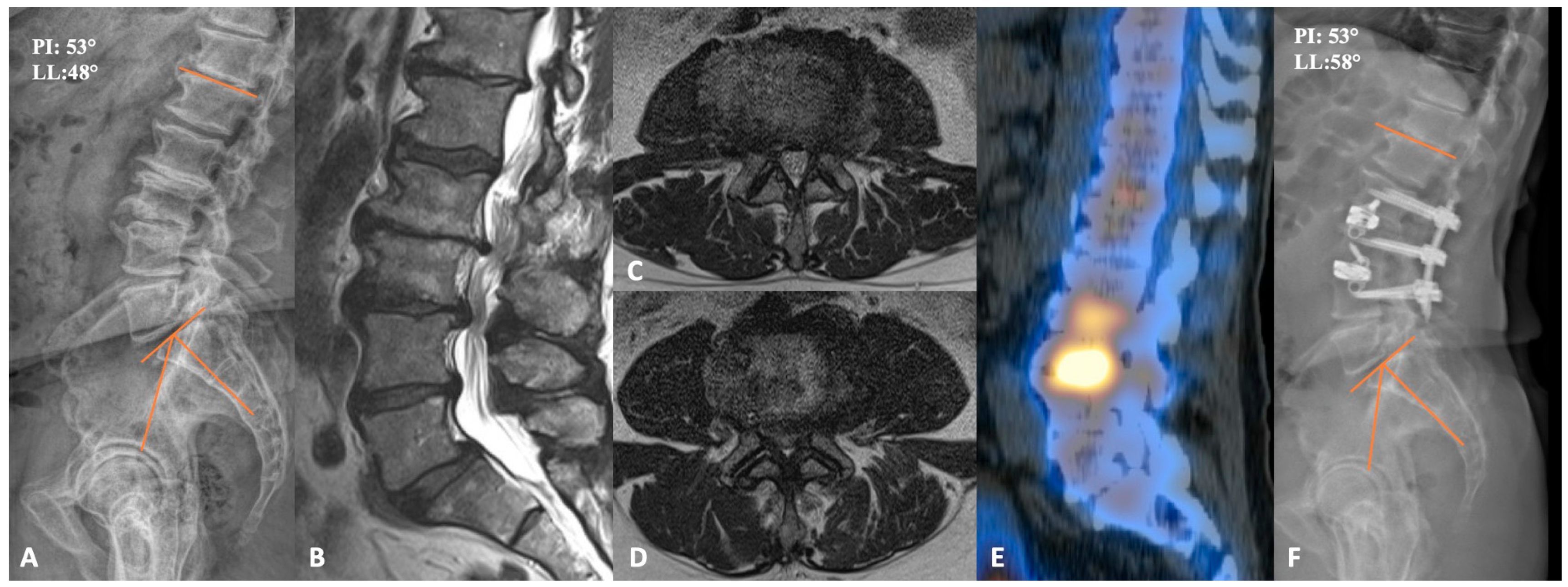
2.3. Case Example
3. Evidence
3.1. Gain in Lordosis
3.2. Retroperitoneal Space and Major Vessels
3.3. Psoas Anatomy and Lumbar Plexus
3.4. Efficiency
3.5. Limitations
4. Complication Profile
4.1. Neuropathy
4.2. Inadvertent Anterior Longitudinal Ligament Release
4.3. Vascular Injury
4.4. Other (Durotomy, Hematoma, and Ureter Injury)
5. Current Applications
5.1. Degenerative Spondylolisthesis
5.2. Adjacent Segment Disease
6. Complex Applications
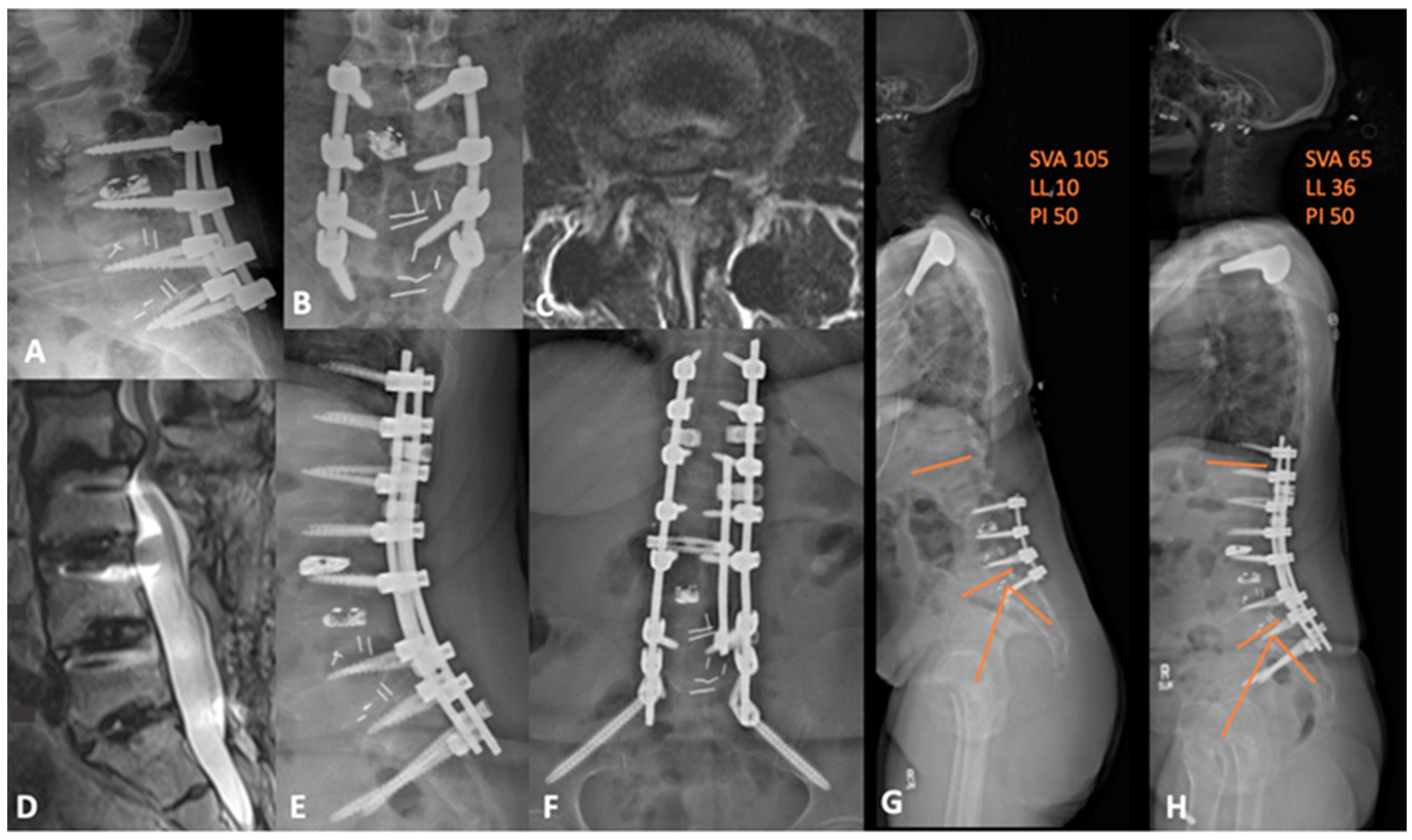
6.1. Deformity
6.2. Anterior Column Realignment
6.3. Corpectomy
6.4. Oncology
6.5. Pseudoarthrosis and Implant Retreival
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ozgur, B.M.; Aryan, H.E.; Pimenta, L.; Taylor, W.R. Extreme Lateral Interbody Fusion (XLIF): A novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006, 6, 435–443. [Google Scholar] [CrossRef]
- Fujibayashi, S.; Kawakami, N.; Asazuma, T.; Ito, M.; Mizutani, J.; Nagashima, H.; Nakamura, M.; Sairyo, K.; Takemasa, R.; Iwasaki, M. Complications Associated with Lateral Interbody Fusion: Nationwide Survey of 2998 Cases during the First 2 Years of Its Use in Japan. Spine 2017, 42, 1478–1484. [Google Scholar] [CrossRef]
- Anand, N.; Baron, E.M. Urological injury as a complication of the transpsoas approach for discectomy and interbody fusion. J. Neurosurg. Spine 2013, 18, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Uribe, J.S.; Deukmedjian, A.R. Visceral, vascular, and wound complications following over 13,000 lateral interbody fusions: A survey study and literature review. Eur. Spine J. 2015, 24 (Suppl. S3), 386–396. [Google Scholar] [CrossRef] [PubMed]
- Hijji, F.Y.; Narain, A.S.; Bohl, D.D.; Ahn, J.; Long, W.W.; DiBattista, J.V.; Kudaravalli, K.T.; Singh, K. Lateral lumbar interbody fusion: A systematic review of complication rates. Spine J. 2017, 17, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Ohba, T.; Oba, H.; Oda, K.; Tanaka, N.; Haro, H. Surgical Outcomes after Minimally Invasive Direct Lateral Corpectomy with Percutaneous Pedicle Screws for Osteoporotic Thoracolumbar Vertebral Collapse with Neurologic Deficits in the Thoracolumbar Spine Compared with Those after Posterior Spinal Fusion with Vertebroplasty. Spine 2021, 46, 1271–1278. [Google Scholar] [CrossRef]
- Cheung, Z.B.; Chen, D.H.; White, S.J.W.; Kim, J.S.; Cho, S.K. Anterior Column Realignment in Adult Spinal Deformity: A Case Report and Review of the Literature. World Neurosurg. 2019, 123, e379–e386. [Google Scholar] [CrossRef]
- Le, H.; Barber, J.; Phan, E.; Hurley, R.K., Jr.; Javidan, Y. Minimally Invasive Lateral Corpectomy of the Thoracolumbar Spine: A Case Series of 20 Patients. Glob. Spine J. 2022, 12, 29–36. [Google Scholar] [CrossRef]
- Lang, G.; Perrech, M.; Navarro-Ramirez, R.; Hussain, I.; Pennicooke, B.; Maryam, F.; Avila, M.J.; Hartl, R. Potential and Limitations of Neural Decompression in Extreme Lateral Interbody Fusion-A Systematic Review. World Neurosurg. 2017, 101, 99–113. [Google Scholar] [CrossRef]
- Rabau, O.; Navarro-Ramirez, R.; Aziz, M.; Teles, A.; Mengxiao Ge, S.; Quillo-Olvera, J.; Ouellet, J. Lateral Lumbar Interbody Fusion (LLIF): An Update. Glob. Spine J. 2020, 10, 17S–21S. [Google Scholar] [CrossRef]
- Sinkov, V.; Lockey, S.D.; Cunningham, B.W. Single Position Lateral Lumbar Interbody Fusion with Posterior Instrumentation Utilizing Computer Navigation and Robotic Assistance: Retrospective case review and surgical technique considerations. Glob. Spine J. 2022, 12, 75S–81S. [Google Scholar] [CrossRef]
- Walker, C.T.; Godzik, J.; Xu, D.S.; Theodore, N.; Uribe, J.S.; Chang, S.W. Minimally Invasive Single-Position Lateral Interbody Fusion with Robotic Bilateral Percutaneous Pedicle Screw Fixation: 2-Dimensional Operative Video. Oper. Neurosurg. 2019, 16, E121. [Google Scholar] [CrossRef]
- Buckland, A.J.; Proctor, D.; Thomas, J.A.; Protopsaltis, T.S.; Ashayeri, K.; Braly, B.A. Single-Position Prone Lateral Lumbar Interbody Fusion Increases Operative Efficiency and Maintains Safety in Revision Lumbar Spinal Fusion. Spine 2023, 49, E19–E24. [Google Scholar] [CrossRef]
- Khajavi, K.; Menezes, C.M.; Braly, B.A.; Thomas, J.A. Postoperative spinal alignment comparison of lateral versus supine patient position L5-S1 anterior lumbar interbody fusion. Eur. Spine J. 2022, 31, 2248–2254. [Google Scholar] [CrossRef]
- Thomas, J.A.; Menezes, C.; Buckland, A.J.; Khajavi, K.; Ashayeri, K.; Braly, B.A.; Kwon, B.; Cheng, I.; Berjano, P. Single-position circumferential lumbar spinal fusion: An overview of terminology, concepts, rationale and the current evidence base. Eur. Spine J. 2022, 31, 2167–2174. [Google Scholar] [CrossRef]
- Amaral, R.; Moriguchi, R.; Pokorny, G.; Arnoni, D.; Barreira, I.; Marcelino, F.; Pokorny, J.; Pimenta, L. Comparison of segmental lordosis gain of prone transpsoas (PTP) vs. lateral lumbar interbody fusion. Arch. Orthop. Trauma. Surg. 2023, 143, 5485–5790. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, L.; Taylor, W.R.; Stone, L.E.; Wali, A.R.; Santiago-Dieppa, D.R. Prone Transpsoas Technique for Simultaneous Single-Position Access to the Anterior and Posterior Lumbar Spine. Oper. Neurosurg. 2020, 20, E5–E12. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.G.; Pollina, J.; Joseph, S.A., Jr.; Howell, K.M. Effects of Surgical Positioning on L4–L5 Accessibility and Lumbar Lordosis in Lateral Transpsoas Lumbar Interbody Fusion: A Comparison of Prone and Lateral Decubitus in Asymptomatic Adults. World Neurosurg. 2021, 149, e705–e713. [Google Scholar] [CrossRef] [PubMed]
- North, R.Y.; Strong, M.J.; Yee, T.J.; Kashlan, O.N.; Oppenlander, M.E.; Park, P. Navigation and Robotic-Assisted Single-Position Prone Lateral Lumbar Interbody Fusion: Technique, Feasibility, Safety, and Case Series. World Neurosurg. 2021, 152, 221–230.e221. [Google Scholar] [CrossRef] [PubMed]
- Rawicki, N.; Dowdell, J.E.; Sandhu, H.S. Current state of navigation in spine surgery. Ann. Transl. Med. 2021, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Khan, S.N. Does less invasive spine surgery result in increased radiation exposure? A systematic review. Clin. Orthop. Relat. Res. 2014, 472, 1738–1748. [Google Scholar] [CrossRef]
- Soliman, M.A.R.; Ruggiero, N.; Aguirre, A.O.; Kuo, C.C.; Khawar, W.I.; Khan, A.; Jowdy, P.K.; Starling, R.V.; Mullin, J.P.; Pollina, J. Prone Transpsoas Lateral Lumbar Interbody Fusion for Degenerative Lumbar Spine Disease: Case Series with an Operative Video Using Fluoroscopy-Based Instrument Tracking Guidance. Oper. Neurosurg. 2022, 23, 382–388. [Google Scholar] [CrossRef]
- Harimaya, K.; Lenke, L.G.; Mishiro, T.; Bridwell, K.H.; Koester, L.A.; Sides, B.A. Increasing lumbar lordosis of adult spinal deformity patients via intraoperative prone positioning. Spine 2009, 34, 2406–2412. [Google Scholar] [CrossRef]
- Sembrano, J.N.; Yson, S.C.; Horazdovsky, R.D.; Santos, E.R.; Polly, D.W., Jr. Radiographic Comparison of Lateral Lumbar Interbody Fusion Versus Traditional Fusion Approaches: Analysis of Sagittal Contour Change. Int. J. Spine Surg. 2015, 9, 16. [Google Scholar] [CrossRef]
- Issa, T.Z.; Lee, Y.; Lambrechts, M.J.; Tran, K.S.; Trenchfield, D.; Baker, S.; Fras, S.; Yalla, G.R.; Kurd, M.F.; Woods, B.I.; et al. The impact of cage positioning on lumbar lordosis and disc space restoration following minimally invasive lateral lumbar interbody fusion. Neurosurg. Focus. 2023, 54, E7. [Google Scholar] [CrossRef]
- Wang, T.Y.; Mehta, V.A.; Sankey, E.W.; Shaffrey, C.I.; Than, K.D.; Taylor, W.R.; Pollina, J.; Pimenta, L.; Abd-El-Barr, M.M. Single-position prone transpsoas fusion for the treatment of lumbar adjacent segment disease: Early experience of twenty-four cases across three tertiary medical centers. Eur. Spine J. 2022, 31, 2255–2261. [Google Scholar] [CrossRef]
- Mills, E.S.; Treloar, J.; Idowu, O.; Shelby, T.; Alluri, R.K.; Hah, R.J. Single position lumbar fusion: A systematic review and meta-analysis. Spine J. 2022, 22, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.A.R.; Aguirre, A.O.; Ruggiero, N.; Kuo, C.C.; Mariotti, B.L.; Khan, A.; Mullin, J.P.; Pollina, J. Comparison of prone transpsoas lateral lumbar interbody fusion and transforaminal lumbar interbody fusion for degenerative lumbar spine disease: A retrospective radiographic propensity score-matched analysis. Clin. Neurol. Neurosurg. 2022, 213, 107105. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.A.R.; Khan, A.; Pollina, J. Comparison of Prone Transpsoas and Standard Lateral Lumbar Interbody Fusion Surgery for Degenerative Lumbar Spine Disease: A Retrospective Radiographic Propensity Score-Matched Analysis. World Neurosurg. 2022, 157, e11–e21. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, L.; Joseph, S.A., Jr.; Moore, J.A.; Miles, J.; Alvernia, J.E.; Howell, K.M. Risk of Injury to Retroperitoneal Structures in Prone and Lateral Decubitus Transpsoas Approaches to Lumbar Interbody Fusion: A Pilot Cadaveric Anatomical Study. Cureus 2023, 15, e41733. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, A.R.; Kepler, C.K.; Rihn, J.A.; Suzuki, H.; Ratliff, J.K.; Harrop, J.S.; Morrison, W.B.; Limthongkul, W.; Albert, T.J. Anatomical relationships of the anterior blood vessels to the lower lumbar intervertebral discs: Analysis based on magnetic resonance imaging of patients in the prone position. J. Bone Jt. Surg. Am. 2012, 94, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Qian, B.P.; Qiu, Y.; Wang, B.; Yu, Y.; Zhu, Z.Z.; Jiang, J. Position of the aorta relative to the spine in patients with thoracolumbar/lumbar kyphosis secondary to ankylosing spondylitis. Spine 2013, 38, E1235–E1241. [Google Scholar] [CrossRef] [PubMed]
- Alluri, R.; Clark, N.; Sheha, E.; Shafi, K.; Geiselmann, M.; Kim, H.J.; Qureshi, S.; Dowdell, J. Location of the Femoral Nerve in the Lateral Decubitus Versus Prone Position. Glob. Spine J. 2021, 13, 1765–1770. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Clymer, J.W.; Po-Han Chen, B.; Sadeghirad, B.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged operative duration is associated with complications: A systematic review and meta-analysis. J. Surg. Res. 2018, 229, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.D.; Hsu, W.K.; De Oliveira, G.S., Jr.; Saha, S.; Kim, J.Y. Operative duration as an independent risk factor for postoperative complications in single-level lumbar fusion: An analysis of 4588 surgical cases. Spine 2014, 39, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Thirukumaran, C.; Mesfin, A.; Molinari, R.W. Complications and readmission after lumbar spine surgery in elderly patients: An analysis of 2,320 patients. Spine J. 2017, 17, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Hersey, A.E.; Durand, W.M.; Eltorai, A.E.M.; DePasse, J.M.; Daniels, A.H. Longer Operative Time in Elderly Patients Undergoing Posterior Lumbar Fusion Is Independently Associated with Increased Complication Rate. Glob. Spine J. 2019, 9, 179–184. [Google Scholar] [CrossRef]
- Watkins, R.G.; Gupta, A.; Watkins, R.G. Cost-effectiveness of image-guided spine surgery. Open Orthop. J. 2010, 4, 228–233. [Google Scholar] [CrossRef]
- Buckland, A.J.; Ashayeri, K.; Leon, C.; Manning, J.; Eisen, L.; Medley, M.; Protopsaltis, T.S.; Thomas, J.A. Single position circumferential fusion improves operative efficiency, reduces complications and length of stay compared with traditional circumferential fusion. Spine J. 2021, 21, 810–820. [Google Scholar] [CrossRef]
- Hiyama, A.; Katoh, H.; Sakai, D.; Sato, M.; Tanaka, M.; Watanabe, M. Comparison of radiological changes after single- position versus dual- position for lateral interbody fusion and pedicle screw fixation. BMC Musculoskelet. Disord. 2019, 20, 601. [Google Scholar] [CrossRef]
- Hiyama, A.; Sakai, D.; Sato, M.; Watanabe, M. The analysis of percutaneous pedicle screw technique with guide wire-less in lateral decubitus position following extreme lateral interbody fusion. J. Orthop. Surg. Res. 2019, 14, 304. [Google Scholar] [CrossRef] [PubMed]
- Ouchida, J.; Kanemura, T.; Satake, K.; Nakashima, H.; Ishikawa, Y.; Imagama, S. Simultaneous single-position lateral interbody fusion and percutaneous pedicle screw fixation using O-arm-based navigation reduces the occupancy time of the operating room. Eur. Spine J. 2020, 29, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Ziino, C.; Arzeno, A.; Cheng, I. Analysis of single-position for revision surgery using lateral interbody fusion and pedicle screw fixation: Feasibility and perioperative results. J. Spine Surg. 2019, 5, 201–206. [Google Scholar] [CrossRef]
- Ziino, C.; Konopka, J.A.; Ajiboye, R.M.; Ledesma, J.B.; Koltsov, J.C.B.; Cheng, I. Single position versus lateral-then-prone positioning for lateral interbody fusion and pedicle screw fixation. J. Spine Surg. 2018, 4, 717–724. [Google Scholar] [CrossRef]
- Lamartina, C.; Berjano, P. Prone single-position extreme lateral interbody fusion (Pro-XLIF): Preliminary results. Eur. Spine J. 2020, 29, 6–13. [Google Scholar] [CrossRef]
- Patel, A.; Rogers, M.; Michna, R. A retrospective review of single-position prone lateral lumbar interbody fusion cases: Early learning curve and perioperative outcomes. Eur. Spine J. 2023, 32, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Buckland, A.J.; Braly, B.A.; O’Malley, N.A.; Ashayeri, K.; Protopsaltis, T.S.; Kwon, B.; Cheng, I.; Thomas, J.A. Lateral decubitus single position anterior posterior surgery improves operative efficiency, improves perioperative outcomes, and maintains radiological outcomes comparable with traditional anterior posterior fusion at minimum 2-year follow-up. Spine J. 2023, 23, 685–694. [Google Scholar] [CrossRef]
- Ashayeri, K.; Leon, C.; Tigchelaar, S.; Fatemi, P.; Follett, M.; Cheng, I.; Thomas, J.A.; Medley, M.; Braly, B.; Kwon, B.; et al. Single position lateral decubitus anterior lumbar interbody fusion (ALIF) and posterior fusion reduces complications and improves perioperative outcomes compared with traditional anterior-posterior lumbar fusion. Spine J. 2022, 22, 419–428. [Google Scholar] [CrossRef]
- Xu, D.S.; Walker, C.T.; Farber, S.H.; Godzik, J.; Gandhi, S.V.; Koffie, R.M.; Turner, J.D.; Uribe, J.S. Surgical anatomy of minimally invasive lateral approaches to the thoracolumbar junction. J. Neurosurg. Spine 2022, 36, 937–944. [Google Scholar] [CrossRef]
- Farber, S.H.; Valenzuela Cecchi, B.; O’Neill, L.K.; Chapple, K.M.; Zhou, J.J.; Alan, N.; Gooldy, T.C.; DiDomenico, J.D.; Snyder, L.A.; Turner, J.D.; et al. Complications associated with single-position prone lateral lumbar interbody fusion: A systematic review and pooled analysis. J. Neurosurg. Spine 2023, 39, 380–386. [Google Scholar] [CrossRef]
- Soliman, M.A.R.; Diaz-Aguilar, L.; Kuo, C.C.; Aguirre, A.O.; Khan, A.; San Miguel-Ruiz, J.E.; Amaral, R.; Abd-El-Barr, M.M.; Moss, I.L.; Smith, T.; et al. Complications of the Prone Transpsoas Lateral Lumbar Interbody Fusion for Degenerative Lumbar Spine Disease: A Multicenter Study. Neurosurgery 2023, 93, 1106–1111. [Google Scholar] [CrossRef]
- Gammal, I.D.; Spivak, J.M.; Bendo, J.A. Systematic Review of Thigh Symptoms after Lateral Transpsoas Interbody Fusion for Adult Patients with Degenerative Lumbar Spine Disease. Int. J. Spine Surg. 2015, 9, 62. [Google Scholar] [CrossRef]
- Goodnough, L.H.; Koltsov, J.; Wang, T.; Xiong, G.; Nathan, K.; Cheng, I. Decreased estimated blood loss in lateral trans-psoas versus anterior approach to lumbar interbody fusion for degenerative spondylolisthesis. J. Spine Surg. 2019, 5, 185–193. [Google Scholar] [CrossRef]
- Uribe, J.S.; Arredondo, N.; Dakwar, E.; Vale, F.L. Defining the safe working zones using the minimally invasive lateral retroperitoneal transpsoas approach: An anatomical study. J. Neurosurg. Spine 2010, 13, 260–266. [Google Scholar] [CrossRef]
- Bydon, M.; Alvi, M.A.; Goyal, A. Degenerative Lumbar Spondylolisthesis: Definition, Natural History, Conservative Management, and Surgical Treatment. Neurosurg. Clin. N. Am. 2019, 30, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Eismont, F.J.; Norton, R.P.; Hirsch, B.P. Surgical management of lumbar degenerative spondylolisthesis. J. Am. Acad. Orthop. Surg. 2014, 22, 203–213. [Google Scholar] [CrossRef]
- Wong, E.; Altaf, F.; Oh, L.J.; Gray, R.J. Adult Degenerative Lumbar Scoliosis. Orthopedics 2017, 40, e930–e939. [Google Scholar] [CrossRef] [PubMed]
- Stone, L.E.; Wali, A.R.; Santiago-Dieppa, D.R.; Taylor, W.R. Prone-transpsoas as single-position, circumferential access to the lumbar spine: A brief survey of index cases. N. Am. Spine Soc. J. 2021, 6, 100053. [Google Scholar] [CrossRef] [PubMed]
- Virk, S.S.; Niedermeier, S.; Yu, E.; Khan, S.N. Adjacent segment disease. Orthopedics 2014, 37, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Cui, J.; Zhang, J.; He, J.; Tang, J.; Ren, H.; Ye, L.; Liang, D.; Jiang, X. Biomechanical evaluation of strategies for adjacent segment disease after lateral lumbar interbody fusion: Is the extension of pedicle screws necessary? BMC Musculoskelet. Disord. 2020, 21, 117. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.S.; Bridwell, K.H.; Lenke, L.G.; Dalton, J.; Kelly, M.P. Health-related quality of life outcomes in complex adult spinal deformity surgery. J. Neurosurg. Spine 2018, 28, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Scheer, J.K.; Hostin, R.; Robinson, C.; Schwab, F.; Lafage, V.; Burton, D.C.; Hart, R.A.; Kelly, M.P.; Keefe, M.; Polly, D.; et al. Operative Management of Adult Spinal Deformity Results in Significant Increases in QALYs Gained Compared to Nonoperative Management: Analysis of 479 Patients with Minimum 2-Year Follow-up. Spine 2018, 43, 339–347. [Google Scholar] [CrossRef]
- Januszewski, J.; Vivas, A.C.; Uribe, J.S. Limitations and complications of minimally invasive spinal surgery in adult deformity. Ann. Transl. Med. 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Leveque, J.C.; Yanamadala, V.; Buchlak, Q.D.; Sethi, R.K. Correction of severe spinopelvic mismatch: Decreased blood loss with lateral hyperlordotic interbody grafts as compared with pedicle subtraction osteotomy. Neurosurg. Focus. 2017, 43, E15. [Google Scholar] [CrossRef] [PubMed]
- Mundis, G.M., Jr.; Turner, J.D.; Kabirian, N.; Pawelek, J.; Eastlack, R.K.; Uribe, J.; Klineberg, E.; Bess, S.; Ames, C.; Deviren, V.; et al. Anterior Column Realignment has Similar Results to Pedicle Subtraction Osteotomy in Treating Adults with Sagittal Plane Deformity. World Neurosurg. 2017, 105, 249–256. [Google Scholar] [CrossRef]
- Saigal, R.; Mundis, G.M., Jr.; Eastlack, R.; Uribe, J.S.; Phillips, F.M.; Akbarnia, B.A. Anterior Column Realignment (ACR) in Adult Sagittal Deformity Correction: Technique and Review of the Literature. Spine 2016, 41 (Suppl. S8), S66–S73. [Google Scholar] [CrossRef]
- Godzik, J.; Hlubek, R.J.; de Andrada Pereira, B.; Xu, D.S.; Walker, C.T.; Farber, S.H.; Turner, J.D.; Mundis, G.; Uribe, J.S. Combined Lateral Transpsoas Anterior Column Realignment with Pedicle Subtraction Osteotomy to Treat Severe Sagittal Plane Deformity: Cadaveric Feasibility Study and Early Clinical Experience. World Neurosurg. 2019, 121, e589–e595. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.; Ottardi, C.; Lovi, A.; Brayda-Bruno, M.; Villa, T.; Galbusera, F. Anterior support reduces the stresses on the posterior instrumentation after pedicle subtraction osteotomy: A finite-element study. Eur. Spine J. 2017, 26, 450–456. [Google Scholar] [CrossRef]
- Piche, J.D.; Butt, B.; Ahmady, A.; Park, P.; Patel, R.; Nassr, A.; Aleem, I. Lateral lumbar corpectomy: Indications and surgical technique with review of the literature. Semin. Spine Surg. 2022, 34, 100949. [Google Scholar] [CrossRef]
- Gandhi, S.D.; Liu, D.S.; Sheha, E.D.; Colman, M.W. Prone transpsoas lumbar corpectomy: Simultaneous posterior and lateral lumbar access for difficult clinical scenarios. J. Neurosurg. Spine 2021, 35, 284–291. [Google Scholar] [CrossRef]
- Chun, D.S.; Baker, K.C.; Hsu, W.K. Lumbar pseudarthrosis: A review of current diagnosis and treatment. Neurosurg. Focus. 2015, 39, E10. [Google Scholar] [CrossRef] [PubMed]
- Derman, P.B.; Singh, K. Surgical Strategies for the Treatment of Lumbar Pseudarthrosis in Degenerative Spine Surgery: A Literature Review and Case Study. HSS J. 2020, 16, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Slosar, P.J.; Reynolds, J.B.; Schofferman, J.; Goldthwaite, N.; White, A.H.; Keaney, D. Patient satisfaction after circumferential lumbar fusion. Spine 2000, 25, 722–726. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drossopoulos, P.N.; Bardeesi, A.; Wang, T.Y.; Huang, C.-C.; Ononogbu-uche, F.C.; Than, K.D.; Crutcher, C.; Pokorny, G.; Shaffrey, C.I.; Pollina, J.; et al. Advancing Prone-Transpsoas Spine Surgery: A Narrative Review and Evolution of Indications with Representative Cases. J. Clin. Med. 2024, 13, 1112. https://doi.org/10.3390/jcm13041112
Drossopoulos PN, Bardeesi A, Wang TY, Huang C-C, Ononogbu-uche FC, Than KD, Crutcher C, Pokorny G, Shaffrey CI, Pollina J, et al. Advancing Prone-Transpsoas Spine Surgery: A Narrative Review and Evolution of Indications with Representative Cases. Journal of Clinical Medicine. 2024; 13(4):1112. https://doi.org/10.3390/jcm13041112
Chicago/Turabian StyleDrossopoulos, Peter N., Anas Bardeesi, Timothy Y. Wang, Chuan-Ching Huang, Favour C. Ononogbu-uche, Khoi D. Than, Clifford Crutcher, Gabriel Pokorny, Christopher I. Shaffrey, John Pollina, and et al. 2024. "Advancing Prone-Transpsoas Spine Surgery: A Narrative Review and Evolution of Indications with Representative Cases" Journal of Clinical Medicine 13, no. 4: 1112. https://doi.org/10.3390/jcm13041112
APA StyleDrossopoulos, P. N., Bardeesi, A., Wang, T. Y., Huang, C.-C., Ononogbu-uche, F. C., Than, K. D., Crutcher, C., Pokorny, G., Shaffrey, C. I., Pollina, J., Taylor, W., Bhowmick, D. A., Pimenta, L., & Abd-El-Barr, M. M. (2024). Advancing Prone-Transpsoas Spine Surgery: A Narrative Review and Evolution of Indications with Representative Cases. Journal of Clinical Medicine, 13(4), 1112. https://doi.org/10.3390/jcm13041112