Cephalic Vein Cutdown Is Superior to Subclavian Puncture as Venous Access for Patients with Cardiac Implantable Devices after Long-Term Follow-Up
Abstract
1. Introduction
2. Materials and Methods
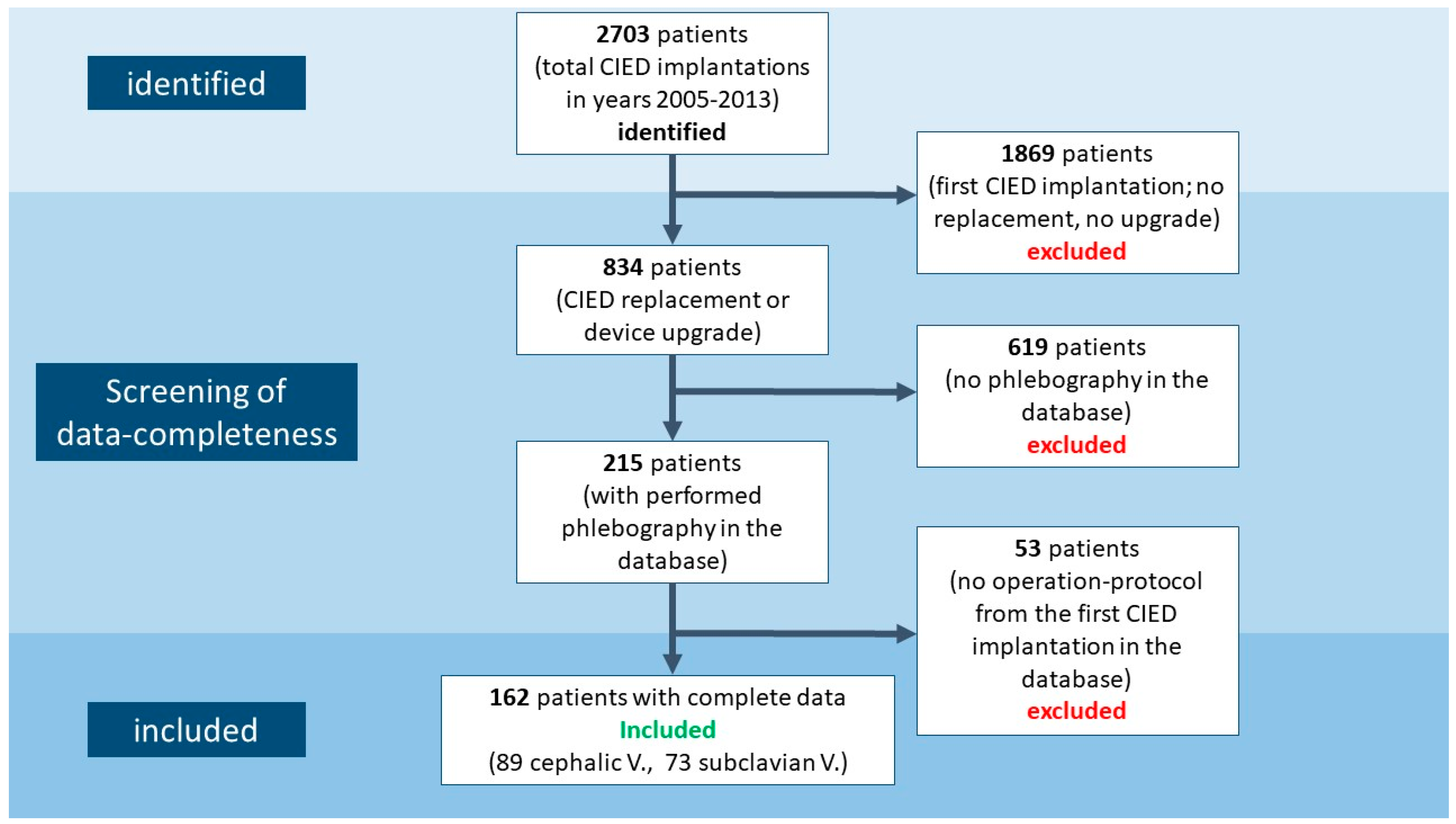
2.1. Study Population
2.2. Phlebography
2.3. Primary Endpoint and Sample Size Calculation
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parsonnet, V.; Roelke, M. The cephalic vein cutdown versus subclavian puncture for pacemaker/ICD lead implantation. Pacing Clin. Electrophysiol. PACE 1999, 22, 695–697. [Google Scholar] [CrossRef]
- Magney, J.E.; Flynn, D.M.; Parsons, J.A.; Staplin, D.H.; Chin-Purcell, M.V.; Milstein, S.; Hunter, D.W. Anatomical mechanisms explaining damage to pacemaker leads, defibrillator leads, and failure of central venous catheters adjacent to the sternoclavicular joint. Pacing Clin. Electrophysiol. PACE 1993, 16, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.Y.; Kwong, N.P.; Cheong, A.P. Venous access and long-term pacemaker lead failure: Comparing contrast-guided axillary vein puncture with subclavian puncture and cephalic cutdown. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2017, 19, 1193–1197. [Google Scholar] [CrossRef]
- Markewitz, A. [Annual Report 2017 of the German Pacemaker and Defibrillator-Register. Part 1: Cardiac Pacemaker: Working Group on Pacemaker and Defibrillators at the IQTIG-Institute for Quality Assurance and Transparency in Healthcare]. Herzschrittmachertherapie Elektrophysiologie 2019, 30, 377–388. [Google Scholar] [CrossRef]
- Markewitz, A. [Annual report 2017 of the German pacemaker- and defibrillator register—Part 2: Implantable cardioverter defibrillators (ICD): Working group on Cardiac pacemaker and implantable cardioverter-defibrillators at the IQTIG—Institute of Quality Assurance and Transparency in Healthcare]. Herzschrittmachertherapie Elektrophysiologie 2019, 30, 389–403. [Google Scholar] [CrossRef]
- Kirkfeldt, R.E.; Johansen, J.B.; Nohr, E.A.; Moller, M.; Arnsbo, P.; Nielsen, J.C. Pneumothorax in cardiac pacing: A population-based cohort study of 28,860 Danish patients. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2012, 14, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhou, Y.F.; Yang, P.; Gao, Y.S.; Zhao, G.R.; Ren, S.Y.; Li, X.L. Optimized Axillary Vein Technique versus Subclavian Vein Technique in Cardiovascular Implantable Electronic Device Implantation: A Randomized Controlled Study. Chin. Med. J. 2016, 129, 2647–2651. [Google Scholar] [CrossRef] [PubMed]
- Liccardo, M.; Nocerino, P.; Gaia, S.; Ciardiello, C. Efficacy of ultrasound-guided axillary/subclavian venous approaches for pacemaker and defibrillator lead implantation: A randomized study. J. Interv. Card. Electrophysiol. 2018, 51, 153–160. [Google Scholar] [CrossRef]
- Pokorney, S.D.; Mi, X.; Lewis, R.K.; Greiner, M.; Epstein, L.M.; Carrillo, R.G.; Zeitler, E.P.; Al-Khatib, S.M.; Hegland, D.D.; Piccini, J.P. Outcomes Associated With Extraction Versus Capping and Abandoning Pacing and Defibrillator Leads. Circulation 2017, 136, 1387–1395. [Google Scholar] [CrossRef]
- Zucchelli, G.; Di Cori, A.; Segreti, L.; Laroche, C.; Blomstrom-Lundqvist, C.; Kutarski, A.; Regoli, F.; Butter, C.; Defaye, P.; Pasquié, J.L.; et al. Major cardiac and vascular complications after transvenous lead extraction: Acute outcome and predictive factors from the ESC-EHRA ELECTRa (European Lead Extraction ConTRolled) registry. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2019, 21, 771–780. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Version 3.4.0; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 19 December 2020).
- Raatikainen, M.J.; Arnar, D.O.; Zeppenfeld, K.; Merino, J.L.; Levya, F.; Hindriks, G.; Kuck, K.H. Statistics on the use of cardiac electronic devices and electrophysiological procedures in the European Society of Cardiology countries: 2014 report from the European Heart Rhythm Association. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2015, 17 (Suppl. 1), i1–i75. [Google Scholar] [CrossRef]
- Erkapic, D.; Duray, G.Z.; Bauernfeind, T.; De Rosa, S.; Hohnloser, S.H. Insulation defects of thin high-voltage ICD leads: An underestimated problem? J. Cardiovasc. Electrophysiol. 2011, 22, 1018–1022. [Google Scholar] [CrossRef]
- Birnie, D.H.; Parkash, R.; Exner, D.V.; Essebag, V.; Healey, J.S.; Verma, A.; Coutu, B.; Kus, T.; Mangat, I.; Ayala-Paredes, F.; et al. Clinical predictors of Fidelis lead failure: Report from the Canadian Heart Rhythm Society Device Committee. Circulation 2012, 125, 1217–1225. [Google Scholar] [CrossRef]
- Benz, A.P.; Vamos, M.; Erath, J.W.; Hohnloser, S.H. Cephalic vs. subclavian lead implantation in cardiac implantable electronic devices: A systematic review and meta-analysis. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2019, 21, 121–129. [Google Scholar] [CrossRef]
- Abu-El-Haija, B.; Bhave, P.D.; Campbell, D.N.; Mazur, A.; Hodgson-Zingman, D.M.; Cotarlan, V.; Giudici, M.C. Venous Stenosis After Transvenous Lead Placement: A Study of Outcomes and Risk Factors in 212 Consecutive Patients. J. Am. Heart Assoc. 2015, 4, e001878. [Google Scholar] [CrossRef] [PubMed]
- Gallik, D.M.; Ben-Zur, U.M.; Gross, J.N.; Furman, S. Lead fracture in cephalic versus subclavian approach with transvenous implantable cardioverter defibrillator systems. Pacing Clin. Electrophysiol. PACE 1996, 19, 1089–1094. [Google Scholar] [CrossRef]
- Jacobs, D.M.; Fink, A.S.; Miller, R.P.; Anderson, W.R.; McVenes, R.D.; Lessar, J.F.; Cobian, K.E.; Staffanson, D.B.; Upton, J.E.; Bubrick, M.P. Anatomical and morphological evaluation of pacemaker lead compression. Pacing Clin. Electrophysiol. PACE 1993, 16, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Magney, J.E.; Parsons, J.A.; Flynn, D.M.; Hunter, D.W. Pacemaker and defibrillator lead entrapment: Case studies. Pacing Clin. Electrophysiol. PACE 1995, 18, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Roelke, M.; O’Nunain, S.S.; Osswald, S.; Garan, H.; Harthorne, J.W.; Ruskin, J.N. Subclavian crush syndrome complicating transvenous cardioverter defibrillator systems. Pacing Clin. Electrophysiol. PACE 1995, 18, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, E.P.; Wang, Y.; Dharmarajan, K.; Anstrom, K.J.; Peterson, E.D.; Daubert, J.P.; Curtis, J.P.; Al-Khatib, S.M. Outcomes 1 Year After Implantable Cardioverter-Defibrillator Lead Abandonment Versus Explantation for Unused or Malfunctioning Leads: A Report from the National Cardiovascular Data Registry. Circ. Arrhythmia Electrophysiol. 2016, 9, e003953. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.; Jorgensen, O.D.; Nielsen, J.C.; Thogersen, A.M.; Philbert, B.T.; Johansen, J.B. Incidence of device-related infection in 97,750 patients: Clinical data from the complete Danish device-cohort (1982-2018). Eur. Heart J. 2019, 40, 1862–1869. [Google Scholar] [CrossRef]
- Knops, R.E.; Reddy, V.Y.; Ip, J.E.; Doshi, R.; Exner, D.V.; Defaye, P.; Canby, R.; Bongiorni, M.G.; Shoda, M.; Hindricks, G.; et al. A Dual-Chamber Leadless Pacemaker. N. Engl. J. Med. 2023, 388, 2360–2370. [Google Scholar] [CrossRef]
- O’Connor, M.; Barbero, U.; Kramer, D.B.; Lee, A.; Hua, A.; Ismail, T.; McCarthy, K.P.; Niederer, S.; Rinaldi, C.A.; Markides, V.; et al. Anatomic, histologic, and mechanical features of the right atrium: Implications for leadless atrial pacemaker implantation. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2023, 25, euad235. [Google Scholar] [CrossRef] [PubMed]
- Weipert, K.F.; Kostic, S.; Gökyildirim, T.; Johnson, V.; Chasan, R.; Gemein, C.; Rosenbauer, J.; Erkapic, D.; Schmitt, J. Safety and Performance of the Subcutaneous Implantable Cardioverter Defibrillator Detection Algorithm INSIGHT(TM) in Pacemaker Patients. J. Clin. Med. 2023, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Abaci, G.; Johnson, V.; Erkapic, D.; Gemein, C.; Chasan, R.; Weipert, K.; Hamm, C.W.; Klein, H.U. Safety of the Wearable Cardioverter Defibrillator (WCD) in Patients with Implanted Pacemakers. Pacing Clin. Electrophysiol. PACE 2017, 40, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Iliodromitis, K.; Deftereos, S.G.; Bogossian, H. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization: What is the correct level of evidence for the superiority of cephalic vein cutdown? C, B or maybe A? Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2022, 24, 697. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.; Nedios, S.; Karosiene, Z.; Scholten, M.; Lemke, B.; Tulka, S.; Knippschild, S.; Macher-Heidrich, S.; Adomeit, H.J.; Zarse, M.; et al. Perioperative complications after pacemaker implantation: Higher complication rates with subclavian vein puncture than with cephalic vein cut-down. J. Interv. Card. Electrophysiol. 2022, 66, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Ussen, B.; Dhillon, P.S.; Anderson, L.; Beeton, I.; Hickman, M.; Gallagher, M.M. Safety and feasibility of cephalic venous access for cardiac resynchronization device implantation. Pacing Clin. Electrophysiol. PACE 2011, 34, 365–369. [Google Scholar] [CrossRef]
- Beig, J.R.; Ganai, B.A.; Alai, M.S.; Lone, A.A.; Hafeez, I.; Dar, M.I.; Tramboo, N.A.; Rather, H.A. Contrast venography vs. microwire assisted axillary venipuncture for cardiovascular implantable electronic device implantation. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2018, 20, 1318–1323. [Google Scholar] [CrossRef]
- Yang, F.; Kulbak, G. A new trick to a routine procedure: Taking the fear out of the axillary vein stick using the 35° caudal view. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2015, 17, 1157–1160. [Google Scholar] [CrossRef]
- Esmaiel, A.; Hassan, J.; Blenkhorn, F.; Mardigyan, V. The Use of Ultrasound to Improve Axillary Vein Access and Minimize Complications during Pacemaker Implantation. Pacing Clin. Electrophysiol. PACE 2016, 39, 478–482. [Google Scholar] [CrossRef] [PubMed]
| Cephalic Vein | Subclavian Vein | |
|---|---|---|
| n = 89 | n = 73 | |
| Basic characteristics | ||
| female | 16 (18%) | 26 (36%) |
| age | 73 ± 7 | 70 ± 7 |
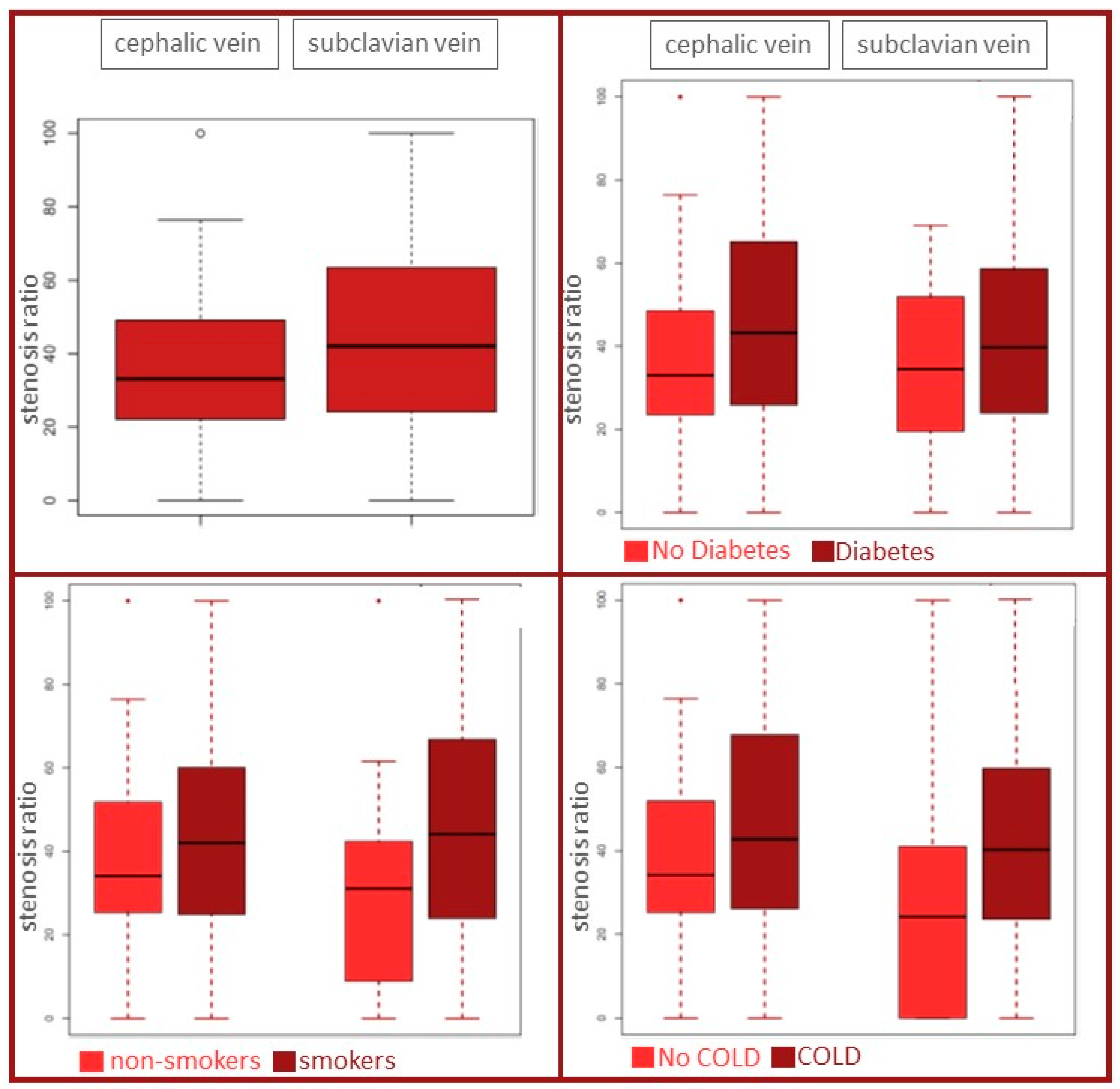
| smoker | 23 (26%) | 18 (25%) |
| adipositas | 40 (45%) | 34 (47%) |
| Diabetes | 31 (35%) | 29 (40%) |
| hypertension | 81 (91%) | 66 (90%) |
| HLP | 64 (72%) | 48 (66%) |
| CAD | 52 (58%) | 45 (62%) |
| renal failure | 29 (33%) | 26 (36%) |
| COLD | 18 (20%) | 24 (33%) |
| ECG | ||
| SR | 64 (72%) | 49 (67%) |
| AF | 25 (28%) | 24 (33%) |
| AVB | 31 (26%) | 21 (29%) |
| Medication | ||
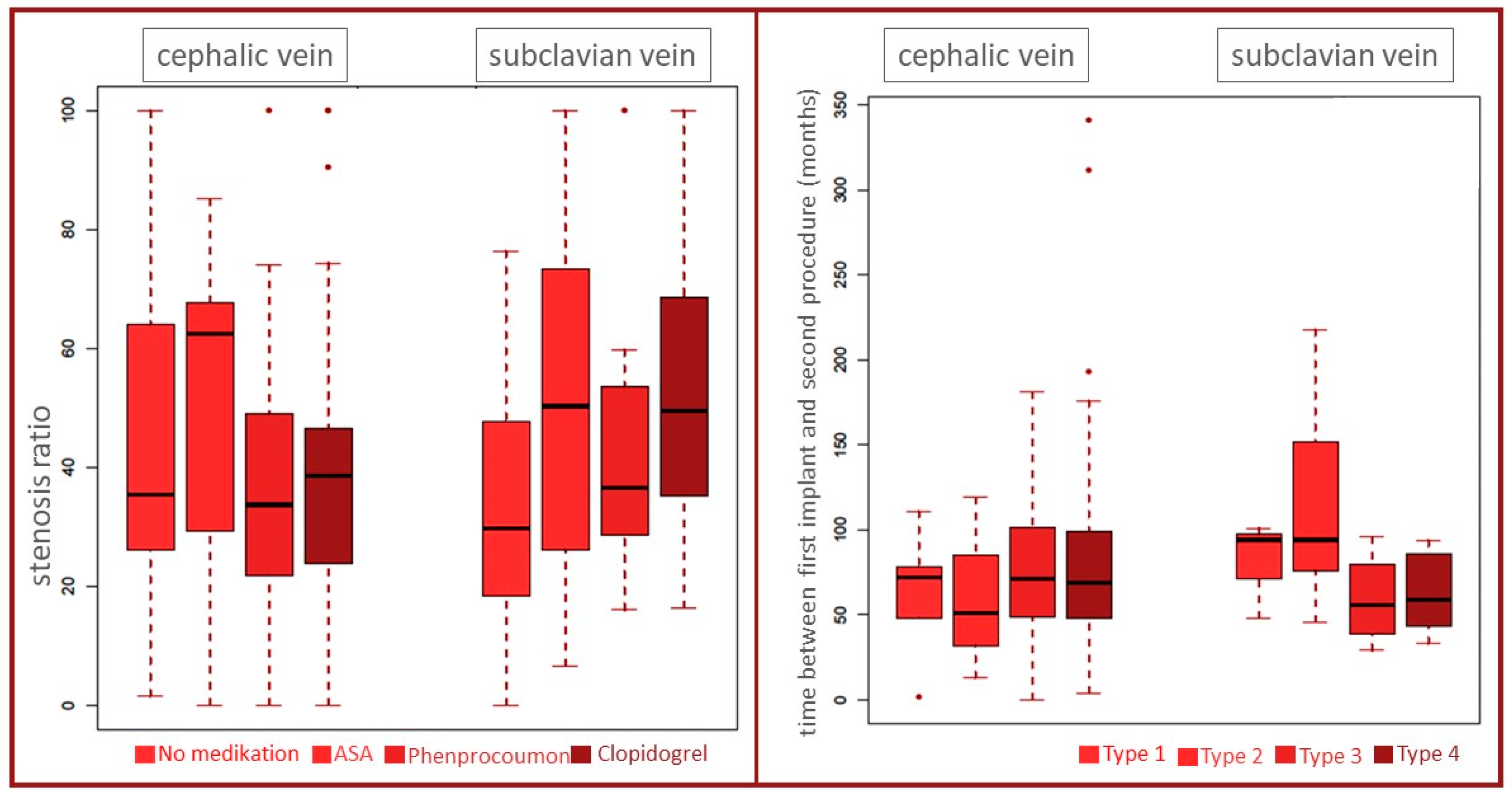
| ASA | 31 (35%) | 33 (45%) |
| Phenprocoumon | 34 (38%) | 22 (30%) |
| Clopidogrel | 12 (13%) | 12 (16%) |
| Cardiomyopathy | ||
| ICM | 26 (29%) | 16 (22%) |
| DCM | 13 (15%) | 15 (21%) |
| HCM | 3 (3%) | 2 (3%) |
| myocarditis | 1 (1%) | 0 |
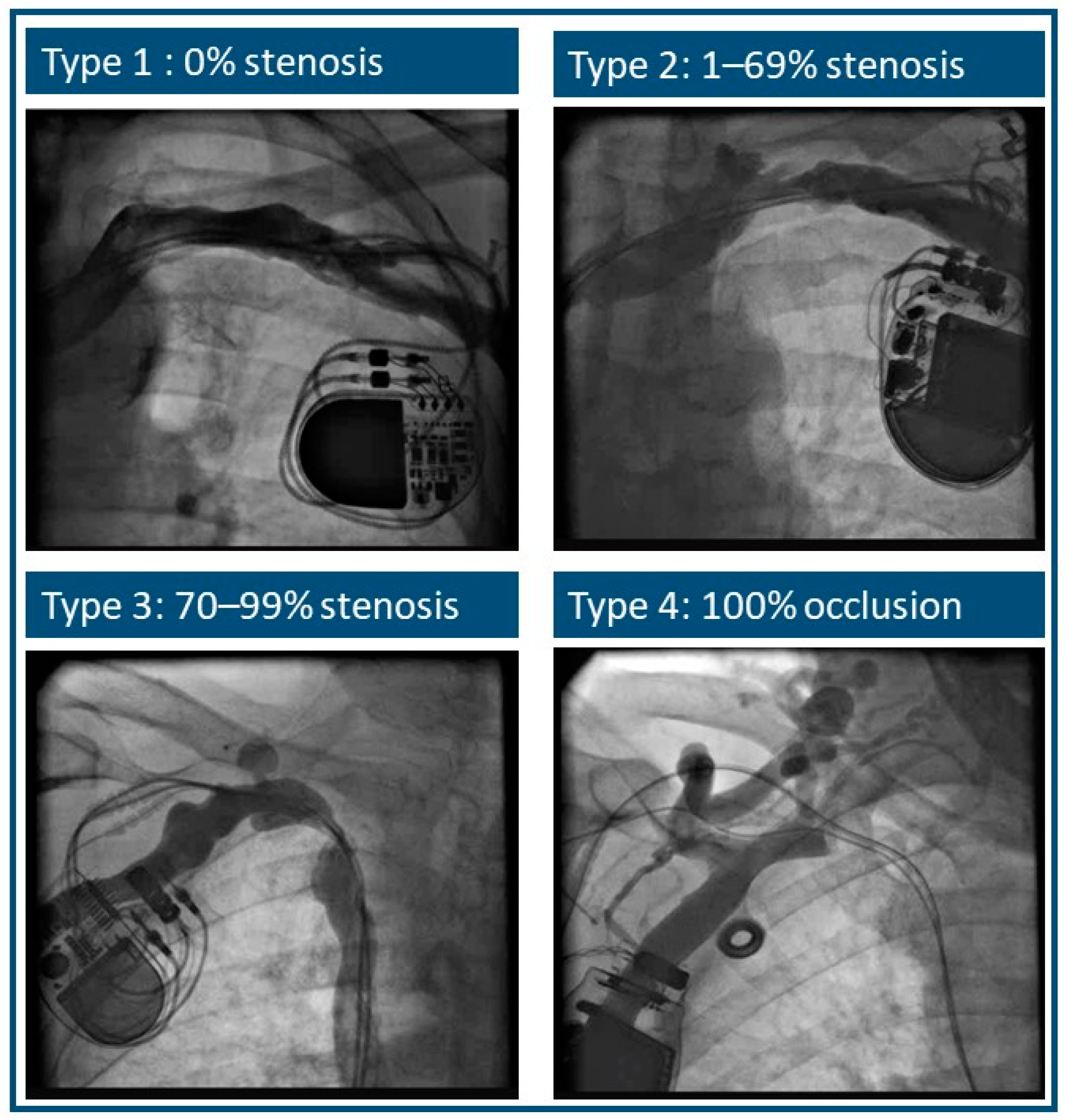
| Types of Stenosis | Cephalic Vein | Subclavian Vein | Total |
|---|---|---|---|
| 1 and 2 | 82 (92%) | 58 (79%) | 140 |
| 3 and 4 | 7 (8%) | 15 (21%) | 22 |
| total | 89 | 73 | 162 |
| Cephalic Vein (n = 89) | Subclavian Vein (n = 73) | |
|---|---|---|
| Mean stenosis ration | ||
| 36 ± 13% | 46 ± 17% | |
| Types of stenosis | ||
| 1 (stenosis 0%) | 6 (6.7%) | 3 (4.1%) |
| 2 (stenosis 1–69%) | 76 (85.4%) | 55 (75.3% |
| 3 (stenosis 70–99%) | 3 (3.4%) | 9 (12.3%) |
| 4 (occlusion) | 4 (4.5%) | 6 (8.2%) |
| Stenosis Type | Number of Leads | |||||
|---|---|---|---|---|---|---|
| Pacemaker | ICD | |||||
| 1 Lead | 2 Leads | 3 Leads | 1 Lead | 2 Leads | 3 Leads | |
| n = 9 | n = 51 | n = 10 | n = 28 | n = 21 | n = 43 | |
| Type 1 | 0 | 3 | 0 | 2 | 0 | 4 |
| Type 2 | 7 | 42 | 7 | 23 | 18 | 34 |
| Type 3 | 1 | 4 | 2 | 2 | 2 | 1 |
| Type 4 | 1 | 2 | 1 | 1 | 1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knorr, D.; Bandorski, D.; Bogossian, H.; Iliodromitis, K.; Schiedat, F.; Karosiene, Z.; Mijic, D.; Lemke, B.; Seyfarth, M.; Voß, S.; et al. Cephalic Vein Cutdown Is Superior to Subclavian Puncture as Venous Access for Patients with Cardiac Implantable Devices after Long-Term Follow-Up. J. Clin. Med. 2024, 13, 1044. https://doi.org/10.3390/jcm13041044
Knorr D, Bandorski D, Bogossian H, Iliodromitis K, Schiedat F, Karosiene Z, Mijic D, Lemke B, Seyfarth M, Voß S, et al. Cephalic Vein Cutdown Is Superior to Subclavian Puncture as Venous Access for Patients with Cardiac Implantable Devices after Long-Term Follow-Up. Journal of Clinical Medicine. 2024; 13(4):1044. https://doi.org/10.3390/jcm13041044
Chicago/Turabian StyleKnorr, Dario, Dirk Bandorski, Harilaos Bogossian, Konstantinos Iliodromitis, Fabian Schiedat, Zana Karosiene, Dejan Mijic, Bernd Lemke, Melchior Seyfarth, Sabrina Voß, and et al. 2024. "Cephalic Vein Cutdown Is Superior to Subclavian Puncture as Venous Access for Patients with Cardiac Implantable Devices after Long-Term Follow-Up" Journal of Clinical Medicine 13, no. 4: 1044. https://doi.org/10.3390/jcm13041044
APA StyleKnorr, D., Bandorski, D., Bogossian, H., Iliodromitis, K., Schiedat, F., Karosiene, Z., Mijic, D., Lemke, B., Seyfarth, M., Voß, S., Knippschild, S., Aweimer, A., Zarse, M., Kloppe, A., & Botsios, S. (2024). Cephalic Vein Cutdown Is Superior to Subclavian Puncture as Venous Access for Patients with Cardiac Implantable Devices after Long-Term Follow-Up. Journal of Clinical Medicine, 13(4), 1044. https://doi.org/10.3390/jcm13041044






