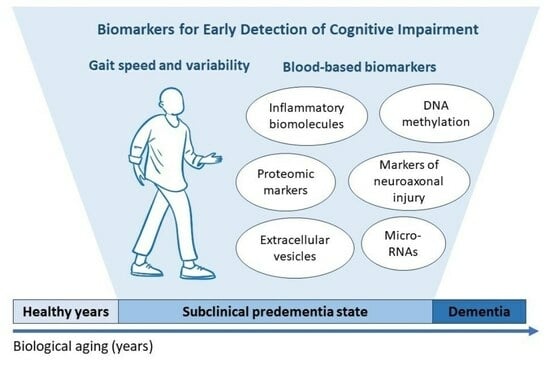
Biological and Physical Performance Markers for Early Detection of Cognitive Impairment in Older Adults
Abstract
1. Introduction
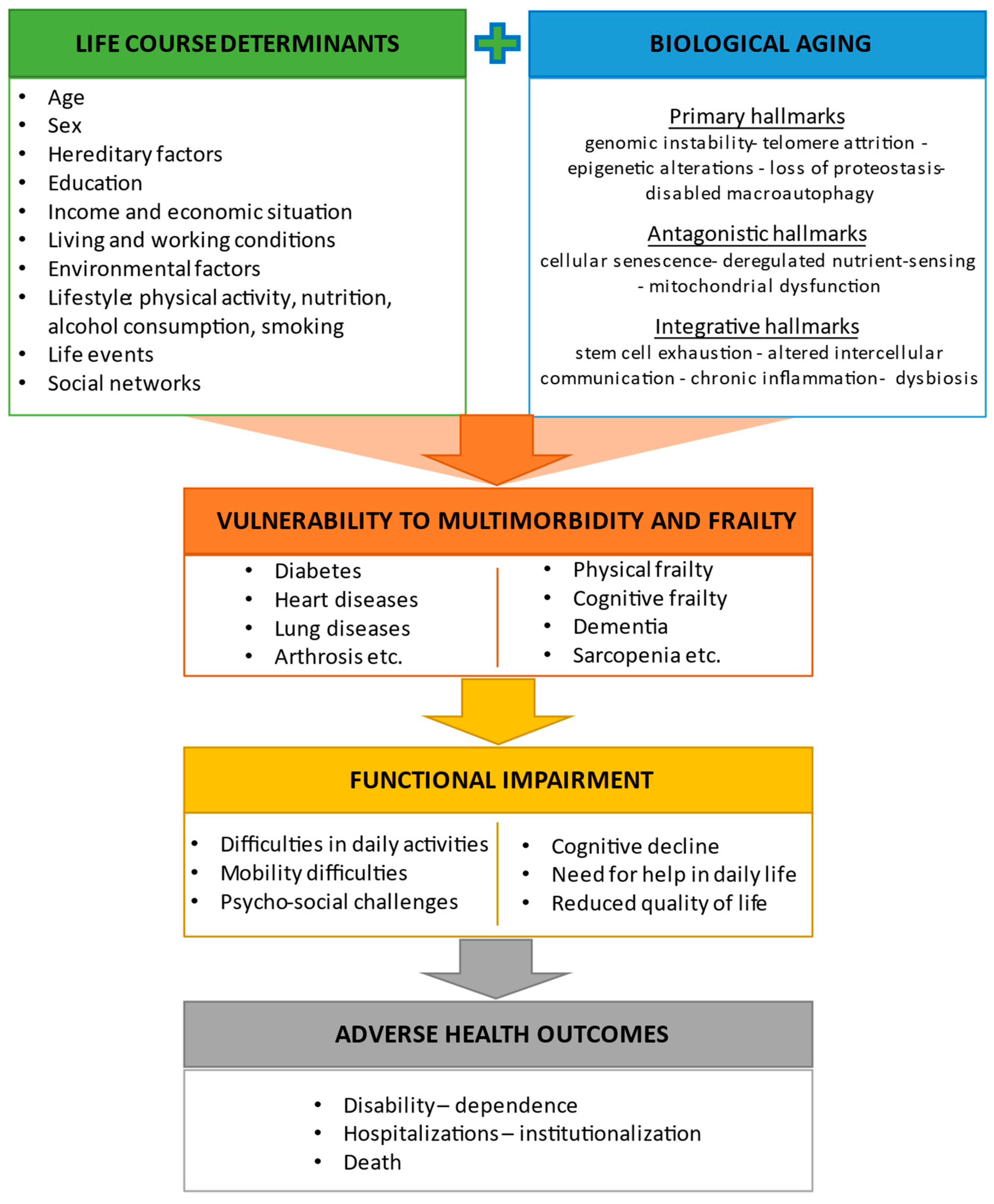
2. Biological Aging and Its Relationship with Physical and Cognitive Frailty
2.1. Hallmarks of Aging and Their Interaction with Life Course Determinants
2.2. Frailty, Cognitive Frailty, and Other Predementia Syndromes
3. Biomarkers of Aging Associated with Cognitive Frailty or Cognitive Decline
3.1. Inflammatory Markers
3.2. Markers of Neurodegeneration
3.3. “Next-Generation” Biomarkers
3.4. Proteomic Biomarkers
4. Frailty and Physical Performance as Predictors of Cognitive Decline
4.1. Frailty
4.2. Gait Speed, Stride-to-Stride Variability, and Gait Speed under Dual-Task Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beard, J.R.; Bloom, D.E. Towards a comprehensive public health response to population ageing. Lancet 2015, 385, 658–661. [Google Scholar] [CrossRef]
- Roller-Wirnsberger, R.; Thurner, B.; Pucher, C.; Lindner, S.; Wirnsberger, G.H. The clinical and therapeutic challenge of treating older patients in clinical practice. Brit. J. Clin. Pharmacol. 2020, 86, 1904–1911. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Belsky, D.W.; Perls, T.T. Quantification and analysis of biological aging: Genetic, genomic, and biomarker geroscience tools. Innov. Aging 2017, 1, 56–57. [Google Scholar] [CrossRef]
- Khan, S.S.; Singer, B.D.; Vaughan, D.E. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell 2017, 16, 624–633. [Google Scholar] [CrossRef]
- Sarkeala, T.; Nummi, T.; Vuorisalmi, M.; Hervonen, A.; Jylhä, M. Disability trends among nonagenarians in 2001–2007: Vitality 90+ Study. Eur. J. Ageing 2011, 8, 87–94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef]
- Nusselder, W.J.; Looman, C.W.N.; Mackenbach, J.P. The level and time course of disability: Trajectories of disability in adults and young elderly. Disabil. Rehabil. 2006, 28, 1015–1026. [Google Scholar] [CrossRef]
- Gill, T.M.; Gahbauer, E.A.; Han, L.; Allore, H.G. Trajectories of disability in the last year of life. N. Engl. J. Med. 2010, 362, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Fried, T.R.; Bradley, E.H.; Williams, C.S.; Tinetti, M.E. Functional disability and health care expenditures for older persons. Arch. Intern. Med. 2001, 161, 2602–2607. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Disability 2011; World Health Organization: Geneva, Switzerland, 2011; ISBN 92-4-068800-5. [Google Scholar]
- Chowdhary, N.; Barbui, C.; Anstey, K.J.; Kivipelto, M.; Barbera, M.; Peters, R.; Zheng, L.; Kulmala, J.; Stephen, R.; Ferri, C.P.; et al. Reducing the risk of cognitive decline and dementia: WHO recommendations. Front. Neurol. 2022, 12, 765584. [Google Scholar] [CrossRef] [PubMed]
- Dua, T.; Seeher, K.M.; Sivananthan, S.; Chowdhary, N.; Pot, A.M.; Saxena, S. World Health Organization’s global action plan on the public health response to dementia 2017–2025. Alzheimers Dement. 2017, 13, P1450–P1451. [Google Scholar] [CrossRef]
- Mebane-Sims, I. 2009 Alzheimer’s disease facts and figures. Alzheimers Dement. 2009, 5, 234–270. [Google Scholar] [CrossRef]
- Kivipelto, M.; Mangialasche, F.; Snyder, H.M.; Allegri, R.; Andrieu, S.; Arai, H.; Baker, L.; Belleville, S.; Brodaty, H.; Brucki, S.M.; et al. World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020, 16, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Price, G.; Udeh-Momoh, C.; Kivipelto, M.; Middleton, L.T. Editorial: Dementia prevention: A global challenge in urgent need of solutions. J. Prev. Alzheimers Dis. 2022, 9, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Sierra, F.; Kohanski, R. Geroscience and the trans-NIH Geroscience Interest Group, GSIG. Geroscience 2017, 39, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef]
- Tchkonia, T.; Palmer, A.K.; Kirkland, J.L. New horizons: Novel approaches to enhance healthspan through targeting cellular senescence and related aging mechanisms. J. Clin. Endocrinol. Metab. 2021, 106, E1481–E1487. [Google Scholar] [CrossRef]
- Riessland, M.; Orr, M.E. Translating the biology of aging into new therapeutics for Alzheimer’s disease: Senolytics. J. Prev. Alzheimers Dis. 2023, 10, 633–646. [Google Scholar] [CrossRef]
- Cummings, J.L.; Osse, A.M.L.; Kinney, J.W. Alzheimer’s disease: Novel targets and investigational drugs for disease modification. Drugs 2023, 83, 1387–1408. [Google Scholar] [CrossRef]
- Cohen, J.A.; Verghese, J.; Zwerling, J.L. Cognition and gait in older people. Maturitas 2016, 93, 73–77. [Google Scholar] [CrossRef]
- Vos, W.H.; Boekel, L.C.; Janssen, M.M.; Leenders, R.T.A.J.; Luijkx, K.G. Exploring the impact of social network change: Experiences of older adults ageing in place. Health Soc. Care Comm. 2020, 28, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Wernher, I.; Lipsky, M.S. Psychological theories of aging. Dis. Mon. 2015, 61, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Witkowski, J.M.; McElhaney, J.; Loeb, M.; Mitnitski, A.; Pawelec, G. Aging, frailty and age-related diseases. Biogerontology 2010, 11, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Plagg, B.; Zerbe, S. How does the environment affect human ageing? An interdisciplinary review. J. Gerontol. Geriatr. 2020, 69, 53–67. [Google Scholar] [CrossRef]
- Bachmann, M.C.; Bellalta, S.; Basoalto, R.; Gómez-Valenzuela, F.; Jalil, Y.; Lépez, M.; Matamoros, A.; von Bernhardi, R. The challenge by multiple environmental and biological factors induce inflammation in aging: Their role in the promotion of chronic disease. Front. Immunol. 2020, 11, 570083. [Google Scholar] [CrossRef]
- Langevin, H.M.; Weber, W.; Chen, W. Integrated multicomponent interventions to support healthy aging of the whole person. Aging Cell 2024, 23, e14001. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Milagro, F.I.; Riezu-Boj, J.I.; Martinez, J.A. Epigenetic signatures underlying inflammation: An interplay of nutrition, physical activity, metabolic diseases, and environmental factors for personalized nutrition. Inflamm. Res. 2021, 70, 29–49. [Google Scholar] [CrossRef]
- Vermeiren, S.; Vella-Azzopardi, R.; Beckwée, D.; Habbig, A.-K.; Scafoglieri, A.; Jansen, B.; Bautmans, I. Frailty and the prediction of negative health outcomes: A meta-analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1163.e1–1163.e17. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty measurement in research and clinical practice: A review. Eur. J. Intern. Med. 2016, 31, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.M.; Gahbauer, E.A.; Allore, H.G.; Han, L. Transitions between frailty states among community-living older persons. Arch. Intern. Med. 2006, 166, 418–423. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Galluzzo, L.; Rodríguez-Laso, Á.; Van Der Heyden, J.; Ranhoff, A.H.; Carcaillon-Bentata, L.; Beltzer, N.; Kennelly, S.; Liew, A. Transitions and trajectories in frailty states over time: A systematic review of the European Joint Action ADVANTAGE. Ann. Ist. 2018, 54, 246–252. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. Can. Med. Assoc. J. 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Sargent, L.; Nalls, M.; Starkweather, A.; Hobgood, S.; Thompson, H.; Amella, E.J.; Singleton, A. Shared biological pathways for frailty and cognitive impairment: A systematic review. Ageing Res. Rev. 2018, 47, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; van Kan, G.A.; Ousset, P.-J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J. Nutr. Health Aging 2013, 17, 726–734. [Google Scholar] [CrossRef]
- Verghese, J.; Annweiler, C.; Ayers, E.; Barzilai, N.; Beauchet, O.; Bennett, D.A.; Bridenbaugh, S.A.; Buchman, A.S.; Callisaya, M.L.; Camicioli, R.; et al. Motoric cognitive risk syndrome: Multicountry prevalence and dementia risk. Neurology 2014, 83, 718–726. [Google Scholar] [CrossRef]
- Mullin, D.S.; Cockburn, A.; Welstead, M.; Luciano, M.; Russ, T.C.; Muniz-Terrera, G. Mechanisms of motoric cognitive risk—Hypotheses based on a systematic review and meta-analysis of longitudinal cohort studies of older adults. Alzheimers Dement. 2022, 18, 2413–2427. [Google Scholar] [CrossRef]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J. Am. Geriatr. Soc. 2010, 58, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-P.; Lee, W.-J.; Peng, L.-N.; Shimada, H.; Tsai, T.-F.; Lin, C.-P.; Arai, H.; Chen, L.-K. Physio-cognitive decline syndrome as the phenotype and treatment target of unhealthy aging. J. Nutr. Health Aging 2021, 25, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-J.; Peng, L.-N.; Lin, M.-H.; Loh, C.-H.; Chung, C.-P.; Wang, P.-N.; Chen, L.-K. Six-year transition of physio-cognitive decline syndrome: Results from I-Lan Longitudinal Aging Study. Arch. Gerontol. Geriat. 2022, 102, 104743. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Wang, C.; Lipton, R.B.; Holtzer, R. Motoric cognitive risk syndrome and the risk of dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Godbout, J.P.; Johnson, R.W. Age and neuroinflammation: A lifetime of psychoneuroimmune consequences. Immunol. Allergy Clin. N. Am. 2009, 29, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.W.; Godbout, J.P. Aging, neuroinflammation, and behavior. In Psychoneuroimmunology, 4th ed.; Ader, R., Ed.; Academic Press: Oxford, MI, USA, 2007; Volume 1, pp. 379–391. [Google Scholar]
- Bai, A.; Shi, H.; Huang, X.; Xu, W.; Deng, Y. Association of C-reactive protein and motoric cognitive risk syndrome in community-dwelling older adults: The China Health and Retirement Longitudinal Study. J. Nutr. Health Aging 2021, 25, 1090–1095. [Google Scholar] [CrossRef]
- Adriaensen, W.; Matheï, C.; van Pottelbergh, G.; Vaes, B.; Legrand, D.; Wallemacq, P.; Degryse, J.-M. Significance of serum immune markers in identification of global functional impairment in the oldest old: Cross-sectional results from the BELFRAIL study. Age 2014, 36, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Diniz, B.S.; Lima-Costa, M.F.; Peixoto, S.V.; Firmo, J.O.A.; Torres, K.C.L.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Grady, J.; Kuchel, G.A.; Castro-Costa, E. Cognitive frailty is associated with elevated proinflammatory markers and a higher risk of mortality. Am. J. Geriatr. Psychiatry 2022, 30, 825–833. [Google Scholar] [CrossRef]
- Bortone, I.; Griseta, C.; Battista, P.; Castellana, F.; Lampignano, L.; Zupo, R.; Sborgia, G.; Lozupone, M.; Moretti, B.; Giannelli, G.; et al. Physical and cognitive profiles in motoric cognitive risk syndrome in an older population from Southern Italy. Eur. J. Neurol. 2021, 28, 2565–2573. [Google Scholar] [CrossRef]
- Groeger, J.L.; Ayers, E.; Barzilai, N.; Beauchet, O.; Callisaya, M.; Torossian, M.R.; Derby, C.; Doi, T.; Lipton, R.B.; Milman, S.; et al. Inflammatory biomarkers and motoric cognitive risk syndrome: Multicohort survey. Cereb. Circ. Cogn. Behav. 2022, 3, 100151. [Google Scholar] [CrossRef]
- Kochlik, B.; Herpich, C.; Moreno-Villanueva, M.; Klaus, S.; Müller-Werdan, U.; Weinberger, B.; Fiegl, S.; Toussaint, O.; Debacq-Chainiaux, F.; Schön, C.; et al. Associations of circulating GDF15 with combined cognitive frailty and depression in older adults of the MARK-AGE study. GeroScience 2023, online ahead of print. [Google Scholar] [CrossRef]
- Merchant, R.A.; Chan, Y.H.; Anbarasan, D.; Aprahamian, I. Association of motoric cognitive risk syndrome with sarcopenia and systemic inflammation in pre-frail older adults. Brain Sci. 2023, 13, 936. [Google Scholar] [CrossRef] [PubMed]
- Sathyan, S.; Barzilai, N.; Atzmon, G.; Milman, S.; Ayers, E.; Verghese, J. Association of anti-inflammatory cytokine IL10 polymorphisms with motoric cognitive risk syndrome in an Ashkenazi Jewish population. Neurobiol. Aging 2017, 58, 238.e1–238.e8. [Google Scholar] [CrossRef] [PubMed]
- Giacomucci, G.; Mazzeo, S.; Bagnoli, S.; Ingannato, A.; Leccese, D.; Berti, V.; Padiglioni, S.; Galdo, G.; Ferrari, C.; Sorbi, S.; et al. Plasma neurofilament light chain as a biomarker of Alzheimer’s disease in subjective cognitive decline and mild cognitive impairment. J. Neurol. 2022, 269, 4270–4280. [Google Scholar] [CrossRef] [PubMed]
- De Wolf, F.; Ghanbari, M.; Licher, S.; McRae-McKee, K.; Gras, L.; Weverling, G.J.; Wermeling, P.; Sedaghat, S.; Ikram, M.K.; Waziry, R.; et al. Plasma tau, neurofilament light chain and amyloid-β levels and risk of dementia; A population-based cohort study. Brain 2020, 143, 1220–1232. [Google Scholar] [CrossRef] [PubMed]
- Visconte, C.; Golia, M.T.; Fenoglio, C.; Serpente, M.; Gabrielli, M.; Arcaro, M.; Sorrentino, F.; Busnelli, M.; Arighi, A.; Fumagalli, G.; et al. Plasma microglial-derived extracellular vesicles are increased in frail patients with mild cognitive impairment and exert a neurotoxic effect. Geroscience 2023, 45, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhu, M.; Yang, J.; Pang, Y.; Wang, Q.; Li, T.; Li, F.; Wang, Q.; Li, Y.; Wei, Y. Exosomal microRNA-based predictive model for preclinical Alzheimer’s disease: A multicenter study. Biol. Psychiatry 2022, 92, 44–53. [Google Scholar] [CrossRef]
- Siedlecki-Wullich, D.; Català-Solsona, J.; Fábregas, C.; Hernández, I.; Clarimon, J.; Lleó, A.; Boada, M.; Saura, C.A.; Rodríguez-Álvarez, J.; Miñano-Molina, A.J. Altered microRNAs related to synaptic function as potential plasma biomarkers for Alzheimer’s disease. Alzheimers Res. Ther. 2019, 11, 46. [Google Scholar] [CrossRef]
- Kenny, A.; McArdle, H.; Calero, M.; Rabano, A.; Madden, S.F.; Adamson, K.; Forster, R.; Spain, E.; Prehn, J.H.M.; Henshall, D.C.; et al. Elevated plasma microRNA-206 levels predict cognitive decline and progression to dementia from mild cognitive impairment. Biomolecules 2019, 9, 734. [Google Scholar] [CrossRef]
- Xie, B.; Liu, Z.; Jiang, L.; Liu, W.; Song, M.; Zhang, Q.; Zhang, R.; Cui, D.; Wang, X.; Xu, S. Increased serum miR-206 level predicts conversion from amnestic mild cognitive impairment to Alzheimer’s disease: A 5-year follow-up study. J. Alzheimers Dis. 2017, 55, 509–520. [Google Scholar] [CrossRef]
- Sugden, K.; Caspi, A.; Elliott, M.L.; Bourassa, K.J.; Chamarti, K.; Corcoran, D.L.; Hariri, A.R.; Houts, R.M.; Kothari, M.; Kritchevsky, S.; et al. Association of pace of aging measured by blood-based DNA methylation with age-related cognitive impairment and dementia. Neurology 2022, 99, E1402–E1413. [Google Scholar] [CrossRef]
- Degerman, S.; Josefsson, M.; Nordin Adolfsson, A.; Wennstedt, S.; Landfors, M.; Haider, Z.; Pudas, S.; Hultdin, M.; Nyberg, L.; Adolfsson, R. Maintained memory in aging is associated with young epigenetic age. Neurobiol. Aging 2017, 55, 167–171. [Google Scholar] [CrossRef]
- Tanaka, T.; Lavery, R.; Varma, V.; Fantoni, G.; Colpo, M.; Thambisetty, M.; Candia, J.; Resnick, S.M.; Bennett, D.A.; Biancotto, A.; et al. Plasma proteomic signatures predict dementia and cognitive impairment. Alzheimers Dement. 2020, 6, e12018. [Google Scholar] [CrossRef]
- DeMarshall, C.A.; Nagele, E.P.; Sarkar, A.; Acharya, N.K.; Godsey, G.; Goldwaser, E.L.; Kosciuk, M.; Thayasivam, U.; Han, M.; Belinka, B.; et al. Detection of Alzheimer’s disease at mild cognitive impairment and disease progression using autoantibodies as blood-based biomarkers. Alzheimers Dement. 2016, 3, 51–62. [Google Scholar] [CrossRef]
- Ehtewish, H.; Mesleh, A.; Ponirakis, G.; Lennard, K.; Al Hamad, H.; Chandran, M.; Parray, A.; Abdesselem, H.; Wijten, P.; Decock, J.; et al. Profiling the autoantibody repertoire reveals autoantibodies associated with mild cognitive impairment and dementia. Front. Neurol. 2023, 14, 1256745. [Google Scholar] [CrossRef]
- Singh, T.; Newman, A.B. Inflammatory markers in population studies of aging. Ageing Res. Rev. 2011, 10, 319–329. [Google Scholar] [CrossRef]
- Xu, W.; Bai, A.; Liang, Y.; Lin, Z. Association between depression and motoric cognitive risk syndrome among community-dwelling older adults in China: A 4-year prospective cohort study. Eur. J. Neurol. 2022, 29, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Westwater, M.L.; Noble, S.; Rosenblatt, M.; Dai, W.; Qi, S.; Sui, J.; Calhoun, V.D.; Scheinost, D. Associations between grip strength, brain structure, and mental health in >40,000 participants from the UK Biobank. BMC Med. 2022, 20, 286. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Harris, K.E.; Hou, L.; Xia, X.; Liu, X.; Ge, M.; Jia, S.; Zhou, L.; Zhao, W.; Zhang, Y.; et al. The prevalence and associated factors of motoric cognitive risk syndrome in multiple ethnic middle-aged to older adults in west China: A cross-sectional study. Eur. J. Neurol. 2022, 29, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Sekhon, H.; Launay, C.P.; Gaudreau, P.; Morais, J.A.; Allali, G. Late-life depressive symptomatology, motoric cognitive risk syndrome, and incident dementia: The “NuAge” study results. Front. Aging Neurosci. 2021, 13, 740181. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.; Steffens, D. Overlay of late-life depression and cognitive impairment. Focus 2017, 15, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Nixon, R.A. Neurofilament proteins as biomarkers to monitor neurological diseases and the efficacy of therapies. Front. Neurosci. 2021, 15, 689938. [Google Scholar] [CrossRef] [PubMed]
- Gafson, A.R.; Barthélemy, N.R.; Bomont, P.; Carare, R.O.; Durham, H.D.; Julien, J.-P.; Kuhle, J.; Leppert, D.; Nixon, R.A.; Weller, R.O.; et al. Neurofilaments: Neurobiological foundations for biomarker applications. Brain 2020, 143, 1975–1998. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Aebi, U. Intermediate filaments: Structure and assembly. Cold Spring Harb. Perspect. Biol. 2016, 8, a018242. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, H.; Skillbäck, T.; Mattsson, N.; Trojanowski, J.Q.; Portelius, E.; Shaw, L.M.; Weiner, M.W.; Blennow, K. Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol. 2016, 73, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Osborn, K.E.; Khan, O.A.; Kresge, H.A.; Bown, C.W.; Liu, D.; Moore, E.E.; Gifford, K.A.; Acosta, L.M.Y.; Bell, S.P.; Hohman, T.J.; et al. Cerebrospinal fluid and plasma neurofilament light relate to abnormal cognition. Alzheimers Dement. 2019, 11, 700–709. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to extracellular vesicles: Biogenesis, RNA cargo selection, content, release, and uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Lunavat, T.R.; Cheng, L.; Kim, D.-K.; Bhadury, J.; Jang, S.C.; Lässer, C.; Sharples, R.A.; López, M.D.; Nilsson, J.; Gho, Y.S.; et al. Small RNA deep sequencing discriminates subsets of extracellular vesicles released by melanoma cells—Evidence of unique microRNA Cargos. RNA Biol. 2015, 12, 810–823. [Google Scholar] [CrossRef]
- O’Brien, S.J.; Bishop, C.; Hallion, J.; Fiechter, C.; Scheurlen, K.; Paas, M.; Burton, J.; Galandiuk, S. Long non-coding RNA (lncRNA) and epithelial-mesenchymal transition (EMT) in colorectal cancer: A systematic review. Cancer Biol. Ther. 2020, 21, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Smith, F.; Kumar, S.; Vijayan, M.; Reddy, P.H. Are microRNAs true sensors of ageing and cellular senescence? Ageing Res. Rev. 2017, 35, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Raj, K. DNA Methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef]
- Horvath, S. DNA Methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Belsky, D.W.; Moffitt, T.E.; Cohen, A.A.; Corcoran, D.L.; Levine, M.E.; Prinz, J.A.; Schaefer, J.; Sugden, K.; Williams, B.; Poulton, R.; et al. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: Do they measure the same thing? Am. J. Epidemiol. 2018, 187, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Honda, T.; Narazaki, K.; Chen, T.; Kishimoto, H.; Haeuchi, Y.; Kumagai, S. Physical frailty is associated with longitudinal decline in global cognitive function in non-demented older adults: A prospective study. J. Nutr. Health Aging 2018, 22, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Siejka, T.P.; Srikanth, V.K.; Hubbard, R.E.; Moran, C.; Beare, R.; Wood, A.G.; Collyer, T.A.; Gujjari, S.; Phan, T.G.; Callisaya, M.L. Frailty is associated with cognitive decline independent of cerebral small vessel disease and brain atrophy. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Scafato, E.; Seripa, D.; Lozupone, M.; Imbimbo, B.P.; D’Amato, A.; Tortelli, R.; Schilardi, A.; Galluzzo, L.; Gandin, C.; et al. Reversible cognitive frailty, dementia, and all-cause mortality. The Italian Longitudinal Study on Aging. J. Am. Med. Dir. Assoc. 2017, 18, 89.e1–89.e8. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Doi, T.; Lee, S.; Makizako, H.; Chen, L.-K.; Arai, H. Cognitive frailty predicts incident dementia among community-dwelling older people. J. Clin. Med. 2018, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, T.W.; Lee, J.S.W.; Kwok, T.; Woo, J. Physical frailty predicts future cognitive decline—A four-year prospective study in 2737 cognitively normal older adults. J. Nutr. Health Aging 2011, 15, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Lipton, R.B.; Hall, C.B.; Kuslansky, G.; Katz, M.J.; Buschke, H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N. Engl. J. Med. 2002, 347, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Roberts, R.O.; Savica, R.; Cha, R.; Drubach, D.I.; Christianson, T.; Pankratz, V.S.; Geda, Y.E.; Machulda, M.M.; Ivnik, R.J.; et al. Assessing the temporal relationship between cognition and gait: Slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 929–937. [Google Scholar] [CrossRef]
- Deshpande, N.; Metter, E.J.; Bandinelli, S.; Guralnik, J.; Ferrucci, L. Gait speed under varied challenges and cognitive decline in older persons: A prospective study. Age Ageing 2009, 38, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Allerhand, M.; Sayer, A.A.; Cooper, C.; Deary, I.J. The dynamic relationship between cognitive function and walking speed: The English Longitudinal Study of Ageing. Age 2014, 36, 9682. [Google Scholar] [CrossRef]
- Van Kan, G.A.; Rolland, Y.; Gillette-Guyonnet, S.; Gardette, V.; Annweiler, C.; Beauchet, O.; Andrieu, S.; Vellas, B. Gait speed, body composition, and dementia. The EPIDOS-Toulouse cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Ojagbemi, A.; D’Este, C.; Verdes, E.; Chatterji, S.; Gureje, O. Gait speed and cognitive decline over 2 years in the Ibadan Study of Aging. Gait Posture 2015, 41, 736–740. [Google Scholar] [CrossRef]
- Rosso, A.L.; Verghese, J.; Metti, A.L.; Boudreau, R.M.; Aizenstein, H.J.; Kritchevsky, S.; Harris, T.; Yaffe, K.; Satterfield, S.; Studenski, S.; et al. Slowing gait and risk for cognitive impairment: The hippocampus as a shared neural substrate. Neurology 2017, 89, 336–342. [Google Scholar] [CrossRef]
- Alfaro-Acha, A.; Al Snih, S.; Raji, M.A.; Markides, K.S.; Ottenbacher, K.J. Does 8-foot walk time predict cognitive decline in older Mexicans Americans? J. Am. Geriatr. Soc. 2007, 55, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Wang, C.; Lipton, R.B.; Holtzer, R.; Xue, X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J. Neurol. Neurosurg. Psychiatry 2007, 78, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-F.; Suprawesta, L.; Chen, S.-J.; Yu, W.-Y.; Lin, M.-R. Predictors of incident reversible and potentially reversible cognitive frailty among Taiwanese older adults. BMC Geriatr. 2023, 23, 24. [Google Scholar] [CrossRef]
- Byun, S.; Han, J.W.; Kim, T.H.; Kim, K.; Kim, T.H.; Park, J.Y.; Suh, S.W.; Seo, J.Y.; So, Y.; Lee, K.H.; et al. Gait variability can predict the risk of cognitive decline in cognitively normal older people. Dement. Geriatr. Cogn. Disord. 2018, 45, 251–261. [Google Scholar] [CrossRef]
- Montero-Odasso, M.M.; Sarquis-Adamson, Y.; Speechley, M.; Borrie, M.J.; Hachinski, V.C.; Wells, J.; Riccio, P.M.; Schapira, M.; Sejdic, E.; Camicioli, R.M.; et al. Association of dual-task gait with incident dementia in mild cognitive impairment: Results from the Gait and Brain Study. JAMA Neurol. 2017, 74, 857–865. [Google Scholar] [CrossRef]
- Allali, G.; Ayers, E.I.; Verghese, J. Motoric cognitive risk syndrome subtypes and cognitive profiles. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Shimada, H.; Makizako, H.; Tsutsumimoto, K.; Verghese, J.; Suzuki, T. Motoric cognitive risk syndrome: Association with incident dementia and disability. J. Alzheimers Dis. 2017, 59, 77–84. [Google Scholar] [CrossRef]
- Verghese, J.; LeValley, A.; Hall, C.B.; Katz, M.J.; Ambrose, A.F.; Lipton, R.B. Epidemiology of gait disorders in community-residing older adults. J. Am. Geriatr. Soc. 2006, 54, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Ostir, G.V.; Berges, I.M.; Ottenbacher, K.J.; Fisher, S.R.; Barr, E.; Hebel, J.R.; Guralnik, J.M. Gait speed and dismobility in older adults. Arch. Phys. Med. Rehabil. 2015, 96, 1641–1645. [Google Scholar] [CrossRef]
- Cesari, M.; Kritchevsky, S.B.; Penninx, B.W.H.J.; Nicklas, B.J.; Simonsick, E.M.; Newman, A.B.; Tylavsky, F.A.; Brach, J.S.; Satterfield, S.; Bauer, D.C.; et al. Prognostic value of usual gait speed in well-functioning older people—Results from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2005, 53, 1675–1680. [Google Scholar] [CrossRef]
- Andrews, A.W.; Vallabhajosula, S.; Boise, S.; Bohannon, R.W. Normal gait speed varies by age and sex but not by geographical region: A systematic review. J. Physiother. 2023, 69, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Alcock, L.; Vitório, R.; Stuart, S.; Rochester, L.; Pantall, A. Faster walking speeds require greater activity from the primary motor cortex in older adults compared to younger adults. Sensors 2023, 23, 6921. [Google Scholar] [CrossRef]
- Herssens, N.; Verbecque, E.; Hallemans, A.; Vereeck, L.; Van Rompaey, V.; Saeys, W. Do spatiotemporal parameters and gait variability differ across the lifespan of healthy adults? A systematic review. Gait Posture 2018, 64, 181–190. [Google Scholar] [CrossRef]
- Kim, M.; Won, C.W. Sarcopenia is associated with cognitive impairment mainly due to slow gait speed: Results from the Korean Frailty and Aging Cohort Study (KFACS). Int. J. Environ. Res. Public. Health 2019, 16, 1491. [Google Scholar] [CrossRef]
- Garcia-Cifuentes, E.; Botero-Rodríguez, F.; Ramirez Velandia, F.; Iragorri, A.; Marquez, I.; Gelvis-Ortiz, G.; Acosta, M.-F.; Jaramillo-Jimenez, A.; Lopera, F.; Cano-Gutiérrez, C.A. Muscular function as an alternative to identify cognitive impairment: A secondary analysis from SABE Colombia. Front. Neurol. 2022, 13, 695253. [Google Scholar] [CrossRef]
- McGough, E.L.; Kelly, V.E.; Logsdon, R.G.; McCurry, S.; Cochrane, B.B.; Engel, J.M.; Teri, L. Associations between physical performance and executive function in older adults with mild cognitive impairment: Gait speed and the timed “up & go” test. Phys. Ther. 2011, 91, 1198–1207. [Google Scholar] [CrossRef]
- Beauchet, O.; Allali, G.; Montero-Odasso, M.; Sejdić, E.; Fantino, B.; Annweiler, C. Motor phenotype of decline in cognitive performance among community-dwellers without dementia: Population-based study and meta-analysis. PLoS ONE 2014, 9, e99318. [Google Scholar] [CrossRef]
- Bovonsunthonchai, S.; Vachalathiti, R.; Hiengkaew, V.; Bryant, M.S.; Richards, J.; Senanarong, V. Quantitative gait analysis in mild cognitive impairment, dementia, and cognitively intact individuals: A cross-sectional case–control study. BMC Geriatr. 2022, 22, 767. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.L.; Buchanan, C.K.; Nahin, R.L.; DeKosky, S.T.; Atkinson, H.H.; Carlson, M.C.; Williamson, J.D. Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 1244–1251. [Google Scholar] [CrossRef]
- Jiang, G.; Wu, X. Slower maximal walking speed is associated with poorer global cognitive function among older adults residing in China. PeerJ 2022, 10, e13809. [Google Scholar] [CrossRef]
- Callisaya, M.L.; Blizzard, L.; Schmidt, M.D.; McGinley, J.L.; Srikanth, V.K. Ageing and gait variability—A population-based study of older people. Age Ageing 2010, 39, 191–197. [Google Scholar] [CrossRef]
- Allali, G.; Annweiler, C.; Blumen, H.M.; Callisaya, M.L.; De Cock, A.-M.; Kressig, R.W.; Srikanth, V.; Steinmetz, J.-P.; Verghese, J.; Beauchet, O. Gait phenotype from mild cognitive impairment to moderate dementia: Results from the GOOD initiative. Eur. J. Neurol. 2016, 23, 527–541. [Google Scholar] [CrossRef]
- Byun, S.; Lee, H.J.; Kim, J.S.; Choi, E.; Lee, S.; Kim, T.H.; Kim, J.H.; Han, J.W.; Kim, K.W. Exploring shared neural substrates underlying cognition and gait variability in adults without dementia. Alzheimers Dement. 2023, 15, 206. [Google Scholar] [CrossRef]
- Martin, K.L.; Blizzard, L.; Wood, A.G.; Srikanth, V.; Thomson, R.; Sanders, L.M.; Callisaya, M.L. Cognitive Function, Gait, and Gait Variability in older people: A population-based study. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 726–732. [Google Scholar] [CrossRef]
- Boripuntakul, S.; Kamnardsiri, T.; Lord, S.R.; Maiarin, S.; Worakul, P.; Sungkarat, S. Gait variability during abrupt slow and fast speed transitions in older adults with mild cognitive impairment. PLoS ONE 2022, 17, e0276658. [Google Scholar] [CrossRef]
- Zhou, H.; Park, C.; Shahbazi, M.; York, M.K.; Kunik, M.E.; Naik, A.D.; Najafi, B. Digital biomarkers of cognitive frailty: The value of detailed gait assessment beyond gait speed. Gerontology 2022, 68, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Pieruccini-Faria, F.; Black, S.E.; Masellis, M.; Smith, E.E.; Almeida, Q.J.; Li, K.Z.H.; Bherer, L.; Camicioli, R.; Montero-Odasso, M. Gait variability across neurodegenerative and cognitive disorders: Results from the Canadian Consortium of Neurodegeneration in Aging (CCNA) and the Gait and Brain Study. Alzheimers Dement. 2021, 17, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Gillain, S.; Dramé, M.; Lekeu, F.; Wojtasik, V.; Ricour, C.; Croisier, J.-L.; Salmon, E.; Petermans, J. Gait speed or gait variability, which one to use as a marker of risk to develop Alzheimer disease? A pilot study. Aging Clin. Exp. Res. 2016, 28, 249–255. [Google Scholar] [CrossRef]
- Beauchet, O.; Allali, G.; Launay, C.; Herrmann, F.R.; Annweiler, C. Gait variability at fast-pace walking speed: A biomarker of mild cognitive impairment? J. Nutr. Health Aging 2013, 17, 235–239. [Google Scholar] [CrossRef]
- Simoni, D.; Rubbieri, G.; Baccini, M.; Rinaldi, L.; Becheri, D.; Forconi, T.; Mossello, E.; Zanieri, S.; Marchionni, N.; Di Bari, M. Different motor tasks impact differently on cognitive performance of older persons during dual task tests. Clin. Biomech. 2013, 28, 692–696. [Google Scholar] [CrossRef]
- Al-Yahya, E.; Dawes, H.; Smith, L.; Dennis, A.; Howells, K.; Cockburn, J. Cognitive motor interference while walking: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2011, 35, 715–728. [Google Scholar] [CrossRef]
- Belghali, M.; Chastan, N.; Cignetti, F.; Davenne, D.; Decker, L.M. Loss of gait control assessed by cognitive-motor dual-tasks: Pros and cons in detecting people at risk of developing Alzheimer’s and Parkinson’s diseases. Geroscience 2017, 39, 305–329. [Google Scholar] [CrossRef]
- Åhman, H.B.; Berglund, L.; Cedervall, Y.; Kilander, L.; Giedraitis, V.; McKee, K.J.; Ingelsson, M.; Rosendahl, E.; Åberg, A.C. Dual-Task tests predict conversion to dementia—A prospective memory-clinic-based cohort study. Int. J. Environ. Res. Public Health 2020, 17, 8129. [Google Scholar] [CrossRef]
- Ma, R.; Zhào, H.; Wei, W.; Liu, Y.; Huang, Y. Gait characteristics under single-/dual-task walking conditions in elderly patients with cerebral small vessel disease: Analysis of gait variability, gait asymmetry and bilateral coordination of gait. Gait Posture 2022, 92, 65–70. [Google Scholar] [CrossRef]
| Biomarker | Study Design and Population | Main Results | Reference |
|---|---|---|---|
| Inflammatory Markers | |||
| CRP | Cross-sectional; individuals ≥60 years (n = 5642) | Higher CRP levels were associated with MCR with memory impairment. | Bai et al. (2021) [48] |
| Panel of inflammatory cytokines and growth factors | Cross-sectional; individuals ≥80 years without severe dementia (n = 415) | IL-6 was associated with cognitive and physical function. | Adriaensen et al. (2014) [49] |
| IL-6 | Cross-sectional; individuals ≥60 years (n = 1340) | Individuals with cognitive frailty had significantly higher serum IL-6 levels compared with controls. | Diniz et al. (2022) [50] |
| CRP, IL-6, TNF-α | Cross-sectional; individuals ≥65 years without dementia (n = 1041) | High CRP and IL-6 serum levels were associated with MCR. | Bortone et al. (2021) [51] |
| Meta-analysis of five cross-sectional studies; older adults (n = 3101) | Circulating IL-6 and CRP were associated with MCR, with associations varying according to the presence of vascular disease. | Groeger et al. (2022) [52] | |
| GDF15 | Cross-sectional; individuals ≥35 years (n = 2736) | Higher plasma GDF15 levels were associated with a combination of cognitive frailty and depression and with cognitive frailty and depressive symptoms separately in younger and older adults. | Kochlik et al. (2023) [53] |
| Progranulin, GDF15, IL-10, IL-6, TNF-α | Cross-sectional; prefrail adults ≥60 years without dementia (n = 397) | Serum TNF-α was significantly elevated in MCR independent of sarcopenia but without obesity. Low IL-10 and IL-10/TNF-α ratio were associated with MCR, independent of sarcopenia and obesity. | Merchant et al. (2023) [54] |
| IL-10 gene polymorphism | Longitudinal (follow-up 3 years); individuals ≥65 years without dementia (n = 530) | Single-nucleotide polymorphisms in the transcriptional regulatory regions of IL-10 gene were associated with incident MCR. | Sathyan et al. (2017) [55] |
| Markers of Neuroaxonal Injury | |||
| NfL | Cross-sectional; individuals ≥45 years with SCD, MCI, or AD (n = 110) | Plasma NfL levels were increased in participants with MCI or AD compared with those with SCD. | Giacomucci et al. (2022) [56] |
| Longitudinal (follow-up 14 years); individuals ≥55 years without dementia (n = 4444) | Higher plasma NfL levels were associated with greater risk of all-cause dementia or AD. Mean NfL concentrations increased 3.4 times faster in participants who developed AD compared with those who remained dementia-free. Plasma values for cases diverged from controls 9.6 years before AD diagnosis. | de Wolf et al. (2020) [57] | |
| Extracellular Vesicles | |||
| Total, neural-, and microglial-derived EVs | Cross-sectional; individuals with and without dementia and frailty, age not reported (n = 60) | Participants with AD had diminished plasma neural EVs levels. Microglial-derived EVs were increased in number in plasma of MCI participants with frailty. | Visconte et al. (2023) [58] |
| MicroRNAs | |||
| Exosomal miRNAs | Longitudinal; four datasets of individuals with and without dementia (n = 544) | A predictive model with six miRNAs (miR29c-5p, miR-143-3p, miR-335-5p, miR-485-5p, miR-138-5p, miR-342-3p) detected preclinical AD 5 to 7 years before the onset of cognitive impairment. | Jia et al. (2022) [59] |
| miRNAs | Longitudinal; two datasets of individuals with and without dementia (n = 147) | The study found that miR-92a-3p, miR-181c-5p, and miR-210-3p were upregulated in plasma of individuals with MCI or AD compared with cognitively healthy participants. Those with MCI who progressed to AD during follow-up showed higher plasma levels of these miRNAs. | Siedlecki-Wullich et al. (2019) [60] |
| miRNA-206 | Longitudinal (follow-up 5 years); individuals with MCI and AD (n = 79) | miRNA-206 was associated with cognitive decline and memory deficits. Changes in plasma levels of miRNA-206 predicted cognitive decline and progression towards dementia in participants with MCI. | Kenny et al. (2019) [61] |
| Longitudinal (follow-up 5 years); individuals with amnestic MCI (n = 458) | During the follow-up, AD was diagnosed in 128/458 participants (28%). Serum levels of miRNA-206 were significantly higher in participants who converted to AD than in those with stable MCI both at baseline and at five years. Serum miRNA-206 was an independent predictor of AD conversion. | Xie et al. (2017) [62] | |
| Epigenetic Clocks | |||
| DunedinPACE | Cross-sectional; individuals ≥55 years with and without dementia (n = 649) Longitudinal (follow-up 14 years; n = 2264) | DunedinPACE was associated with clinical diagnosis of AD and worse cognitive tests. Participants with more advanced age on the clocks and faster DunedinPACE at baseline were at increased risk of developing dementia during the follow-up. | Sugden et al. (2022) [63] |
| DNA methylation | Longitudinal (follow-up 15 years); individuals ≥55 years with and without dementia (n = 52) | A lower delta age (DNAm age—chronological age) was observed in those with maintained memory functions compared with participants with average or accelerated decline. DNAm age at follow-up, but not chronologic age, was a predictor of dementia. | Degerman et al. (2017) [64] |
| Proteomic Markers | |||
| Plasma proteins | Longitudinal (follow-up 15 years); individuals 20–102 years with and without dementia (n = 997) | Myostatin, peptidase inhibitor 3, trefoil factor 3, and pregnancy-associated plasma protein A were associated with cognitive decline in participants who were cognitively healthy at baseline. | Tanaka et al. (2020) [65] |
| Plasma autoantibodies | Cross-sectional; individuals ≥55 years with and without MCI (n = 236) | Autoantibody biomarkers differentiated participants with MCI from age- and sex-matched controls (accuracy 100%). The autoantibody panel also differentiated those with MCI from participants with mild to moderate AD or other neurologic and non-neurologic diseases. | DeMarshall et al. (2016) [66] |
| Cross-sectional; individuals ≥55 years with and without MCI and dementia (n = 127) | Differential expression analysis identified 33 altered autoantibodies in participants with dementia compared with cognitively healthy controls, and 38 autoantibodies in those with dementia compared with individuals with MCI. | Ehtewish et al. (2023) [67] | |
| Biomarker | Study Population and Follow-Up | Frailty, Cognition, and Gait Measures | Main Results | Reference |
|---|---|---|---|---|
| Frailty | ||||
| Physical or cognitive frailty | Cognitively healthy adults ≥65 years (n = 2737); follow-up 4 years | Physical frailty: handgrip strength, BMI, ASM, gait speed, chair-stand test. Cognition: MMSE. | Most frailty measures at baseline were associated with lower MMSE scores four years later. | Auyeung et al. (2011) [90] |
| Cognitively healthy adults ≥65 years (n = 1045); follow-up 3 years | Physical frailty: Fried’s frailty criteria. Cognition: MoCA. | Chances of incident cognitive decline were more that twofold greater in individuals with physical frailty than in those with no frailty. | Chen et al. (2018) [86] | |
| Cognitively healthy adults ≥60 years (n = 385); follow-up 7 years | Frailty: Rockwood’s frailty index. Cognition: a neuropsychological test battery. | Frailty was associated with incident decline in global cognition independent of brain atrophy and cerebral small vessel disease. | Siejka et al. (2022) [87] | |
| Cognitively healthy adults ≥60 years (n = 2150); follow-up 3.5 and 7 years | Reversible cognitive frailty: presence of physical frailty and SCD. Cognition: a cognitive test battery. | Over a 3.5-year and a 7-year follow-up, participants with reversible cognitive frailty showed an increased risk of incident dementia, particularly vascular dementia. | Solfrizzi et al. (2017) [88] | |
| Cognitively healthy adults ≥65 years (n = 4570); follow-up 3 years | Physical frailty: slow gait speed and muscle weakness. Cognition: a cognitive test battery. | Cognitive frailty, but not physical frailty without MCI, was a predictor of incident dementia. | Shimada et al. (2018) [89] | |
| Gait Measures | ||||
| Neurologic gait | Cognitively healthy adults ≥75 years (n = 422); follow-up 6.6 years | Gait: neurological gait assessment. Cognition: a neuropsychological test battery and clinical assessment. | The presence of neurologic gait at baseline was a predictor of dementia, especially vascular dementia. | Verghese et al. (2002) [91] |
| Gait speed | Cognitively healthy adults ≥70 years (n = 1478); follow-up 4 years | Gait: usual gait speed. Cognition: a neuropsychological test battery. | A faster gait speed at baseline was associated with less cognitive decline. | Mielke et al. (2013) [92] |
| Cognitively healthy adults ≥65 years (n = 660); follow-up 3 years | Gait: usual and fast gait speed, walking-while-talking. Cognition: incident cognitive impairment defined as a ≥3 points loss on MMSE. | Gait speed at fast pace was associated with cognitive performance at follow-up. | Deshpande et al. (2009) [93] | |
| Cognitively healthy adults ≥60 years (n = 2654); follow-up 6 years | Gait: usual gait speed. Cognition: a cognitive test battery. | Better performance on executive function, memory, and processing speed was associated with slower decline in gait speed. | Gale et al. (2014) [94] | |
| Cognitively healthy adults ≥75 years (n = 1462); follow-up 7 years | Gait: usual gait speed. Cognition: a cognitive test battery. | Gait speed was associated with incident dementia independent of body composition parameters. | Van Kan et al. (2012) [95] | |
| Cognitively healthy adults ≥65 years (n = 1042); follow-up 2 years | Gait: usual gait speed. Cognition: 10-word delay recall test. | A slower baseline gait speed was associated with poorer cognition at follow-up. | Ojagbemi et al. (2015) [96] | |
| Cognitively healthy adults ≥65 years (n = 175); follow-up 14 years | Gait: usual gait speed. Cognition: clinical assessment. | Gait slowing was associated with cognitive impairment at year 14. A decreased gray matter volume in the right hippocampus on brain MRI was associated with both gait slowing and cognitive impairment. | Rosso et al. (2017) [97] | |
| Individuals ≥65 years with MMSE ≥21(n = 2070); follow-up 7 years | Gait: usual gait speed. Cognition: MMSE. | Participants with slower gait speed at baseline had a greater rate of cognitive decline at follow-up. | Alfaro-Acha et al. (2007) [98] | |
| Gait speed and variability | Individuals ≥70 with and without dementia (n = 427); follow-up 5 years | Gait: steady state walking using an electronic system. Cognition: a neuropsychological test battery. | Higher gait variability at baseline was associated with increased risk of incident dementia. | Verghese et al. (2007) [99] |
| Cognitively healthy adults ≥65 years (n = 758); follow-up 2 years | Gait: steady-state walking using an electronic system. Cognition: a cognitive test battery. Frailty: Fried’s frailty criteria. | Slower gait speed, lower balance confidence, and greater double-support time during walking at baseline were associated with incident cognitive frailty. | Hwang et al. (2023) [100] | |
| Cognitively healthy adults ≥60 years (n = 91); follow-up 4 years | Gait: steady-state walk using triaxial accelerometry-based gait analysis. Cognition: a standardized diagnostic interview. | Individuals with high gait variability had about a 12-fold greater risk of incident MCI than those with mid to low variability. | Byun et al. (2018) [101] | |
| Dual-task walk | Individuals ≥70 years with MCI (n = 112); follow-up 6 years | Gait: steady-state single- and dual-task walking using an electronic system. Cognition: a neuropsychological test battery. | A high dual-task walk cost was associated with progression to dementia. | Montero-Odasso et al. (2017) [102] |
| MCR | Individuals ≥65 years with and without cognitive impairment (n = 314); follow-up 1–2 years | Gait: steady-state walking using an electronic system. Cognition: a neuropsychological test battery. | At baseline, MCR was associated with deficits in attention, language, and overall cognitive status. A slow gait speed and a high gait variability were associated with incident cognitive impairment. | Allali et al. (2016) [103] |
| Cognitively healthy adults ≥60 years (n = 26,802); follow-up 12 years | Gait: steady state walking. Cognition: incident cognitive impairment defined as ≥4 points loss on MMSE. | MCR predicted incident cognitive impairment and dementia. | Verghese et al. (2014) [40] | |
| Cognitively healthy adults ≥60 years (n = 4326); follow-up 2.5 years | Gait: self-reported slow gait. Cognition: incident cases of dementia identified from insurance data. | MCR was associated with a greater risk of incident dementia. | Doi et al. (2017) [104] | |
| Cognitively healthy adults ≥70 years (n = 997); follow-up 3 years | Gait: steady-state walking using an electronic system. Cognition: a neuropsychological test battery. | Participants with MCR were at greater risk of developing dementia and vascular dementia. | Verghese et al. (2013) [45] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerminen, H.; Marzetti, E.; D’Angelo, E. Biological and Physical Performance Markers for Early Detection of Cognitive Impairment in Older Adults. J. Clin. Med. 2024, 13, 806. https://doi.org/10.3390/jcm13030806
Kerminen H, Marzetti E, D’Angelo E. Biological and Physical Performance Markers for Early Detection of Cognitive Impairment in Older Adults. Journal of Clinical Medicine. 2024; 13(3):806. https://doi.org/10.3390/jcm13030806
Chicago/Turabian StyleKerminen, Hanna, Emanuele Marzetti, and Emanuela D’Angelo. 2024. "Biological and Physical Performance Markers for Early Detection of Cognitive Impairment in Older Adults" Journal of Clinical Medicine 13, no. 3: 806. https://doi.org/10.3390/jcm13030806
APA StyleKerminen, H., Marzetti, E., & D’Angelo, E. (2024). Biological and Physical Performance Markers for Early Detection of Cognitive Impairment in Older Adults. Journal of Clinical Medicine, 13(3), 806. https://doi.org/10.3390/jcm13030806