Abstract
Background: Congestion is an essential issue in patients with heart failure (HF). Standard treatments do not usually achieve decongestion, and various strategies have been proposed to guide treatment, such as determination of natriuresis. After starting treatment with loop diuretics, we postulate that initial natriuresis might help treatment titration, decongestion, and improve prognosis. Methods: It was a prospective and observational study. Patients admitted with the diagnosis of HF decompensation were eligible. An assessment of congestion was performed during the first 48 h. Results: A total of 113 patients were included. A poor diuretic response was observed in 39.8%. After the first 48 h, patients with a greater diuretic response on admission (NaU > 80 mmol/L) showed fewer pulmonary b lines (12 vs. 15; p = 0.084), a lower IVC diameter (18 mm vs. 22 mm; p = 0.009), and lower IAP figures (11 mmHg vs. 13 mmHg; p = 0.041). Survival analysis tests demonstrated significant differences showing a higher proportion of all-cause mortality (ACM) and HF rehospitalization in the poor-diuretic-response group (log-rank test = 0.020). Conclusions: Up to 40% of the patients presented a poorer diuretic response at baseline, translating into worse outcomes. Patients with an optimal diuretic response showed significantly higher abdominal decongestion at 48 h and a better prognosis regarding ACM and/or HF rehospitalizations.
1. Introduction
Congestion is the main therapeutic target in patients with HF who are admitted with acute symptoms [1]. Endovenous (e.v.) loop diuretics are the gold-standard treatment to improve clinical congestion [2,3]. However, this strategy is inefficient as several studies have shown that around 30–40% of acute HF patients have signs of persistent congestion at discharge, leading to worse outcomes [4,5,6]. Hence, alternative therapeutical strategies have been proposed to improve congestion removal, achieve an efficient diuretic response, and improve outcomes. In this sense, serum biomarkers such as the amino-terminal fragment of pro-brain natriuretic peptide (NT-proBNP) [7], Carbohydrate antigen 125 (CA125) [8], or point-of-care ultrasound (POC) [9] have been proposed as additional tools, besides clinical signs of congestion, to guide e.v. diuretics during acute decompensated heart failure (ADHF), and the results have been promising [8,10].
The analysis of initial natriuresis after the first doses of e.v. furosemide has been demonstrated to have prognostic implications in ADHF [11,12]. Furthermore, some studies suggest that it could help clinicians adjust decongestive treatment early after admission for ADHF. Testani et al. [13,14] demonstrated that urinary sodium concentrations in a random sample after the two hours of e.v. loop diuretic predicts total diuresis [13] during the following six hours, as well as total natriuresis, diuresis, and prognosis [14,15]. Even more, induced natriuresis during the initial 24 h indicates a diuretic response and prognosis that is better than diuresis alone [16]. Natriuresis is becoming central for assessing diuretic response in patients with ADHF, and, along with the glomerular filtration rate (GFR), it allows for stratification of vital prognosis better than these two parameters on their own [17]. However, natriuresis-guided therapy has not yet demonstrated improvement regarding all-cause mortality or first-heart-failure rehospitalizations [18,19]. Therefore, a multimodal approach to evaluating these patients, including other parameters, might improve the assessment and management of decompensation episodes.
We hypothesized that initial natriuresis after starting e.v. furosemide has prognostic implications in ADHF patients due to its link to tissular and intravascular decongestion. The main objectives of this study are (1) to analyze the prognostic impact of baseline natriuresis. (2) To analyze the association of natriuresis with markers of congestion. (3) To analyze the association between natriuresis and abdominal congestion through intraabdominal pressure (IAP).
2. Materials and Methods
Study population: Observational and retrospective analyses were carried out at the Internal Medicine Ward of a tertiary hospital, between 2016 and 2023. Inclusion criteria were (1) Age > 18 age years old. (2) NT-proBNP > 1000 pg/mL. (3) Symptons (dyspnea in NYHA functional class II, III, IV) and/or signs of congestion (edema, ascites, jugular engorgement, lung crackles, or pulmonary congestion signs on chest X-ray) due to HF. (4) Informed consent signed. Exclusion criteria were (1) intensive-care previous admission. (2) Impaired cognitive or functional status. (3) End-stage kidney disease (CrCl < 10 mL/min, dialysis and/or renal transplant) [20]. (4) Advanced Chronic obstructive pulmonary disease (COPD) is defined as FEV1 < 30%. Medical data, including previous antecedents, physical examination, and vital signs, were recorded during the first 48 h of admission.
Multimodal assessment of congestion: Tissular lung congestion was detected through the presence of b-lines [21]. We used a portable ultrasound system (Lumify, Philliphs©) and a sectorial probe for explorations. A 12-zone protocol has been previously validated to evaluate patients with acute respiratory failure performing lung ultrasound [22]. Nevertheless, previous studies have shown that an 8-zone protocol is as accurate as the former option [23]. In our examination, 8 thoracic quadrants ((4 right zones and 4 left zones) were explored by trained staff. If 3 or more B-lines were identified in each field, it was considered a positive result. Total b-lines detected were registered at baseline and after 48 h of admission. Intravascular congestion was quantified by analyzing inferior vena cava (IVC) morphology. The portable ultrasound device Lumify (Phillips©) was again used to measure IVC diameter at baseline and after 48 h of e.v. diuretic treatment. The subcostal view allows the estimation of the IVC diameter, and it should be evaluated proximal to the entrance of hepatic veins into the IVC. The collapsibility of IVC was also estimated with a cut-off of < 50% as pathological [24]. The deadline to perform these explorations (lung and IVC ultrasound) was six hours after the first morning e.v. diuretic dose.
Intraabdominal pressure measurement: Intra-abdominal pressure was measured using the vesical catheterization technique using pre-specified equipment designed for this purpose (Unometer Abdo-pressure©). Urine catheters are commonly used during episodes of AHF and are considered a low-risk procedure. A bladder catheter has to be placed in every case; those patients who had not had a medical indication for the utilization of a catheter before their study inclusion would be offered to use one. This technique consists of placing a small volume of saline solution (25 mL) through a closed system with a water column that registers IAP in real time. This technique has been previously validated in HF patients [25].
Laboratory analysis: A complete blood test analysis was performed on the first morning after admission. The estimated glomerular filtration rate (eGFR) was calculated with the Chronic Kidney Disease Epidemiology Collaboration Creatinine formula (CKD-EPI-creatinine). NT-proBNP and CA125 concentrations were determined with specific laboratory kits (Roche Elecsys® NT-proBNP assay; Roche Elecsys®CA 125 assay). The urinary natriuresis was determined from urinary spot samples collected between two and three hours after the administration of the first bolus of e.v. morning furosemide. The bladder was emptied before the application of furosemide.
Statistical analysis: Continuous variables were expressed as the mean (±standard deviation) or median (Interquartile range) as appropriate. Qualitative variables were expressed as a percentage. Baseline patient characteristics were stratified based on a cut-off point selected from previous studies (NaU ≤ 80 mmol/L vs. NaU > 80 mmol/L) [26] and compared using the Student’s t-test or Mann–Whitney U test for continuous variables and chi-square test for categorical variables. The primary endpoint of the study was the composite of all-cause mortality (ACM) and/or HF readmissions at 90 days. As secondary objectives, ACM at 90 days, HF rehospitalizations after 90 days, and mean length of stay were analyzed separately. Kaplan–Meier survival curves were compared using the log-rank test. The Cox regression model was used to identify potential predictors of the primary endpoint (ACM and/or HF rehospitalizations at 90 days). First, the candidate variables were chosen using a univariate analysis, selecting as possible independent predictors those variables with a p-value < 0.100. The multivariate analysis was carried out in steps, conditionally, and backwards. Continuous variables were transformed with fractional polynomials if needed. The confidence intervals were 95%, establishing statistical significance when p < 0.005. All analyses were carried out using SPSS (Statistical Package for the Social Sciences; version 24) and JAMOVI. The study was carried out in compliance with the recommendations contained in the international declaration of Helsinki. The study was approved by the Aragon HealthResearch Ethics Committee (9 September 2015; Ref. C.P.-C.I. PI15/0227).
This manuscript has been elaborated considering the STROBE checklist guidelines [27].
3. Results
Inclusion and exclusion criteria were applied after selecting eligible patients for the study. Patients who met those criteria were included in the study. A total of 113 patients were recruited (Figure 1).
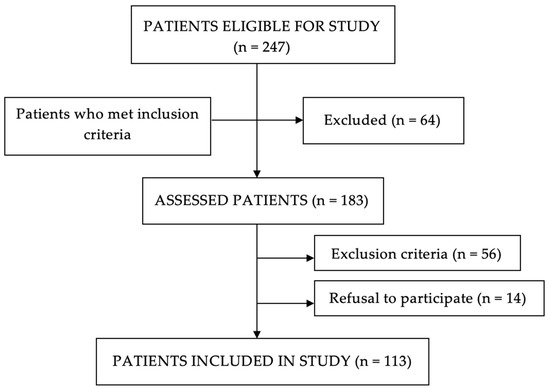
Figure 1.
Flow chart of patient selection.
3.1. Baseline Characteristics
The mean age was 81.7 ± 8.3 years; 54% were women, and 61.1% of the sample had HF with preserved ejection fraction (HFpEF). The most frequent comorbidities were arterial hypertension (81.4%), atrial fibrillation (66.4%), dyslipidemia (54.9%), and diabetes mellitus (36.3%). Impaired renal function (eGFR < 60 mL/min/1.73 m2) was present in 64% of patients, and of these, 49% presented with an eGFR between 59 and 30 mL/min. The percentage of use of angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor antagonists, or neprilysin inhibitors (ACEI/ARB/ARNI) was approximately 60%; this percentage was similar for β-blockers use. On the other hand, 21.2% were being treated with mineralocorticoid receptor blockers (MRB), and approximately 10% had previously received treatment with sodium-glucose cotransporter type 2 inhibitors (SGLT2i). (Table 1).

Table 1.
Baseline characteristics according to sodium urinary concentrations at admission.
Baseline characteristics according to initial spontaneous urine sodium concentrations (NaU) after the first e.v. dose of furosemide are shown in Table 1. A poor diuretic response (NaU ≤ 80 mmol/L) was observed in 39.8% of patients. These patients had higher NT-proBNP concentrations on admission (6227 pg/mL vs. 4113 pg/mL; p = 0.056), lower natremia (138 mmol/L vs. 141 mmol/L; p < 0.001), and lower chloremia (97 mmol/L vs. 100 mmol/L; p = 0.002). (Table 1).
3.2. Multimodal Assessment of Congestion and Intraabdominal Pressure
Intraabdominal pressure was registered in 57 patients. Baseline multimodal assessment of congestion and IAP did not differ between both groups at admission. However, after the first 48 h of admission, patients with a greater diuretic response on admission (NaU > 80 mmol/L) showed a trend of having fewer pulmonary b-lines (12 vs. 15; p = 0.084), a lower IVC diameter (18 mm vs. 22 mm; p = 0.009), and lower IAP values (11 mmHg vs. 13 mmHg; p = 0.041). In addition, urinary sodium concentrations in patients with the greatest diuretic response continued to be significantly higher after the first 48 h (84 mmol/L vs. 75 mmol/L; p = 0.042). (Table 2).

Table 2.
Markers of congestion and intraabdominal pressure level, according to initial urine sodium concentrations.
3.3. Outcomes
During the follow-up period (90 days), a total of 19 patients (16.8%) died, 22 patients (18.6%) were readmitted for HF, and a total of 35 (31%) achieved the primary endpoint (ACM and/or HF rehospitalization at 90 days). Kaplan–Meier curves and a log-rank test showed significant differences between groups. Patients with a poor diuretic response (NaU ≤ 80 mmol/L) experienced a higher proportion of events (Log-rank test = 0.020) (Figure 2).
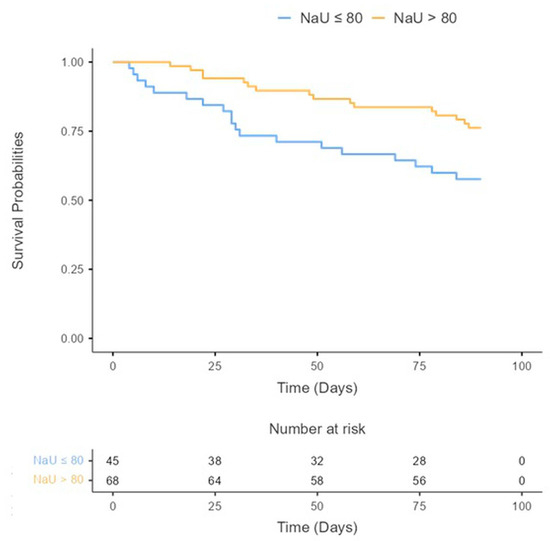
Figure 2.
All-cause mortality and/or HF rehospitalization during 90 days after discharge according to baseline urinary sodium concentrations.
Univariate analysis identified previous oral furosemide doses (HR 2.85 [1.01–8.07]; p = 0.049), eGFR at admission (HR 0.39 [0.18–0.86]; p = 0.020), admission urinary sodium concentrations > 80 mmol/L (HR 0.46 [0.24–0.90]; p = 0.023), and admission NT-proBNP concentrations (HR 1.43 [1.07–1.90]; p = 0.016) as potential predictors for the primary outcomes. After adjusting for seven variables, the multivariate Cox regression analysis identified urinary sodium concentration > 80 mmol/L (HR 0.50 [0.25–1.02]; p = 0.056) and initial CA125 concentrations (HR 1.44 [0.98–2.10]; p = 0.073) as independent risk predictors for the primary endpoint. The area under the curve for that model was 0.759 (0.654–0.862) (p < 0.001). (Table 3 and Figure 3).

Table 3.
Univariable and multivariate Cox regression analysis for the primary endpoint (all-cause mortality and/or HF rehospitalizations at 90 days).
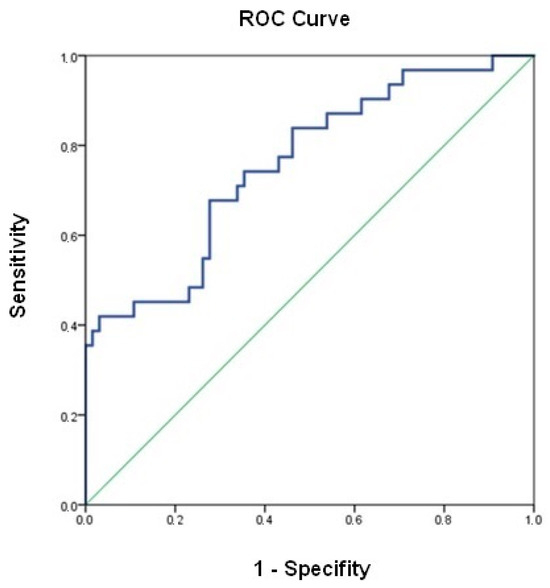
Figure 3.
ROC curve for the multivariable Cox regression model.
4. Discussion
The main findings of this study were that an optimal diuretic response after initial e.v. loop diuretics (NaU > 80 mmol/L) was associated with effective decongestion with less pulmonary and intravascular congestion and a significant fall of IAP. This behavior of IAP has not been described previously. Notably, an insufficient diuretic response (NaU ≤ 80 mmol/L) was frequent (40% of the cohort), and we independently predicted ACM and/or HF rehospitalizations at 90 days.
4.1. Natriuresis and Decongestion in Acute Heart Failure
Congestion is the primary therapeutic target in ADHF patients [1]. Currently, HF guidelines recommend the use of e.v. loop diuretics for symptomatic relief in ADHF, adjusting the initial doses based on previous furosemide oral intake [1]. Despite the awareness of the decongestive efficacy of loop diuretics, the reality is that up to one-third of the patients discharged after an episode of ADHF still have subtle signs and/or symptoms of congestion, so-called “persistent congestion”, leading to worse outcomes [4]. Consequently, during the last decade, efforts have been focused on the search for efficient treatment strategies to improve the schedule of diuretics dosage aimed to remove residual congestion at discharge. Several strategies adding biomarker-guided therapy have been used to address this issue. In the CHANCE-HF study [8], CA125 concentrations on admission were used to adjust the initial e.v. loop diuretic doses. In the LUS-HF trial [28], tailored lung-ultrasound-guided diuretic treatment reduced the number of decompensations and improved functional status in outpatient HF patients. More recently, the analysis of urinary metrics, especially natriuresis, has shown promising results in guiding current strategies for the adjustment of diuretics dosage. They are based on the natriuretic response to the initial doses of loop diuretics administered early after admission.
In our study, up to 40% of patients had a poor diuretic response (NaU ≤ 80 mmol/L) associated with impaired prognosis during the first 90 days after discharge. These results are similar to that of the study by Verbrugge et al. [20], which was a posthoc analysis of the cohort of ADVOR clinical trial [29] (Acetazolamide in Decompensated Heart Failure with volume overload). The authors found that patients with an insufficient diuretic response had a worse prognosis due to an increase in death or readmission for HF.
Our results support the additional value of multimodal assessment of congestion performed early after admission. To date, most of the published studies rely on the assessment of the natriuresis from a spontaneous urine sample and congestion assessed either through a physical examination (congestion scores) or serum biomarkers (NT-proBNP or CA125) [11,12,30].
In our cohort, the assessment of congestion was addressed with a multimodal approach that included ultrasounds, serum biomarkers, the natriuretic response, and, for the first time, the measurement of IAP. All these parameters were evaluated at baseline and 48 h after the initial doses of loop diuretics had been administered. We did not find differences in congestion at baseline, but those patients with a good natriuretic response (>80 mEq/L) showed a clear trend of decongestion in terms of in ultrasound and through biomarkers, and they had a significant fall in IAP and fewer outcomes than those with natriuresis below that level. In addition, urine Na concentration continued to be higher in patients with an initial good response.
The change in IAP early after diuretic administration deserves a comment. Abdominal congestion [31,32] has been described as an important pathophysiological mechanism for the development of congestive nephropathy. So far, our study is the first to show that an optimal diuretic response (NaU > 80 mmol/L) is associated with a significant reduction in IAP during the first 48 h of admission. Unfortunately, this measurement was available in only 57 patients, which limits the generalization and interpretation of our results. Even though it seems plausible that natriuresis and reduction in IAP, induced by the initial doses of loop diuretics, allow clinicians to identify the subgroup of patients more prone to residual congestion, and whether the intensification of diuretics in this group is beneficial should be tested in adequately designed studies.
4.2. Clinical Implications
Our results agree with other retrospective analyses on urinary metrics and diuretic response [33,34]. Together, they suggest that these parameters will play a much more relevant role in future HF guidelines. Indeed, two ongoing clinical trials are testing such a strategy, the ENACT-HF [35] clinical trial and the PUSH-HF [18] clinical trial, whose final results are expected in the upcoming months.
What is clear is that achieving an optimal diuretic response as quickly as possible after admission is crucial to improving the prognosis of our patients with decompensated HF. In this context, the combined diuretic strategy will probably become more prominent, as the ADVOR [29] (e.v. furosemide plus acetazolamide) and CLOROTIC [36] (furosemide plus hydrochlorothiazide) trials have shown.
5. Conclusions
Poor diuretic response is common in patients admitted for ADHF and is associated with a higher degree of lung and intravascular residual congestion and a higher risk of mortality after discharge. Patients with an optimal diuretic response achieve decongestion more easily, as is shown by the reduction in the IVC diameter and IAP 48 h after admission. Natriuresis 2 h after initiation of e.v. loop diuretics and the change in IAP 48 h after admission seem to be feasible and valuable tools to identify diuretic response and residual congestion in ADHF. Both tests should probably be implemented more frequently in clinical settings.
6. Limitations
The study has several limitations. First, it is a retrospective study with a small sample size that limits statistical power. Second, natriuresis was measured the morning following admission to the Internal Medicine ward; thus, most patients had received some dose of e.v. furosemide in the Emergency department, which could have reduced the power of the study. Third, the analysis of IAP is limited to only 57 patients due to the difficulty of obtaining it in the currently overloaded clinical departments. Also, patients with contraindications for bladder catheterization were not assessed for this parameter. Despite this, the study should try to overcome such barriers in the future.
Author Contributions
Conceptualization, J.I.P.-C. and J.R.-G.; methodology, M.S.-M., I.G.-L. and J.R.-G.; investigation, S.C.-A., A.C.-S.d.S., C.J.-L., V.G.-H., R.T.-P., M.A.J.-A. and I.G.-L.; Visualization S.C.-A.; Writing S.C.-A. and J.R.-G.; supervision F.R.-L., B.A.-A., J.I.P.-C. and J.R.-G.; Formal Analysis J.R.-G.; Data curation S.C.-A., A.C.-S.d.S. and C.J.-L. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the Spanish Society of Internal Medicine through the “Ayudas a la investigación FEMI para la atención de pacientes crónicos 2015” grant.
Institutional Review Board Statement
The study was approved by the Aragon Health Research Ethics Committee (9 September 2015; Ref. C.P.-C.I. PI15/0227).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants, but they are available from JRG (corresponding author).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Boorsma, E.M.; Ter Maaten, J.M.; Damman, K.; Dinh, W.; Gustafsson, F.; Goldsmith, S.; Burkhoff, D.; Zannad, F.; Udelson, J.E.; Voors, A.A. Congestion in heart failure: A contemporary look at physiology, diagnosis and treatment. Nat. Rev. Cardiol. 2020, 17, 641–655. [Google Scholar] [CrossRef]
- Costanzo, M.R.; Jessup, M. Treatment of congestion in heart failure with diuretics and extracorporeal therapies: Effects on symptoms, renal function, and prognosis. Heart Fail. Rev. 2012, 17, 313–324. [Google Scholar] [CrossRef]
- Rubio-Gracia, J.; Demissei, B.G.; Ter Maaten, J.M.; Cleland, J.G.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int. J. Cardiol. 2018, 258, 185–191. [Google Scholar] [CrossRef]
- Lala, A.; McNulty, S.E.; Mentz, R.J.; Dunlay, S.M.; Vader, J.M.; AbouEzzeddine, O.F.; DeVore, A.D.; Khazanie, P.; Redfield, M.M.; Goldsmith, S.R.; et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure insights from diuretic optimization strategy evaluation in acute decompensated heart failure (DOSE-AHF) and cardiorenal rescue study in acute decompensated heart failure (CARESS-HF). Circ. Heart Fail. 2015, 8, 741–748. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Pang, P.S.; Khan, S.; Konstam, M.A.; Fonarow, G.C.; Traver, B.; Maggioni, A.P.; Cook, T.; Swedberg, K.; Burnett, J.C., Jr. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur. Heart J. 2013, 34, 835–843. [Google Scholar] [CrossRef]
- Januzzi, J.L.; van Kimmenade, R.; Lainchbury, J.; Bayes-Genis, A.; Ordonez-Llanos, J.; Santalo-Bel, M.; Pinto, Y.M.; Richards, M. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: An international pooled analysis of 1256 patients: The International Collaborative of NT-proBNP Study. Eur. Heart J. 2006, 27, 330–337. [Google Scholar] [CrossRef]
- Núñez, J.; Llàcer, P.; Bertomeu-González, V.; Bosch, M.J.; Merlos, P.; García-Blas, S.; Montagud, V.; Bodí, V.; Bertomeu-Martínez, V.; Pedrosa, V.; et al. Carbohydrate Antigen-125-Guided Therapy in Acute Heart Failure: CHANCE-HF: A Randomized Study. JACC Heart Fail. 2016, 4, 833–843. [Google Scholar] [CrossRef]
- Spevack, R.; Al Shukairi, M.; Jayaraman, D.; Dankoff, J.; Rudski, L.; Lipes, J. Serial lung and IVC ultrasound in the assessment of congestive heart failure. Crit Ultrasound J. 2017, 9, 7. [Google Scholar] [CrossRef]
- Li, Y.; Ai, H.; Ma, N.; Li, P.; Ren, J. Lung ultrasound-guided treatment for heart failure: An updated meta-analysis and trial sequential analysis. Front. Cardiovasc. Med. 2022, 9, 943633. [Google Scholar] [CrossRef]
- Brinkley, D.M., Jr.; Burpee, L.J.; Chaudhry, S.P.; Smallwood, J.A.; Lindenfeld, J.; Lakdawala, N.K.; Desai, A.S.; Stevenson, L.W. Spot Urine Sodium as Triage for Effective Diuretic Infusion in an Ambulatory Heart Failure Unit. J. Card. Fail. 2018, 24, 349–354. [Google Scholar] [CrossRef]
- Luk, A.; Groarke, J.D.; Desai, A.S.; Mahmood, S.S.; Gopal, D.M.; Joyce, E.; Shah, S.P.; Lindenfeld, J.; Stevenson, L.; Lakdawala, N.K. First spot urine sodium after initial diuretic identifies patients at high risk for adverse outcome after heart failure hospitalization. Am. Heart J. 2018, 203, 95–100. [Google Scholar] [CrossRef]
- Testani, J.M.; Brisco, M.A.; Turner, J.M.; Spatz, E.S.; Bellumkonda, L.; Parikh, C.R.; Tang, W.H. Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ. Heart Fail. 2014, 7, 261–270. [Google Scholar] [CrossRef]
- Rao, V.S.; Ivey-Miranda, J.B.; Cox, Z.L.; Riello, R.; Griffin, M.; Fleming, J.; Soucier, R.; Sangkachand, P.; O’Brien, M.; LoRusso, F.; et al. Natriuretic Equation to Predict Loop Diuretic Response in Patients With Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 695–708. [Google Scholar] [CrossRef]
- Damman, K.; Ter Maaten, J.M.; Coster, J.E.; Krikken, J.A.; van Deursen, V.M.; Krijnen, H.K.; Hofman, M.; Nieuwland, W.; van Veldhuisen, D.J.; Voors, A.A.; et al. Clinical importance of urinary sodium excretion in acute heart failure. Eur. J. Heart Fail. 2020, 22, 1438–1447. [Google Scholar] [CrossRef]
- Hodson, D.Z.; Griffin, M.; Mahoney, D.; Raghavendra, P.; Ahmad, T.; Turner, J.; Wilson, F.P.; Tang, W.H.W.; Rao, V.S.; Collins, S.P.; et al. Natriuretic Response Is Highly Variable and Associated With 6-Month Survival: Insights From the ROSE-AHF Trial. JACC Heart Fail. 2019, 7, 383–391. [Google Scholar] [CrossRef]
- Biegus, J.; Zymliński, R.; Testani, J.; Marciniak, D.; Zdanowicz, A.; Jankowska, E.A.; Banasiak, W.; Ponikowski, P. Renal profiling based on estimated glomerular filtration rate and spot urine sodium identifies high-risk acute heart failure patients. Eur. J. Heart Fail. 2021, 23, 729–739. [Google Scholar] [CrossRef]
- Ter Maaten, J.M.; Beldhuis, I.E.; van der Meer, P.; Krikken, J.A.; Coster, J.E.; Nieuwland, W.; van Veldhuisen, D.J.; Voors, A.A.; Damman, K. Natriuresis-guided therapy in acute heart failure: Rationale and design of the Pragmatic Urinary Sodium-based treatment algorithm in Acute Heart Failure (PUSH-AHF) trial. Eur. J. Heart Fail. 2022, 24, 385–392. [Google Scholar] [CrossRef]
- Ter Maaten, J.M.; Beldhuis, I.E.; van der Meer, P.; Krikken, J.A.; Postmus, D.; Coster, J.E.; Nieuwland, W.; van Veldhuisen, D.J.; Voors, A.A.; Damman, K. Natriuresis-guided diuretic therapy in acute heart failure: A pragmatic randomized trial. Nat. Med. 2023, 29, 2625–2632. [Google Scholar] [CrossRef]
- Stevens, P.E.; Levin, A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef]
- Picano, E.; Scali, M.C.; Ciampi, Q.; Lichtenstein, D. Lung Ultrasound for the Cardiologist. JACC Cardiovasc. Imaging 2018, 11, 1692–1705. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Mezière, G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest J. 2008, 134, 117–125. [Google Scholar] [CrossRef]
- Levy Adatto, N.; Preisler, Y.; Shetrit, A.; Shepshelovich, D.; Hershkoviz, R.; Isakov, O. Rapid 8-Zone Lung Ultrasound Protocol is Comparable to a Full 12-Zone Protocol for Outcome Prediction in Hospitalized COVID-19 Patients. J. Ultrasound Med. 2022, 41, 1677–1687. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Zymliński, R.; Biegus, J.; Sokolski, M.; Jankowska, E.A.; Banasiak, W.; Ponikowski, P. Validation of transurethral intra abdominal pressure measurement in acute heart failure. Pol. Arch. Intern. Med. 2018, 128, 403–405. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Martens, P.; Dauw, J.; Nijst, P.; Meekers, E.; Augusto, S.N., Jr.; Ter Maaten, J.M.; Damman, K.; Filippatos, G.; Lassus, J.; et al. Natriuretic Response to Acetazolamide in Patients With Acute Heart Failure and Volume Overload. J. Am. Coll. Cardiol. 2023, 81, 2013–2024. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13 (Suppl. 1), S31–S34. [Google Scholar] [CrossRef]
- Rivas-Lasarte, M.; Álvarez-García, J.; Fernández-Martínez, J.; Maestro, A.; López-López, L.; Solé-González, E.; Pirla, M.J.; Mesado, N.; Mirabet, S.; Fluvià, P. Lung ultrasound-guided treatment in ambulatory patients with heart failure: A randomized controlled clinical trial (LUS-HF study). Eur. J. Heart Fail. 2019, 21, 1605–1613. [Google Scholar] [CrossRef]
- Mullens, W.; Dauw, J.; Martens, P.; Verbrugge, F.H.; Nijst, P.; Meekers, E.; Tartaglia, K.; Chenot, F.; Moubayed, S.; Dierckx, R.; et al. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. N. Engl. J. Med. 2022, 387, 1185–1195. [Google Scholar] [CrossRef]
- Biegus, J.; Zymliński, R.; Sokolski, M.; Todd, J.; Cotter, G.; Metra, M.; Jankowska, E.A.; Banasiak, W.; Ponikowski, P. Serial assessment of spot urine sodium predicts effectiveness of decongestion and outcome in patients with acute heart failure. Eur. J. Heart Fail. 2019, 21, 624–633. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Dupont, M.; Steels, P.; Grieten, L.; Malbrain, M.; Tang, W.H.; Mullens, W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J. Am. Coll. Cardiol. 2013, 62, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Abrahams, Z.; Skouri, H.N.; Francis, G.S.; Taylor, D.O.; Starling, R.C.; Paganini, E.; Tang, W.H. Elevated Intra-Abdominal Pressure in Acute Decompensated Heart Failure. A Potential Contributor to Worsening Renal Function? J. Am. Coll. Cardiol. 2008, 51, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Martens, P.; Ameloot, K.; Haemels, V.; Penders, J.; Dupont, M.; Tang, W.H.W.; Droogné, W.; Mullens, W. Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. Eur. J. Heart Fail. 2019, 21, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- García-Magallón, B.; Cobo-Marcos, M.; Martiarena, A.D.; Hernández, E.M.; Martín Jiménez, M.L.; García, A.M.; De Castro Campos, D.; Martín, P.V.; Terciado, F.H.; González, R.G.; et al. Role of Early Assessment of Diuresis and Natriuresis in Detecting In-Hospital Diuretic Resistance in Acute Heart Failure. Front. Physiol. 2022, 13, 887734. [Google Scholar] [CrossRef]
- Dauw, J.; Lelonek, M.; Zegri-Reiriz, I.; Paredes-Paucar, C.P.; Zara, C.; George, V.; Cobo-Marcos, M.; Knappe, D.; Shchekochikhin, D.; Lekhakul, A.; et al. Rationale and Design of the Efficacy of a Standardized Diuretic Protocol in Acute Heart Failure Study. ESC Heart Fail. 2021, 8, 4685–4692. [Google Scholar] [CrossRef]
- Trullàs, J.C.; Morales-Rull, J.L.; Casado, J.; Carrera-Izquierdo, M.; Sánchez-Marteles, M.; Conde-Martel, A.; Dávila-Ramos, M.F.; Llácer, P.; Salamanca-Bautista, P.; Pérez-Silvestre, J.; et al. Combining loop with thiazide diuretics for decompensated heart failure: The CLOROTIC trial. Eur. Heart J. 2023, 44, 411–421. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).