Long-Term Clinical Outcomes in Patients with Chronic Rhinosinusitis with Nasal Polyps Associated with Expanded Types of Endoscopic Sinus Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Surgical Technique and Postoperative Cares
2.3. Data and Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. QoL Outcomes
3.2. Endoscopic and Radiological Outcomes
3.3. Revision Surgery Rates and Complications
4. Discussion
4.1. QoL Outcomes
4.2. Endoscopic and Radiological Outcomes
4.3. Revision Surgery Rates and Surgical Complications
4.4. Limitations and Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58 (Suppl. S29), 1–464. [Google Scholar] [CrossRef]
- Alobid, I.; Colás, C.; Castillo, J.A.; Arismendi, E.; Del Cuvillo, A.; Gómez-Outes, A.; Sastre, J.; Mullol, J.; POLINA Group. Spanish Consensus on the Management of Chronic Rhinosinusitis with Nasal Polyps (POLIposis NAsal/POLINA 2.0). J. Investig. Allergol. Clin. Immunol. 2023, 33, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, R.R.; Kingdom, T.T.; Smith, T.L.; Bleier, B.; DeConde, A.; Luong, A.U.; Poetker, D.M.; Soler, Z.; Welch, K.C.; Wise, S.K.; et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis 2021. Int. Forum Allergy Rhinol. 2021, 11, 213–739. [Google Scholar] [CrossRef]
- Bachert, C.; Marple, B.; Hosemann, W.; Cavaliere, C.; Wen, W.; Zhang, N. Endotypes of Chronic Rhinosinusitis with Nasal Polyps: Pathology and Possible Therapeutic Implications. J. Allergy Clin. Immunol. Pract. 2020, 8, 1514–1519. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Viskens, A.-S.; Backer, V.; Conti, D.; De Corso, E.; Gevaert, P.; Scadding, G.K.; Wagemann, M.; Bernal-Sprekelsen, M.; Chaker, A.; et al. EPOS/EUFOREA Update on Indication and Evaluation of Biologics in Chronic Rhinosinusitis with Nasal Polyps 2023. Rhinology 2023, 61, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Lourijsen, E.S.; Reitsma, S.; Vleming, M.; Hannink, G.; Adriaensen, G.F.J.P.M.; Cornet, M.E.; Hoven, D.R.; Videler, W.J.M.; Bretschneider, J.H.; Reinartz, S.M.; et al. Endoscopic Sinus Surgery with Medical Therapy versus Medical Therapy for Chronic Rhinosinusitis with Nasal Polyps: A Multicentre, Randomised, Controlled Trial. Lancet Respir. Med. 2022, 10, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Tomassen, P.; Vandeplas, G.; Van Zele, T.; Cardell, L.-O.; Arebro, J.; Olze, H.; Förster-Ruhrmann, U.; Kowalski, M.L.; Olszewska-Ziąber, A.; Holtappels, G.; et al. Inflammatory Endotypes of Chronic Rhinosinusitis Based on Cluster Analysis of Biomarkers. J. Allergy Clin. Immunol. 2016, 137, 1449–1456.e4. [Google Scholar] [CrossRef]
- Chapurin, N.; Wu, J.; Labby, A.B.; Chandra, R.K.; Chowdhury, N.I.; Turner, J.H. Current Insight into Treatment of Chronic Rhinosinusitis: Phenotypes, Endotypes, and Implications for Targeted Therapeutics. J. Allergy Clin. Immunol. 2022, 150, 22–32. [Google Scholar] [CrossRef]
- Jonstam, K.; Alsharif, S.; Bogaert, S.; Suchonos, N.; Holtappels, G.; Jae-Hyun Park, J.; Bachert, C. Extent of Inflammation in Severe Nasal Polyposis and Effect of Sinus Surgery on Inflammation. Allergy 2021, 76, 933–936. [Google Scholar] [CrossRef]
- Moreno-Luna, R.; Martin-Jimenez, D.I.; Callejon-Leblic, M.A.; Gonzalez-Garcia, J.; Maza-Solano, J.M.; Porras-Gonzalez, C.; Del Cuvillo-Bernal, A.; Sanchez-Gomez, S. Usefulness of Bilateral Mucoplasty plus Reboot Surgery in Severe Type-2 Chronic Rhinosinusitis with Nasal Polyps. Rhinology 2022, 60, 368–376. [Google Scholar] [CrossRef]
- DeConde, A.S.; Mace, J.C.; Levy, J.M.; Rudmik, L.; Alt, J.A.; Smith, T.L. Prevalence of Polyp Recurrence after Endoscopic Sinus Surgery for Chronic Rhinosinusitis with Nasal Polyposis. Laryngoscope 2017, 127, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Huang, J.H.; Price, C.P.E.; Schauer, J.M.; Suh, L.A.; Harmon, R.; Conley, D.B.; Welch, K.C.; Kern, R.C.; Shintani-Smith, S.; et al. Prognostic Factors for Polyp Recurrence in Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. 2022, 150, 352–361.e7. [Google Scholar] [CrossRef] [PubMed]
- Stammberger, H.; Posawetz, W. Functional Endoscopic Sinus Surgery. Concept, Indications and Results of the Messerklinger Technique. Eur. Arch. Otorhinolaryngol. 1990, 247, 63–76. [Google Scholar] [CrossRef]
- Batra, P.S.; Kern, R.C.; Tripathi, A.; Conley, D.B.; Ditto, A.M.; Haines, G.K.; Yarnold, P.R.; Grammar, L. Outcome Analysis of Endoscopic Sinus Surgery in Patients with Nasal Polyps and Asthma. Laryngoscope 2003, 113, 1703–1706. [Google Scholar] [CrossRef] [PubMed]
- Proimos, E.; Papadakis, C.E.; Chimona, T.S.; Kiagiadaki, D.; Ferekidis, E.; Yiotakis, J. The Effect of Functional Endoscopic Sinus Surgery on Patients with Asthma and CRS with Nasal Polyps. Rhinology 2010, 48, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, S.; Jonstam, K.; van Zele, T.; Gevaert, P.; Holtappels, G.; Bachert, C. Endoscopic Sinus Surgery for Type-2 CRS wNP: An Endotype-Based Retrospective Study. Laryngoscope 2019, 129, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.-H.; Weitzel, E.K.; Lai, J.-T.; Wormald, P.-J.; Lin, C.-H. Retrospective Study of Full-House Functional Endoscopic Sinus Surgery for Revision Endoscopic Sinus Surgery. Int. Forum Allergy Rhinol. 2011, 1, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, N.; Xu, Z.; Zhang, L.; Bachert, C. The Development of the Mucosal Concept in Chronic Rhinosinusitis and Its Clinical Implications. J. Allergy Clin. Immunol. Pract. 2022, 10, 707–715. [Google Scholar] [CrossRef]
- DeConde, A.S.; Suh, J.D.; Mace, J.C.; Alt, J.A.; Smith, T.L. Outcomes of Complete vs Targeted Approaches to Endoscopic Sinus Surgery. Int. Forum Allergy Rhinol. 2015, 5, 691–700. [Google Scholar] [CrossRef]
- Vlaminck, S.; Acke, F.; Prokopakis, E.; Speleman, K.; Kawauchi, H.; van Cutsem, J.-C.; Hellings, P.W.; Jorissen, M.; Seys, S.; Bachert, C.; et al. Surgery in Nasal Polyp Patients: Outcome After a Minimum Observation of 10 Years. Am. J. Rhinol. Allergy 2021, 35, 449–457. [Google Scholar] [CrossRef]
- Lilja, M.J.; Virkkula, P.; Hammaren-Malmi, S.; Laulajainen-Hongisto, A.; Hafren, L.; Kauppi, P.; Sahlman, J.; Fokkens, W.J.; Reitsma, S.; Toppila-Salmi, S.K. The Extent of Endoscopic Sinus Surgery in Patients with Severe Chronic Rhinosinusitis with Nasal Polyps (AirGOs Operative). Rhinol. Online 2021, 4, 154–160. [Google Scholar] [CrossRef]
- Blauwblomme, M.; Gevaert, P.; Van Zele, T. Chronic Rhinosinusitis: Matching the Extent of Surgery with Pathology or Does the Extent of Surgery Matter? Curr. Otorhinolaryngol. Rep. 2023, 11, 273–285. [Google Scholar] [CrossRef]
- Ramkumar, S.P.; Marks, L.; Lal, D.; Marino, M.J. Outcomes of Limited versus Extensive Surgery for Chronic Rhinosinusitis: A Systematic Review and Meta-analysis. Int. Forum Allergy Rhinol. 2023, 13, 2096–2100. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.C.; Cavaliere, C.; Masieri, S.; Van Zele, T.; Gevaert, P.; Holtappels, G.; Zhang, N.; Ramasamy, P.; Voegels, R.L.; Bachert, C. Reboot Surgery for Chronic Rhinosinusitis with Nasal Polyposis: Recurrence and Smell Kinetics. Eur. Arch. Otorhinolaryngol. 2022, 279, 5691–5699. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Gao, Y.; Wang, K.; Lou, H.; Meng, Y.; Wang, C. Long-Term Outcomes of Different Endoscopic Sinus Surgery in Recurrent Chronic Rhinosinusitis with Nasal Polyps and Asthma. Rhinology 2020, 58, 126–135. [Google Scholar] [CrossRef]
- Martin-Jimenez, D.; Moreno-Luna, R.; Cuvillo, A.; Gonzalez-Garcia, J.; Maza-Solano, J.; Sanchez-Gomez, S. Endoscopic Extended Sinus Surgery for Patients with Severe Chronic Rhinosinusitis with Nasal Polyps, the Choice of Mucoplasty: A Systematic Review. Curr. Allergy Asthma Rep. 2023, 23, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Martin-Jimenez, D.; Moreno-Luna, R.; Callejon-Leblic, A.; Del Cuvillo, A.; Ebert Jr, C.S.; Maza-Solano, J.; Gonzalez-Garcia, J.; Sanchez-Gomez, S. Improved Quality of Life in Patients with Chronic Rhinosinusitis with Nasal Polyps Associated with Expanded Types of Endoscopic Sinus Surgery: A Two-Year Retrospective Study. Int. Forum Allergy Rhinol. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Hamilos, D.L.; Hadley, J.A.; Lanza, D.C.; Marple, B.F.; Nicklas, R.A.; Adinoff, A.D.; Bachert, C.; Borish, L.; Chinchilli, V.M.; et al. Rhinosinusitis: Developing Guidance for Clinical Trials. J. Allergy Clin. Immunol. 2006, 118 (Suppl. 5), S17–S61. [Google Scholar] [CrossRef]
- Toma, S.; Hopkins, C. Stratification of SNOT-22 Scores into Mild, Moderate or Severe and Relationship with Other Subjective Instruments. Rhinology 2016, 54, 129–133. [Google Scholar] [CrossRef]
- Rudmik, L.; Soler, Z.M.; Hopkins, C.; Schlosser, R.J.; Peters, A.; White, A.A.; Orlandi, R.R.; Fokkens, W.J.; Douglas, R.; Smith, T.L. Defining Appropriateness Criteria for Endoscopic Sinus Surgery during Management of Uncomplicated Adult Chronic Rhinosinusitis: A RAND/UCLA Appropriateness Study. Rhinology 2016, 54, 117–128. [Google Scholar] [CrossRef]
- Chen, F.-H.; Deng, J.; Hong, H.-Y.; Xu, R.; Guo, J.-B.; Hou, W.-J.; Sun, Y.-Q.; Lai, Y.-Y.; Li, H.-B.; Shi, J.-B. Extensive versus Functional Endoscopic Sinus Surgery for Chronic Rhinosinusitis with Nasal Polyps and Asthma: A 1-Year Study. Am. J. Rhinol. Allergy 2016, 30, 143–148. [Google Scholar] [CrossRef]
- Hopkins, C.; Gillett, S.; Slack, R.; Lund, V.J.; Browne, J.P. Psychometric Validity of the 22-Item Sinonasal Outcome Test. Clin. Otolaryngol. 2009, 34, 447–454. [Google Scholar] [CrossRef]
- de los Santos, G.; Reyes, P.; del Castillo, R.; Fragola, C.; Royuela, A. Cross-Cultural Adaptation and Validation of the Sino-Nasal Outcome Test (SNOT-22) for Spanish-Speaking Patients. Eur. Arch. Otorhinolaryngol. 2015, 272, 3335–3340. [Google Scholar] [CrossRef]
- Psaltis, A.J.; Li, G.; Vaezeafshar, R.; Cho, K.-S.; Hwang, P.H. Modification of the Lund-Kennedy Endoscopic Scoring System Improves Its Reliability and Correlation with Patient-Reported Outcome Measures. Laryngoscope 2014, 124, 2216–2223. [Google Scholar] [CrossRef]
- Lund, V.; Mackay, I. Staging in Rhinosinusitis. Rhinology 1994, 31, 183–184. [Google Scholar]
- Phillips, K.M.; Houssein, F.A.; Boeckermann, L.M.; Singerman, K.W.; Liu, D.T.; Sedaghat, A.R. Multi-Institutional Minimal Clinically Important Difference of the 22-Item Sinonasal Outcome Test in Medically Managed Chronic Rhinosinusitis. Rhinology 2021, 59, 552–559. [Google Scholar] [CrossRef]
- Peterson, A.M.; Miller, B.; Ioerger, P.; Hentati, F.; Doering, M.M.; Kallogjeri, D.; Piccirillo, J.F. Most-Cited Patient-Reported Outcome Measures Within Otolaryngology—Revisiting the Minimal Clinically Important Difference: A Review. JAMA Otolaryngol. Neck Surg. 2023, 149, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Pirola, F.; Pace, G.M.; Giombi, F.; Heffler, E.; Paoletti, G.; Nappi, E.; Sanità, W.; Giulietti, G.; Giunta, G.; Ferreli, F.; et al. Outcomes of Non-Mucosa Sparing Endoscopic Sinus Surgery (Partial Reboot) in Refractory Chronic Rhinosinusitis with Nasal Polyposis: An Academic Hospital Experience. Laryngoscope 2022, 133, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, B.W.; Pang, K.P. The Impact of Sinus Surgery on Sleep Outcomes. Int. Forum Allergy Rhinol. 2015, 5, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Bizaki, A.J.; Taulu, R.; Numminen, J.; Rautiainen, M. Quality of Life after Endoscopic Sinus Surgery or Balloon Sinuplasty: A Randomized Clinical Study. Rhinology 2014, 52, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Alanin, M.C.; Hopkins, C. Effect of Functional Endoscopic Sinus Surgery on Outcomes in Chronic Rhinosinusitis. Curr. Allergy Asthma Rep. 2020, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Calus, L.; Van Bruaene, N.; Bosteels, C.; Dejonckheere, S.; Van Zele, T.; Holtappels, G.; Bachert, C.; Gevaert, P. Twelve-Year Follow-up Study after Endoscopic Sinus Surgery in Patients with Chronic Rhinosinusitis with Nasal Polyposis. Clin. Transl. Allergy 2019, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Soler, Z.M.; Jones, R.; Le, P.; Rudmik, L.; Mattos, J.L.; Nguyen, S.A.; Schlosser, R.J. Sino-Nasal Outcome Test-22 Outcomes after Sinus Surgery: A Systematic Review and Meta-Analysis. Laryngoscope 2018, 128, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Rudmik, L.; Lund, V.J. The Predictive Value of the Preoperative Sinonasal Outcome Test-22 Score in Patients Undergoing Endoscopic Sinus Surgery for Chronic Rhinosinusitis. Laryngoscope 2015, 125, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Loftus, C.A.; Soler, Z.M.; Koochakzadeh, S.; Desiato, V.M.; Yoo, F.; Nguyen, S.A.; Schlosser, R.J. Revision Surgery Rates in Chronic Rhinosinusitis with Nasal Polyps: Meta-Analysis of Risk Factors. Int. Forum Allergy Rhinol. 2020, 10, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Gurrola, J.; Borish, L. Chronic Rhinosinusitis: Endotypes, Biomarkers, and Treatment Response. J. Allergy Clin. Immunol. 2017, 140, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and Safety of Dupilumab in Patients with Severe Chronic Rhinosinusitis with Nasal Polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from Two Multicentre, Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Phase 3 Trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef]
- Arancibia, C.; Langdon, C.; Mullol, J.; Alobid, I. Twelve-Year Long-Term Postoperative Outcomes in Patients with Chronic Rhinosinusitis with Nasal Polyps. Rhinology 2022, 60, 261–269. [Google Scholar] [CrossRef]
- Ta, N.H.; Gao, J.; Philpott, C. A Systematic Review to Examine the Relationship between Objective and Patient-reported Outcome Measures in Sinonasal Disorders: Recommendations for Use in Research and Clinical Practice. Int. Forum Allergy Rhinol. 2021, 11, 910–923. [Google Scholar] [CrossRef]
- Moreno-Luna, R.; Gonzalez-Garcia, J.; Maza-Solano, J.M.; Molina-Fernandez, E.; Pinheiro-Neto, C.D.; Del Cuvillo Bernal, A.; Langdon, C.; Sanchez-Gomez, S. Free Nasal Floor Mucosal Grafting after Endoscopic Total Ethmoidectomy for Severe Nasal Polyposis: A Pilot Study. Rhinology 2019, 57, 219–224. [Google Scholar] [CrossRef]
- Moreno-Luna, R.; González-García, J.; Palacios-García, J.; Maza-Solano, J.M.; Del Cuvillo Bernal, A.; Sánchez-Gómez, S. Usefulness of Endonasal Mucoplasty in the Surgical Treatment of Chronic Rhinosinusitis with Nasal Polyps. Prospective Study. Acta Otorrinolaringol. Esp. 2021, 72, 256–261. [Google Scholar] [CrossRef]
- Cardell, L.-O.; Stjärne, P.; Jonstam, K.; Bachert, C. Endotypes of Chronic Rhinosinusitis: Impact on Management. J. Allergy Clin. Immunol. 2020, 145, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Han, J.K.; Wagenmann, M.; Hosemann, W.; Lee, S.E.; Backer, V.; Mullol, J.; Gevaert, P.; Klimek, L.; Prokopakis, E.; et al. EUFOREA Expert Board Meeting on Uncontrolled Severe Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) and Biologics: Definitions and Management. J. Allergy Clin. Immunol. 2021, 147, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.; Walgama, E.; Thamboo, A.; Chitsuthipakorn, W.; Patel, Z.M.; Nayak, J.V.; Hwang, P.H. Correlation between Extent of Sinus Surgery, Radiographic Disease, and Postoperative Outcomes. Rhinology 2020, 58, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Masterson, L.; Tanweer, F.; Bueser, T.; Leong, P. Extensive Endoscopic Sinus Surgery: Does This Reduce the Revision Rate for Nasal Polyposis? Eur. Arch. Otorhinolaryngol. 2010, 267, 1557–1561. [Google Scholar] [CrossRef]
- Suzuki, S.; Yasunaga, H.; Matsui, H.; Fushimi, K.; Kondo, K.; Yamasoba, T. Complication Rates after Functional Endoscopic Sinus Surgery: Analysis of 50,734 Japanese Patients. Laryngoscope 2015, 125, 1785–1791. [Google Scholar] [CrossRef]
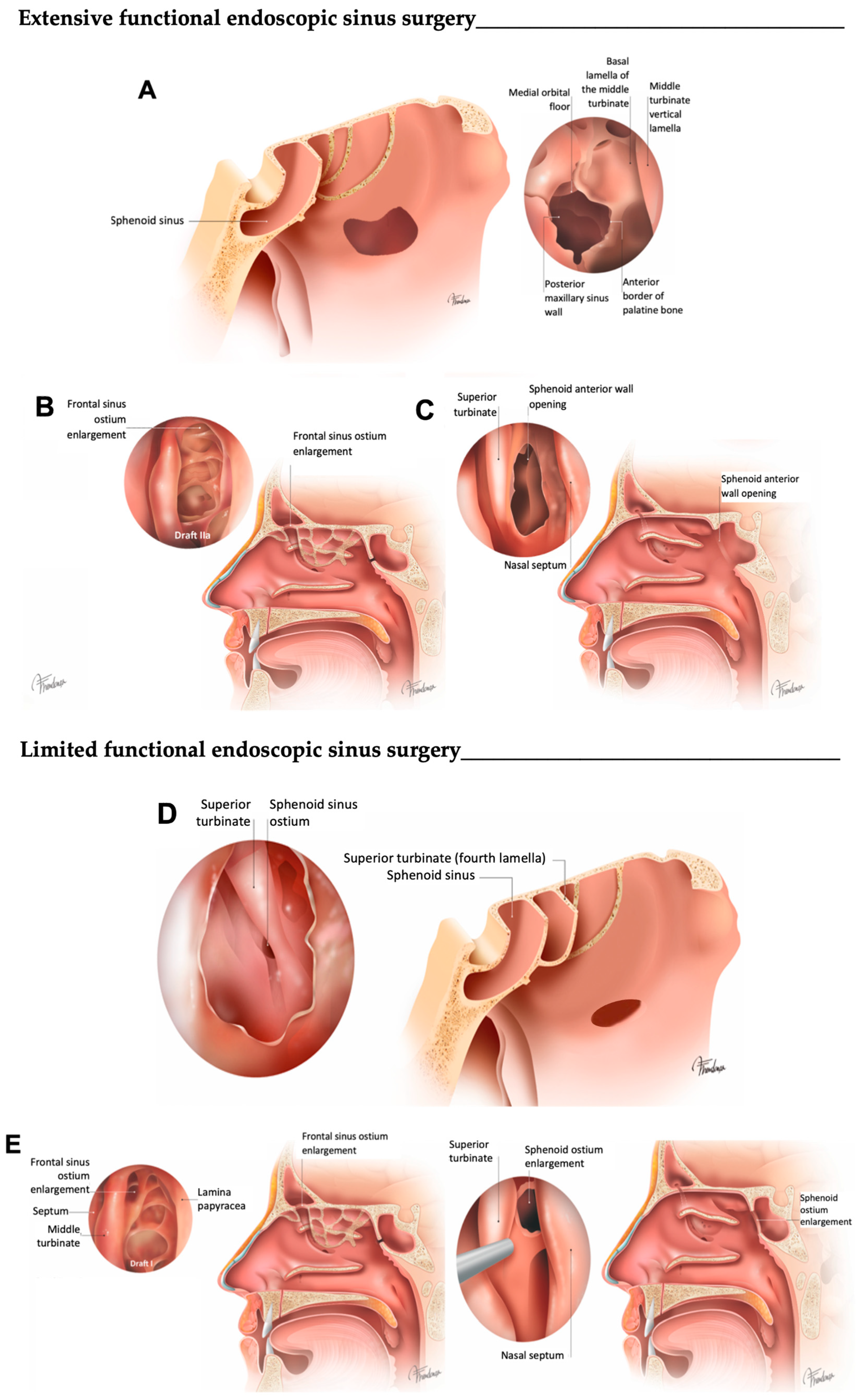

| L–FESS | E–FESS | |
|---|---|---|
| Rationale | Conservative approach targeting osteomeatal complex disease to allow for proper sinus ventilation, mucociliary clearance and easy topical therapy instillation | To address the affected sinuses (CT images), irrespective of the presence of specific sinus-related symptoms; complete removal of all ethmoidal lamellae can prevent unintended obstruction and facilitate postoperative diagnostics and treatment in the patient |
| Objective | To clear diseased ethmoid clefts and compartments and to re-establish ventilation and drainage of the diseased larger sinus via their physiological routes; damage to the surrounding tissue is minimized | E-FESS is a term used to describe uncinectomy, maxillary antrostomy, total ethmoidectomy, wide sphenoidotomy and a Draf IIA frontal sinusotomy |
| Mucosa | Disease is targeted for removal in key areas of the anterior ethmoid and middle meatus; preservation of as much mucosa as possible | Targeted removal of disease from key areas of the ethmoid and middle meatus |
| Uncinectomy | Performed systematically | Performed systematically |
| Ethmoidal bulla | Once the cell walls are fractured, they are removed | Once the cell walls are fractured, they are removed |
| Middle turbinate | Preservation is preferred | Consider the possibility of medializing the middle turbinate or securing it to the septum through the induction of synechiae |
| Vertical plate of the basal lamella of the middle turbinate | The basal lamella is perforated to enter the posterior ethmoid cells whenever needed | The basal lamella is perforated to enter the posterior ethmoid cells and the opening is enlarged |
| Ethmoid bony lamellae | On demand | Removed systematically |
| Middle meatal antrostomy | On demand | As large as possible |
| Maxillary sinus mucosa | Localized irreversible disease is removed to the periosteum; frequently, apparently irreversible mucosal disease resolves | On demand |
| Ethmoid sinus mucosa | On demand | Removed systematically to the periosteum |
| Sphenoidotomy | On demand | Wide |
| Sphenoid sinus mucosa | Preserved | Preserved unless grossly abnormal |
| Frontal sinus opening | Clearing the frontal recess in most cases of inflammatory processes will also produce healing of the sinus without the need for additionally enlarging the sinus ostium itself | Specified Draf IIA Only Draf III when indicated |
| Frontal sinus mucosa | Preserved | Frontal pathway clearance |
| Adjunct procedures | Canine fossa trephination and frontal minitrephination whenever necessary |
| Simple Linear Regression Model β, μx ± SE | Multiple Linear Regression Model Adjusted β, μx ± SE | 95% CI | p Value | R2 | |
|---|---|---|---|---|---|
| E-FESS (type of surgery) | 8.2 ± 3.7 | - | (0.9 to 15.5) | 0.028 | 0.028 |
| - | 12.2 ± 5.0 *1 | (2.3 to 22.0) | 0.016 | 0.396 | |
| Baseline SNOT-22 | 0.5 ± 0.1 | - | (0.4 to 0.7) | <0.001 | 0.233 |
| - | 0.7 ± 0.1 *1 | (0.4 to 0.9) | <0.001 | 0.396 | |
| Age | −0.02 ± 0.1 | - | (−0.3 to 0.3) | 0.917 | 0.000 |
| - | 0.01 ± 0.2 | (−0.4 to 0.4) | 0.944 | 0.396 | |
| Female (gender) | 3.3 ± 3.9 | - | (−4.5 to 11.0) | 0.404 | 0.004 |
| - | −7.1 ± 5.4 | (−17.7 to 3.6) | 0.190 | 0.396 | |
| Asthma | 5.1 ± 3.7 | - | (−2.2 to 12.5) | 0.170 | 0.011 |
| - | −3.3 ± 5.8 | (−14.8 to 8.3) | 0.577 | 0.396 | |
| N-ERD | −2.4 ± 4.6 | - | (−11.4 to 6.6) | 0.600 | 0.002 |
| - | 0.3 ± 6.2 | (−12.0 to 12.5) | 0.966 | 0.396 | |
| Previous ESS | −3.6 ± 4.1 | - | (−11.6 to 4.4) | 0.370 | 0.005 |
| - | −9.3 ± 5.5 | (−20.3 to 1.6) | 0.095 | 0.396 | |
| Eosinophils in peripheral blood (cells/μL) | 5.4 ± 5.5 | - | (−5.5 to 16.3) | 0.328 | 0.006 |
| - | 6.4 ± 6.3 | (−6.0 to 18.9) | 0.306 | 0.396 | |
| Total IgE (UI/L) | 0.01 ± 0.0 | - | (0.0 to 0.01) | 0.067 | 0.032 |
| - | 0.01 ± 0.0 | (0.0 to 0.01) | 0.062 | 0.396 | |
| Proven allergic sensitization (atopy) | 5.6 ± 4.7 | - | (−3.7 to 14.8) | 0.234 | 0.011 |
| - | 3.6 ± 5.0 | (−6.3 to 13.6) | 0.470 | 0.396 | |
| ≥1 cycles of SCS in the pre–surgery year | 3.0 ± 3.8 | - | (−4.5 to 10.4) | 0.431 | 0.004 |
| - | 3.6 ± 4.9 | (−6.1 to 13.4) | 0.458 | 0.396 | |
| Baseline NPS | 2.8 ± 1.1 | - | (0.7 to 5.0) | 0.009 | 0.040 |
| - | 1.7 ± 1.7 | (−1.7 to 5.2) | 0.314 | 0.396 | |
| Baseline MLK scale | 0.2 ± 0.8 | - | (−1.4 to 1.9) | 0.793 | 0.000 |
| - | −0.3 ± 1.3 | (−2.8 to 2.3) | 0.844 | 0.396 | |
| Baseline LM scale | 0.5 ± 0.3 | - | (−0.2 to 1.2) | 0.142 | 0.013 |
| - | 0.2 ± 0.5 | (−0.8 to 1.3) | 0.648 | 0.396 |
| Adjusted β (μx ± SE) | 95% CI | p Value | R2 | |
|---|---|---|---|---|
| E-FESS (type of surgery) | 14.8 ± 4.8 | (5.5 to 24.1) | 0.002 | 0.313 |
| Baseline SNOT-22 | 0.6 ± 0.1 | (0.4 to 0.8) | <0.001 | |
| Previous ESS | −11.2 ± 5.0 | (−21.2 to −1.3) | 0.027 |
| Adjusted β (μx ± SE) | 95% CI | p Value | R2 | |
|---|---|---|---|---|
| NPS * | ||||
| Baseline NPS | 0.8 ± 0.1 | (0.6 to 1.1) | <0.001 | 0.317 |
| Baseline SNOT-22 | −0.02 ± 0.01 | (−0.04 to 0.0) | 0.046 | |
| MLK scale t | ||||
| Baseline MLK scale | 1.1 ± 0.1 | (0.9 to 1.3) | <0.001 | 0.511 |
| Baseline LM scale | −0.1 ± 0.04 | (−0.2 to −0.02) | 0.013 | |
| LM scale α | ||||
| Baseline LM scale | 0.6 ± 0.1 | (0.4 to 0.9) | <0.001 | 0.307 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Jimenez, D.; Moreno-Luna, R.; Callejon-Leblic, A.; del Cuvillo, A.; Ebert, C.S., Jr.; Maza-Solano, J.; Gonzalez-Garcia, J.; Infante-Cossio, P.; Sanchez-Gomez, S. Long-Term Clinical Outcomes in Patients with Chronic Rhinosinusitis with Nasal Polyps Associated with Expanded Types of Endoscopic Sinus Surgery. J. Clin. Med. 2024, 13, 866. https://doi.org/10.3390/jcm13030866
Martin-Jimenez D, Moreno-Luna R, Callejon-Leblic A, del Cuvillo A, Ebert CS Jr., Maza-Solano J, Gonzalez-Garcia J, Infante-Cossio P, Sanchez-Gomez S. Long-Term Clinical Outcomes in Patients with Chronic Rhinosinusitis with Nasal Polyps Associated with Expanded Types of Endoscopic Sinus Surgery. Journal of Clinical Medicine. 2024; 13(3):866. https://doi.org/10.3390/jcm13030866
Chicago/Turabian StyleMartin-Jimenez, Daniel, Ramon Moreno-Luna, Amparo Callejon-Leblic, Alfonso del Cuvillo, Charles S. Ebert, Jr., Juan Maza-Solano, Jaime Gonzalez-Garcia, Pedro Infante-Cossio, and Serafin Sanchez-Gomez. 2024. "Long-Term Clinical Outcomes in Patients with Chronic Rhinosinusitis with Nasal Polyps Associated with Expanded Types of Endoscopic Sinus Surgery" Journal of Clinical Medicine 13, no. 3: 866. https://doi.org/10.3390/jcm13030866
APA StyleMartin-Jimenez, D., Moreno-Luna, R., Callejon-Leblic, A., del Cuvillo, A., Ebert, C. S., Jr., Maza-Solano, J., Gonzalez-Garcia, J., Infante-Cossio, P., & Sanchez-Gomez, S. (2024). Long-Term Clinical Outcomes in Patients with Chronic Rhinosinusitis with Nasal Polyps Associated with Expanded Types of Endoscopic Sinus Surgery. Journal of Clinical Medicine, 13(3), 866. https://doi.org/10.3390/jcm13030866












