Vitamin D Supplementation: Effect on Cytokine Profile in Multiple Sclerosis
Abstract
1. Introduction
2. Literature Search Methodology
3. Results
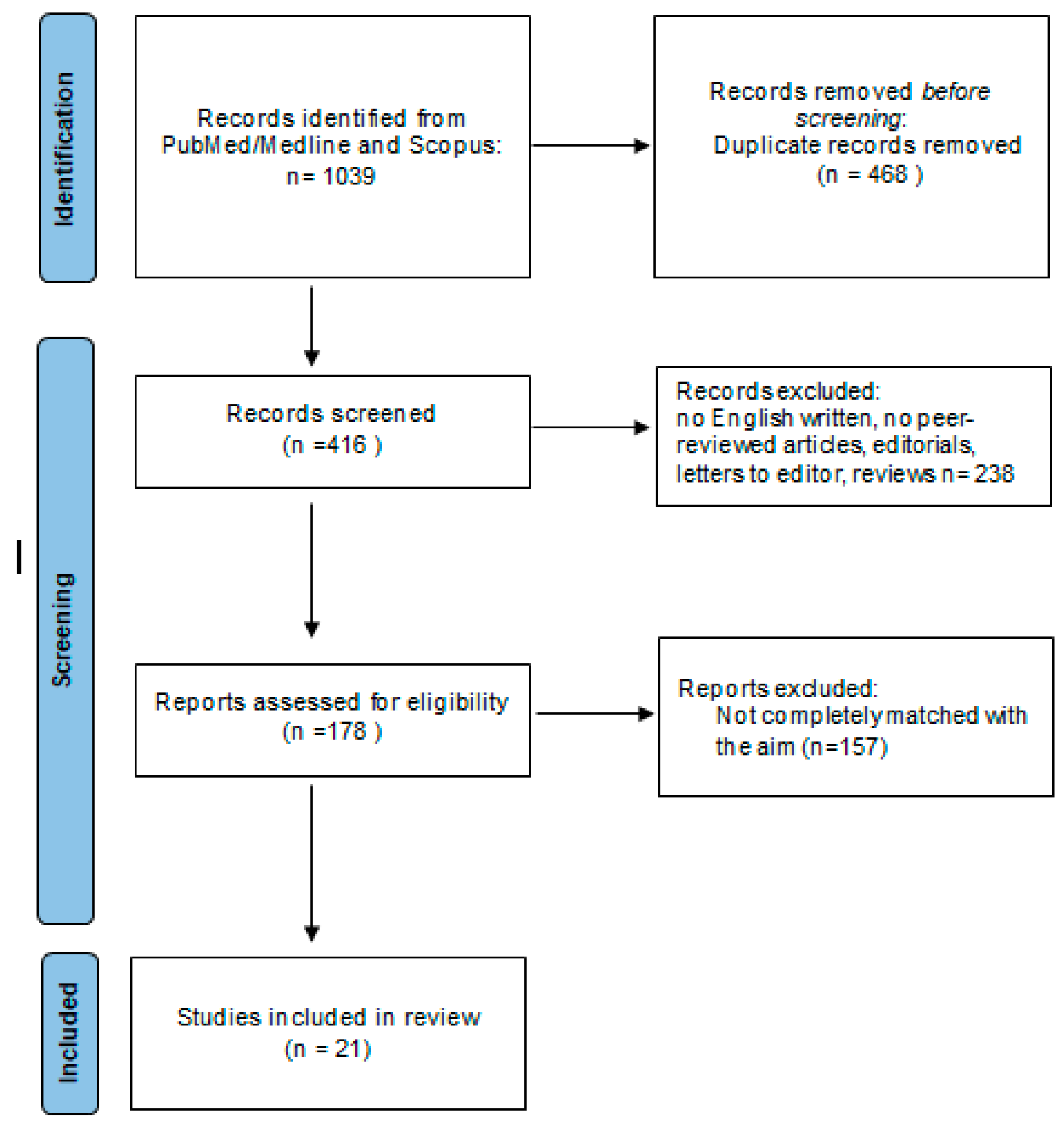
3.1. Study Selection
3.2. Vitamin D effect on Cytokine Profile in MS
- IL-10
- IL-17
- IL-6
- TGF-β1
- IFN-γ
- Other cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Aloisi, F.; Giovannoni, G.; Salvetti, M. Epstein-Barr virus as a cause of multiple sclerosis: Opportunities for prevention and therapy. Lancet Neurol. 2023, 22, 338–349. [Google Scholar] [CrossRef]
- Nejati, A.; Shoja, Z.; Shahmahmoodi, S.; Tafakhori, A.; Mollaei-Kandelous, Y.; Rezaei, F.; Hamid, K.M.; Mirshafiey, A.; Doosti, R.; Sahraian, M.A.; et al. EBV and vitamin D status in relapsing-remitting multiple sclerosis patients with a unique cytokine signature. Med. Microbiol. Immunol. 2016, 205, 143–154. [Google Scholar] [CrossRef]
- Tremlett, H.; Zhu, F.; Ascherio, A.; Munger, K.L. Sun exposure over the life course and associations with multiple sclerosis. Neurology 2018, 90, e1191–e1199. [Google Scholar] [CrossRef]
- Sparaco, M.; Bonavita, S. The role of sex hormones in women with multiple sclerosis: From puberty to assisted reproductive techniques. Front. Neuroendocrinol. 2021, 60, 100889. [Google Scholar] [CrossRef]
- Saponaro, F.; Saba, A.; Zucchi, R. An Update on Vitamin D Metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.; Adorini, L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.; O’Garra, A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [PubMed]
- Lysandropoulos, A.P.; Jaquiéry, E.; Jilek, S.; Pantaleo, G.; Schluep, M.; Du Pasquier, R.A. Vitamin D has a direct immunomodulatory effect on CD8+ T cells of patients with early multiple sclerosis and healthy control subjects. J. Neuroimmunol. 2011, 233, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Boltjes, R.; Knippenberg, S.; Gerlach, O.; Hupperts, R.; Damoiseaux, J. Vitamin D supplementation in multiple sclerosis: An expert opinion based on the review of current evidence. Expert Rev. Neurother. 2021, 21, 715–725. [Google Scholar] [CrossRef]
- Soilu-Hänninen, M.; Airas, L.; Mononen, I.; Heikkilä, A.; Viljanen, M.; Hänninen, A. 25-Hydroxyvitamin D levels in serum at the onset of multiple sclerosis. Mult. Scler. 2005, 11, 266–271. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L.; White, R.; Köchert, K.; Simon, K.C.; Polman, C.H.; Freedman, M.S.; Hartung, H.P.; Miller, D.H.; Montalbán, X.; et al. Vitamin D as an Early Predictor of Multiple Sclerosis Activity and Progression. JAMA Neurol. 2014, 71, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Soilu-Hänninen, M.; Aivo, J.; Lindström, B.M.; Elovaara, I.; Sumelahti, M.L.; Färkkilä, M.; Tienari, P.; Atula, S.; Sarasoja, T.; Herrala, L.; et al. A randomized double-blind placebo-controlled trial with vitamin D3 as an add-on treatment to interferon beta for the treatment of MS. J. Neurol. Neurosurg. Psychiatry 2012, 83, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Åivo, J.; Lindström, B.M.; Soilu-Hänninen, M. A randomised, double-blind, placebocontrolled trial with vitamin D3 in MS: Subgroup analysis of patients with baseline disease activity despite interferon treatment. Mult. Scler. Int. 2012, 2012, 802796. [Google Scholar] [PubMed]
- Miele, G.; Abbadessa, G.; Cavalla, P.; Valentino, P.; Marfia, G.A.; Landi, D.; Bosa, C.; Vercellino, M.; De Martino, A.; Ponzano, M.; et al. Association of vitamin D serum levels and vitamin D supplementation with B cell kinetics and disease activity in Multiple Sclerosis patients treated with ocrelizumab: An Italian multi-center study. Mult. Scler. Relat. Disord. 2022, 68, 104395. [Google Scholar] [CrossRef] [PubMed]
- Muris, A.H.; Rolf, L.; Broen, K.; Hupperts, R.; Damoiseaux, J.; Smolders, J. A low vitamin D status at diagnosis is associated with an early conversion to secondary progressive multiple sclerosis. J. Steroid Biochem. Mol. Biol. 2016, 164, 254–257. [Google Scholar] [CrossRef]
- Sparaco, M.; Carbone, L.; Landi, D.; Ingrasciotta, Y.; Di Girolamo, R.; Vitturi, G.; Marfia, G.A.; Alviggi, C.; Bonavita, S. Assisted Reproductive Technology and Disease Management in Infertile Women with Multiple Sclerosis. CNS Drugs 2023, 37, 849–866. [Google Scholar] [CrossRef]
- Munger, K.L.; Åivo, J.; Hongell, K.; Soilu-Hänninen, M.; Surcel, H.M.; Ascherio, A. Vitamin D status during pregnancy and risk of multiple sclerosis in offspring of women in the Finnish maternity cohort. JAMA Neurol. 2016, 73, 515–519. [Google Scholar] [CrossRef]
- Etemadifar, M.; Janghorbani, M. Efficacy of high-dose vitamin D3 supplementation in vitamin D deficient pregnant women with multiple sclerosis: Preliminary findings of a randomized-controlled trial. Iran. J. Neurol. 2015, 14, 67–73. [Google Scholar] [PubMed]
- Pacis, M.M.; Fortin, C.N.; Zarek, S.M.; Mumford, S.L.; Segars, J.H. Vitamin D and assisted reproduction: Should vitamin D be routinely screened and repleted prior to ART? A systematic review. J. Assist. Reprod. Genet. 2015, 32, 323–335. [Google Scholar] [CrossRef]
- Chu, J.; Gallos, I.; Tobias, A.; Tan, B.; Eapen, A.; Coomarasamy, A. Vitamin D and assisted reproductive treatment outcome: A systematic review and meta-analysis. Hum. Reprod. 2018, 33, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Bove, R.; Rankin, K.; Lin, C.; Zhao, C.; Correale, J.; Hellwig, K.; Michel, L.; Laplaud, D.A.; Chitnis, T. Effect of assisted reproductive technology on multiple sclerosis relapses: Case series and meta-analysis. Mult. Scler. 2020, 26, 1410–1419. [Google Scholar] [CrossRef]
- Shirazi, H.A.; Rasouli, J.; Ciric, B.; Wei, D.; Rostami, A.; Zhang, G.X. 1,25-Dihydroxyvitamin D3 suppressed experimental autoimmune encephalomyelitis through both immunomodulation and oligodendrocyte maturation. Exp. Mol. Pathol. 2017, 102, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Rutz, S.; Ouyang, W. Regulation of Interleukin-10 Expression. Adv. Exp. Med. Biol. 2016, 941, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Ireland, S.J.; Monson, N.L.; Davis, L.S. Seeking balance: Potentiation and inhibition of multiple sclerosis autoimmune responses by IL-6 and IL-10. Cytokine 2015, 73, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.B.; Rose, J.W.; Jaskowski, T.D.; Wilson, A.R.; Husebye, D.; Seraj, H.S.; Hill, H.R. Analysis of proinflammatory and anti-inflammatory cytokine serum concentrations in patients with multiple sclerosis by using a multiplexed immunoassay. Am. J. Clin. Pathol. 2011, 136, 696–704. [Google Scholar] [CrossRef]
- Petereit, H.F.; Pukrop, R.; Fazekas, F.; Bamborschke, S.U.; Röpele, S.; Kölmel, H.W.; Merkelbach, S.; Japp, G.; Jongen, P.J.; Hartung, H.P.; et al. Low interleukin-10 production is associated with higher disability and MRI lesion load in secondary progressive multiple sclerosis. J. Neurol. Sci. 2003, 206, 209–214. [Google Scholar] [CrossRef]
- Wei, Y.; Chang, H.; Feng, H.; Li, X.; Zhang, X.; Yin, L. Low Serum Interleukin-10 Is an Independent Predictive Factor for the Risk of Second Event in Clinically Isolated Syndromes. Front. Neurol. 2019, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Ysrraelit, M.C.; Gaitán, M.I. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain 2009, 132 Pt 5, 1146–1160. [Google Scholar] [CrossRef]
- Walawska-Hrycek, A.; Galus, W.; Hrycek, E.; Kaczmarczyk, A.; Krzystanek, E. The Impact of Vitamin D Low Doses on Its Serum Level and Cytokine Profile in Multiple Sclerosis Patients. J. Clin. Med. 2021, 10, 2781. [Google Scholar] [CrossRef] [PubMed]
- Smolders, J.; Peelen, E.; Thewissen, M.; Cohen Tervaert, J.W.; Menheere, P.; Hupperts, R.; Damoiseaux, J. Safety and T cell modulating effects of high dose vitamin D3 supplementation in multiple sclerosis. PLoS ONE 2010, 5, e15235. [Google Scholar] [CrossRef] [PubMed]
- Ashtari, F.; Toghianifar, N.; Zarkesh-Esfahani, S.H.; Mansourian, M. Short-Term Effect of High-Dose Vitamin D on the Level of Interleukin 10 in Patients with Multiple Sclerosis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Neuroimmunomodulation 2015, 22, 400–404. [Google Scholar] [CrossRef]
- Shirvani-Farsani, Z.; Behmanesh, M.; Sahraian, M.A. Interleukin-10 but not transforming growth factor-β1 gene expression is up-regulated by vitamin D treatment in multiple sclerosis patients. J. Neurol. Sci. 2015, 350, 18–23. [Google Scholar] [CrossRef]
- Hashemi, R.; Morshedi, M.; Asghari Jafarabadi, M.; Altafi, D.; Saeed Hosseini-Asl, S.; Rafie-Arefhosseini, S. Anti-inflammatory effects of dietary vitamin D3 in patients with multiple sclerosis. Neurol. Genet. 2018, 4, e278. [Google Scholar] [CrossRef]
- Hashemi, R.; Hosseini-Asl, S.S.; Arefhosseini, S.R.; Morshedi, M. The impact of vitamin D3 intake on inflammatory markers in multiple sclerosis patients and their first-degree relatives. PLoS ONE 2020, 15, e0231145. [Google Scholar] [CrossRef] [PubMed]
- Mosayebi, G.; Ghazavi, A.; Ghasami, K.; Jand, Y.; Kokhaei, P. Therapeutic effect of vitamin D3 in multiple sclerosis patients. Immunol. Invest. 2011, 40, 627–639. [Google Scholar] [CrossRef]
- Golan, D.; Halhal, B.; Glass-Marmor, L.; Staun-Ram, E.; Rozenberg, O.; Lavi, I.; Dishon, S.; Barak, M.; Ish-Shalom, S.; Miller, A. Vitamin D supplementation for patients with multiple sclerosis treated with interferon-beta: A randomized controlled trial assessing the effect on flu-like symptoms and immunomodulatory properties. BMC Neurol. 2013, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Mrad, M.F.; El Ayoubi, N.K.; Esmerian, M.O.; Kazan, J.M.; Khoury, S.J. Effect of vitamin D replacement on immunological biomarkers in patients with multiple sclerosis. Clin. Immunol. 2017, 181, 9–15. [Google Scholar] [CrossRef]
- Åivo, J.; Hänninen, A.; Ilonen, J.; Soilu-Hänninen, M. Vitamin D3 administration to MS patients leads to increased serum levels of latency activated peptide (LAP) of TGF-beta. J. Neuroimmunol. 2015, 280, 12–15. [Google Scholar] [CrossRef]
- O’Connell, K.; Sulaimani, J.; Basdeo, S.A.; Kinsella, K.; Jordan, S.; Kenny, O.; Kelly, S.B.; Murphy, D.; Heffernan, E.; Killeen, R.P.; et al. Effects of vitamin D3 in clinically isolated syndrome and healthy control participants: A double-blind randomised controlled trial. Mult. Scler. J. Exp. Transl. Clin. 2017, 3, 2055217317727296. [Google Scholar] [CrossRef]
- Naghavi Gargari, B.; Behmanesh, M.; Shirvani Farsani, Z.; Pahlevan Kakhki, M.; Azimi, A.R. Vitamin D Supplementation Up-Regulates IL-6 and IL-17A Gene Expression in Multiple Sclerosis Patients. Int. Immunopharmacol. 2015, 28, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Sotirchos, E.S.; Bhargava, P.; Eckstein, C.; Van Haren, K.; Baynes, M.; Ntranos, A.; Gocke, A.; Steinman, L.; Mowry, E.M.; Calabresi, P.A. Safety and Immunologic Effects of High- vs Low-Dose Cholecalciferol in Multiple Sclerosis. Neurology 2016, 86, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Mahon, B.D.; Gordon, S.A.; Cruz, J.; Cosman, F.; Cantorna, M.T. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J. Neuroimmunol. 2003, 134, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Muris, A.H.; Smolders, J.; Rolf, L.; Thewissen, M.; Hupperts, R.; Damoiseaux, J. SOLARIUM study group. Immune regulatory effects of high dose vitamin D3 supplementation in a randomized controlled trial in relapsing remitting multiple sclerosis patients receiving IFNβ; the SOLARIUM study. J. Neuroimmunol. 2016, 300, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Rolf, L.; Muris, A.H.; Bol, Y.; Damoiseaux, J.; Smolders, J.; Hupperts, R. Vitamin D3 supplementation in multiple sclerosis: Symptoms and biomarkers of depression. J. Neurol. Sci. 2017, 378, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Akgün, K.; Proschmann, U.; Sellner, J.; Ziemssen, T. The role of TH17 cells in multiple sclerosis: Therapeutic implications. Autoimmun. Rev. 2020, 19, 102647. [Google Scholar] [CrossRef]
- Waisman, A.; Hauptmann, J.; Regen, T. The role of IL-17 in CNS diseases. Acta Neuropathol. 2015, 129, 625–637. [Google Scholar] [CrossRef]
- Brucklacher-Waldert, V.; Sturner, K.; Kolster, M.; Wolthausen, J.; Tolosa, E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain 2009, 132, 3329–3341. [Google Scholar] [CrossRef]
- Toghianifar, N.; Ashtari, F.; Zarkesh-Esfahani, S.H.; Mansourian, M. Effect of high dose vitamin D intake on interleukin-17 levels in multiple sclerosis: A randomized, double-blind, placebo-controlled clinical trial. J. Neuroimmunol. 2015, 285, 125–128. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Krei, K.; Fredrikson, S.; Fontana, A.; Link, H. Interleukin-6 is elevated in plasma in multiple sclerosis. J. Neuroimmunol. 1991, 31, 147–153. [Google Scholar] [CrossRef]
- Wullschleger, A.; Kapina, V.; Molnarfi, N.; Courvoisier, D.S.; Seebach, J.D.; Santiago-Raber, M.L.; Hochstrasser, D.F.; Lalive, P.H. Cerebrospinal fluid interleukin-6 in central nervous system inflammatory diseases. PLoS ONE 2013, 8, e72399. [Google Scholar] [CrossRef] [PubMed]
- Maimone, D.; Guazzi, G.C.; Annunziata, P. IL-6 detection in multiple sclerosis brain. J. Neurol. Sci. 1997, 146, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.P.; Matias, I.; Siqueira, M.; Stipursky, J.; Gomes, F.C. Astrocytes and the TGF-β1 Pathway in the Healthy and Diseased Brain: A Double-Edged Sword. Mol. Neurobiol. 2019, 56, 4653–4679. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Tan, N.B.; Howell, K.B.; Barresi, S.; Freeman, J.L.; Vecchio, D.; Piccione, M.; Radio, F.C.; Calame, D.; Zong, S.; et al. Bi-allelic LoF NRROS Variants Impairing Active TGF-β1 Delivery Cause a Severe Infantile-Onset Neurodegenerative Condition with Intracranial Calcification. Am. J. Hum. Genet. 2020, 106, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Huter, E.N.; Stummvoll, G.H.; DiPaolo, R.J.; Glass, D.D.; Shevach, E.M. Cutting edge: Antigen-specific TGF beta-induced regulatory T cells suppress Th17-mediated autoimmune disease. J. Immunol. 2008, 181, 8209–8213. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Y.; Jiang, H.; Sun, M.; Gao, J.; Xie, A. TGF-β in Mice Ameliorates Experimental Autoimmune Encephalomyelitis in Regulating NK Cell Activity. Cell Transplant. 2019, 28, 1155–1160. [Google Scholar] [CrossRef]
- Carrieri, P.B.; Provitera, V.; Bruno, R.; Perrella, M.; Tartaglia, G.; Busto, A.; Perrella, O. Possible role of transforming growth factor-beta in relapsing-remitting multiple sclerosis. Neurol. Res. 1997, 19, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Woodward, W.D.; Hayes, C.E.; DeLuca, H.F. 1,25-dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-beta 1 and IL-4. J. Immunol. 1998, 160, 5314–5319. [Google Scholar] [CrossRef] [PubMed]
- Hawes, J.E.; Tesic, D.; Whitehouse, A.J.; Zosky, G.R.; Smith, J.T.; Wyrwoll, C.S. Maternal vitamin D deficiency alters fetal brain development in the BALB/c mouse. Behav. Brain Res. 2015, 286, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Shirvani-Farsani, Z.; Behmanesh, M.; Mohammadi, S.M.; Naser Moghadasi, A. Vitamin D levels in multiple sclerosis patients: Association with TGF-β2, TGF-βRI, and TGF-βRII expression. Life Sci. 2015, 134, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ros, A.; Martínez-Ginés, M.L.; García-Domínguez, J.M.; Salvador-Martín, S.; Goicochea-Briceño, H.; Cuello, J.P.; Meldaña-Rivera, A.; Higueras-Hernández, Y.; Sanjurjo-Sáez, M.; Álvarez-Sala-Walther, L.A.; et al. Changes in the Expression of TGF-Beta Regulatory Pathway Genes Induced by Vitamin D in Patients with Relapsing-Remitting Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 14447. [Google Scholar] [CrossRef]
- Arellano, G.; Ottum, P.A.; Reyes, L.I.; Burgos, P.I.; Naves, R. Stage-Specific Role of Interferon-Gamma in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. Front. Immunol. 2015, 6, 492. [Google Scholar] [CrossRef] [PubMed]
- Galoppin, M.; Kari, S.; Soldati, S.; Pal, A.; Rival, M.; Engelhardt, B.; Astier, A.; Thouvenot, E. Full spectrum of vitamin D immunomodulation in multiple sclerosis: Mechanisms and therapeutic implications. Brain Commun. 2022, 4, fcac171. [Google Scholar] [CrossRef]
- Rolf, L.; Muris, A.H.; Theunissen, R.; Hupperts, R.; Damoiseaux, J.; Smolders, J. Vitamin D3 supplementation and the IL-2/IL-2R pathway in multiple sclerosis: Attenuation of progressive disturbances? J. Neuroimmunol. 2018, 314, 50–57. [Google Scholar] [CrossRef]
- de la Rubia Ortí, J.E.; García, M.F.; Drehmer, E.; Navarro-Illana, E.; Casani-Cubel, J.; Proaño, B.; Sanchis-Sanchis, C.E.; Escrivá, J.D. Intake of Vitamin D in Patients with Multiple Sclerosis in the Valencian Region and Its Possible Relationship with the Pathogenesis of the Disease. Life 2021, 11, 1380. [Google Scholar] [CrossRef]
- Niino, M.; Fukazawa, T.; Miyazaki, Y.; Takahashi, E.; Minami, N.; Amino, I.; Fujiki, N.; Doi, S.; Kikuchi, S. Suppression of IL-10 production by calcitriol in patients with multiple sclerosis. J. Neuroimmunol. 2014, 270, 86–94. [Google Scholar] [CrossRef]
- Cipriani, C.; Romagnoli, E.; Pepe, J.; Russo, S.; Carlucci, L.; Piemonte, S.; Nieddu, L.; McMahon, D.J.; Singh, R.; Minisola, S. Long-term bioavailability after a single oral or intramuscular administration of 600,000 IU of ergocalciferol or cholecalciferol: Implications for treatment and prophylaxis. J. Clin. Endocrinol. Metab. 2013, 98, 2709–2715. [Google Scholar] [CrossRef]
- Nicoletti, F.; Di Marco, R.; Patti, F.; Zaccone, P.; L’Episcopo, M.R.; Reggio, E.; Xiang, M.; Nicoletti, A.; Reggio, A. Short term treatment of relapsing remitting multiple sclerosis patients with interferon (IFN)-β1B transiently increases the blood levels of interleukin (IL)-6, IL-10 and IFN-γ without significantly modifying those of IL-1β, IL-2, IL-4 and tumour necrosis factor-α. Cytokine 2000, 12, 682–687. [Google Scholar]
- Waschbisch, A.; Sanderson, N.; Krumbholz, M.; Vlad, G.; Theil, D.; Schwab, S.; Mäurer, M.; Derfuss, T. Interferon beta and vitamin D synergize to induce immunoregulatory receptors on peripheral blood monocytes of multiple sclerosis patients. PLoS ONE 2014, 9, e115488. [Google Scholar] [CrossRef]
- Al-Otaibi, K.M.; Alghamdi, B.S.; Al-Ghamdi, M.A.; Mansouri, R.A.; Ashraf, G.M.; Omar, U.M. Therapeutic effect of combination vitamin D3 and siponimod on remyelination and modulate microglia activation in cuprizone mouse model of multiple sclerosis. Front. Behav. Neurosci. 2023, 16, 1068736. [Google Scholar] [CrossRef] [PubMed]
- Laursen, J.H.; Søndergaard, H.B.; Sørensen, P.S.; Sellebjerg, F.; Oturai, A.B. Vitamin D supplementation reduces relapse rate in relapsing-remitting multiple sclerosis patients treated with natalizumab. Mult. Scler. Relat. Disord. 2016, 10, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Tzartos, J.S.; Friese, M.A.; Craner, M.J.; Palace, J.; Newcombe, J.; Esiri, M.M.; Fugger, L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008, 172, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Amsen, D.; de Visser, K.E.; Town, T. Approaches to determine expression of inflammatory cytokines. Methods Mol. Biol. 2009, 511, 107–142. [Google Scholar] [CrossRef] [PubMed]
- Favre, N.; Bordmann, G.; Rudin, W. Comparison of cytokine measurements using ELISA, ELISPOT and semi-quantitative RT-PCR. J. Immunol. Methods 1997, 204, 57–66. [Google Scholar] [CrossRef]
- Rolf, L.; Smolders, J.; van den Ouweland, J.; Hupperts, R.; Damoiseaux, J. Correlation of different cellular assays to analyze T cell-related cytokine profiles in vitamin D3-supplemented patients with multiple sclerosis. Mol. Immunol. 2019, 105, 198–204. [Google Scholar] [CrossRef]
- Muris, A.H.; Damoiseaux, J.; Smolders, J.; Cohen Tervaert, J.W.; Hupperts, R.; Thewissen, M. Intracellular IL-10 detection in T cells by flowcytometry: The use of protein transport inhibitors revisited. J. Immunol. Methods. 2012, 381, 59–65. [Google Scholar] [CrossRef]
- Schuerwegh, A.J.; Stevens, W.J.; Bridts, C.H.; De Clerck, L.S. Evaluation of monensin and brefeldin A for flow cytometric determination of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha in monocytes. Cytometry 2001, 46, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Mandala, W.; Harawa, V.; Munyenyembe, A.; Soko, M.; Longwe, H. Optimization of stimulation and staining conditions for intracellular cytokine staining (ICS) for determination of cytokine-producing T cells and monocytes. Curr. Res. Immunol. 2021, 2, 184–193. [Google Scholar] [CrossRef] [PubMed]
- da Costa, D.S.; Hygino, J.; Ferreira, T.B.; Kasahara, T.M.; Barros, P.O.; Monteiro, C.; Oliveira, A.; Tavares, F.; Vasconcelos, C.C.; Alvarenga, R.; et al. Vitamin D modulates different IL-17-secreting T cell subsets in multiple sclerosis patients. J. Neuroimmunol. 2016, 299, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Spach, K.M.; Nashold, F.E.; Dittel, B.N.; Hayes, C.E. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J. Immunol. 2006, 177, 6030–6037. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Bonavita, S.; Sparaco, M.; Gallo, A.; Tedeschi, G. The role of diet in multiple sclerosis: A review. Nutr. Neurosci. 2018, 21, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Sparaco, M.; Maniscalco, G.T.; Signoriello, E.; Lanzillo, R.; Russo, C.; Carmisciano, L.; Cepparulo, S.; Lavorgna, L.; Gallo, A.; et al. Lifestyle and Mediterranean diet adherence in a cohort of Southern Italian patients with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2021, 47, 102636. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Takacs, I.; Boyanov, M.; Belaya, Z.; Diaconu, C.C.; Mokhort, T.; Zherdova, N.; Rasa, I.; Payer, J.; Pilz, S. Clinical Practice in the Prevention, Diagnosis and Treatment of Vitamin D Deficiency: A Central and Eastern European Expert Consensus Statement. Nutrients 2022, 14, 1483. [Google Scholar] [CrossRef] [PubMed]
| Cytokine | Study | Vitamin D Treatment | Results | Note | ||
|---|---|---|---|---|---|---|
| Dosage | Duration | Route of Administration | ||||
| IL-10 | Walawska-Hrycek, 2021 [33] | 500 to 1000 UI/die | 12 months | oral | ↑ | |
| Smolders, 2010 [34] | 20,000 IU/die | 12 weeks | oral | ↑ | ||
| Ashtari, 2015 [35] | 50,000 IU/5 days | 3 months | oral | ↑ | ||
| Shirvani-Farsani, 2015 [36] | 50,000 IU/week | 8 weeks | i.m. | ↑ | ||
| Hashemi, 2018 [37] | 50,000 IU/week | 8 weeks | oral | ↑ | ||
| Hashemi, 2020 [38] | 50,000 IU/week | 8 weeks | oral | ↑ | ||
| Mosayebi, 2011 [39] | 300,000 IU every month | 6 months | i.m. | ↑ | ||
| Golan, 2013 [40] | 800 IU daily vs 800 IU daily + 75,000 UI every 3 weeks | 12 months | oral | ↔ | ||
| Mrad, 2017 [41] | 10,000 IU/die | 12 weeks | oral | ↔ | ||
| Åivo, 2015 [42] | 20,000 IU/week | 12 months | oral | ↔ | ||
| IL-17 | Walawska-Hrycek, 2021 [33] | 500 to 1000 UI/die | 12 months | oral | ↔ | |
| Smolders, 2008 [34] | 20,000 IU/die | 12 weeks | oral | ↔ | ||
| O’Connell, 2017 [43] | 5000–10,000 IU/die | 24 weeks | oral | ↔ | ||
| Mrad, 2017 [41] | 10,000 IU/die for | 12 weeks | oral | ↔ | ||
| Naghavi Gargari, 2015 [44] | 50,000 IU/week | 2 months | oral | ↑ | ||
| Golan, 2013 [40] | 800 IU/die vs 800 IU/die + 75,000 UI every 3 weeks | 12 months | oral | ↑ | Only in the low-dose group after 3 months | |
| Åivo, 2015 [42] | 20,000 IU/week | 12 months | oral | ↑ * | ||
| Hashemi, 2018 [37] | 50,000 IU/week | 8 weeks | oral | ↓ | ||
| Hashemi, 2020 [38] | 50,000 IU/week | 8 weeks | oral | ↓ | ||
| Sotirchos, 2016 [45] | 10,400 IU or 800 IU daily | 6 months | n.s. | ↓ | Only in the group treated with high-dose | |
| IL-6 | Naghavi Gargari, 2015 [44] | 50,000 IU/ week | 2 months | oral | ↑ | |
| Åivo, 2015 [42] | 20,000 IU/week | 12 months | oral | ↔ | ||
| Hashemi, 2018 [37] | 50,000 IU/week | 8 weeks | oral | ↓ | ||
| Hashemi, 2020 [38] | 50,000 IU/week | 8 weeks | oral | ↓ | ||
| TGF-β1 | Hashemi, 2020 [38] | 50,000 IU/week | 8 weeks | oral | ↑ | |
| Mahon, 2003 [46] | 1000 IU/die | 6 months | oral | ↑ | ||
| Walawska-Hrycek, 2021 [33] | 500 to 1000 UI/die | 12 months | oral | ↑ | ||
| Mosayebi, 2011 [39] | 300,000 IU every month | 6 months | i.m. | ↑ | ||
| Åivo, 2015 [42] | 20,000 IU/week | 12 months | oral | ↑ | ||
| Muris, 2016 [47] | 7000 IU/die for 4 weeks, followed by 14,000 IU/die | 48 weeks | oral | ↔ | ||
| Shirvani-Farsani, 2015 [36] | 50,000 IU/week | 8 weeks | i.m. | ↔ | ||
| IFN-γ | Mahon, 2003 [46] | 1000 IU/die | 6 months | oral | ↔ | |
| Åivo, 2015 [42] | 20,000 IU/week | 12 months | oral | ↔ | ||
| Mosayebi, 2011 [39] | 300,000 IU every month | 6 months | i.m. | ↔ | ||
| O’Connell, 2017 [43] | 5000–10,000 IU/die | 24 weeks | oral | ↔ | ||
| Golan, 2013 [40] | 800 IU/die vs. 800 IU/die + 75,000 UI every 3 weeks | 12 months | oral | ↔ | ||
| Mrad, 2017 [41] | 10,000 IU/die | 12 weeks | oral | ↓ | ||
| Walawska-Hrycek, 2021 [33] | 500 to 1000 UI/die | 12 months | oral | ↑ | ||
| Study | Country | Sample Size | Sex (F/M) | Age (Years) | Disease Duration | EDSS at Baseline | Phenotype of MS | ARR | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Walawska-Hrycek, 2021 [33] | Poland | 44 | 33/11 | 38.4 mn | 5.6 ys mn | 1.5 mdn | RR | n.i. | ||
| Smolders, 2008 [34] | The Netherlands | 15 | 8/7 | 35.15 mdn | 3.47 ys mdn | 2.0 mdn | RR | 1.0 mdn | ||
| Ashtari, 2015 [35] | Iran | DG | 44 | 35/9 | 31.5 mn | 4.10 ys mn | 1.70 mn | RR | 0.75 mn | |
| PlG | 45 | 40/5 | 34.6 mn | 4.46 ys mn | 2.09 mn | 0.83 mn | ||||
| Shirvani-Farsani, 2015 [36] | Iran | DG | 32 | 27/5 | 29.78 (F) mn 37.4 (M) mn | 6.40 ys mn | 2.12 mn | RR | n.i. | |
| CG | 32 | 23/9 | 28.6 (F) mn 27.57 (M) mn | - | - | - | ||||
| Hashemi, 2018 [37] | Iran | MSP | 25 | 21/4 | 32.6 mn | 8.1 ys mn | n.i | n.i | n.i | |
| RP | 25 | 17/8 | 27.4 mn | - | ||||||
| CG | 25 | 20/5 | 31.7 mn | - | ||||||
| Hashemi, 2020 [38] | Iran | MSP | 25 | 21/4 | 32.6 mn | 8.1 ys mn | n.i | n.i | n.i | |
| RP | 25 | 17/8 | 27.4 mn | - | ||||||
| CG | 25 | 20/5 | 31.7 mn | - | ||||||
| Mosayebi, 2011 [39] | Iran | DG | 26 | 17/9 | 37 mn | 4.15 ys mn | 2.1 mn | RR | n.i. # | |
| CG | 33 | 25/8 | 35 mn | 6.4 ys mn | 2.5 mn | - | ||||
| Golan, 2013 [40] | Israel | LDG | 21 | 13/8 | 44.7 mn | 9.3 ys mn | 3.6 mn | RR | 0.38 mn | |
| HDG | 24 | 19/5 | 43.1 mn | 6 ys mn | 2.9 mn | RR | 0.28 mn | |||
| Mrad, 2017 [41] | Lebanon | 46 | 24/22 | 34.6 mn | 3.8 mn | 1 | RR | n.i. | ||
| Åivo, 2015 [42] | Finland | DG | 30 | 18/12 | 38 mdn | 3 ys mdn | 2.0 mdn | RR | 0.5 mdn | |
| PlG | 29 | 19/10 | 35 mdn | 2 ys mdn | 1.5 mdn | RR | 0.5 mdn | |||
| Naghavi Gargari, 2015 [44] | Iran | 32 | 26/6 | 30.68 mn | 5.65 ys mn | 2.21 mn | RR | n.i. | ||
| Sotirchos, 2016 [45] | USA | HDG | 19 | 14/5 | 41.3 mn | 8.2 ys mn | 3 mdn | RR | n.i. | |
| LDG | 21 | 14/7 | 38.8 mn | 7.8 ys mn | 2 mdn | RR | ||||
| Mahon, 2003 [46] | USA | 39 | n.i. | n.i. | n.i. | n.i. | n.i./ | n.i. | ||
| Muris, 2016 [47] | The Netherlands | DG | 30 | 21/9 | 37.7 | 7.3 mts | ≤3.5:22 4–5.5:1 | RR | 1.26 * | |
| PlG | 23 | 14/9 | 37.2 | 7.3 mts | ≤3.5:28 4–5.5:2 | RR | 1.67 * | |||
| O’Connell, 2017 [43] | Ireland | CIS | HDG | 12 | 8/4 | 37.2 mn | - | 0.9 | CIS | |
| LDG | 10 | 5/5 | 32.7 mn | 0.9 | ||||||
| PlG | 7 | 6/1 | 34.3 mn | 0.4 | ||||||
| Ctr | HDG | 13 | 6/7 | 30.5 mn | - | |||||
| LDG | 13 | 10/3 | 30.3 mn | |||||||
| PlG | 12 | 10/2 | 29.1 mn | |||||||
| Study | Method |
|---|---|
| Walawska-Hrycek, 2021 [33] | ELISA |
| Smolders, 2008 [34] | antiCD3 iFACS (PMA + iono/mone) |
| Ashtari, 2015 [35] | |
| Shirvani-Farsani, 2015 [36] | PCR |
| Hashemi, 2018 [37] | PCR |
| Hashemi, 2020 [38] | PCR |
| Mosayebi, 2011 [39] | ELISA |
| Golan, 2013 [40] | ELISA |
| Mrad, 2017 [41] | antiCD3/antiCD28 CBA iFACs (PMA + iono/BA) |
| Åivo, 2015 [42] | FBA |
| O’Connell, 2017 [43] | antiCD3 ELISA iFACs (PMA + iono/BA) |
| Naghavi Gargari, 2015 [44] | PCR |
| Sotirchos, 2016 [45] | antiCD3/antiCD28 iFACs (PMA + iono/BA-mone) |
| Manhor, 2003 [46] | PCR |
| Muris, 2016 [47] | antiCD3 iFACs -PMA + iono/BA -> B-cell -PMA/mone -> T cell (no mone for IL-10) Multiple immunoassay |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparaco, M.; Bonavita, S. Vitamin D Supplementation: Effect on Cytokine Profile in Multiple Sclerosis. J. Clin. Med. 2024, 13, 835. https://doi.org/10.3390/jcm13030835
Sparaco M, Bonavita S. Vitamin D Supplementation: Effect on Cytokine Profile in Multiple Sclerosis. Journal of Clinical Medicine. 2024; 13(3):835. https://doi.org/10.3390/jcm13030835
Chicago/Turabian StyleSparaco, Maddalena, and Simona Bonavita. 2024. "Vitamin D Supplementation: Effect on Cytokine Profile in Multiple Sclerosis" Journal of Clinical Medicine 13, no. 3: 835. https://doi.org/10.3390/jcm13030835
APA StyleSparaco, M., & Bonavita, S. (2024). Vitamin D Supplementation: Effect on Cytokine Profile in Multiple Sclerosis. Journal of Clinical Medicine, 13(3), 835. https://doi.org/10.3390/jcm13030835







