Prophylactic Hyperthermic Intraperitoneal Chemotherapy for Patients at High Risk of Developing Gallbladder Cancer Peritoneal Metastases: Case Report and Rationale for a Prospective Clinical Trial
Abstract
1. Introduction
2. Discussion
2.1. Clinical Presentation of Gallbladder Cancer
2.2. Surgical Challenges in Incidental Gallbladder Cancer
2.3. Impact of Bile Spillage on Recurrence Patterns
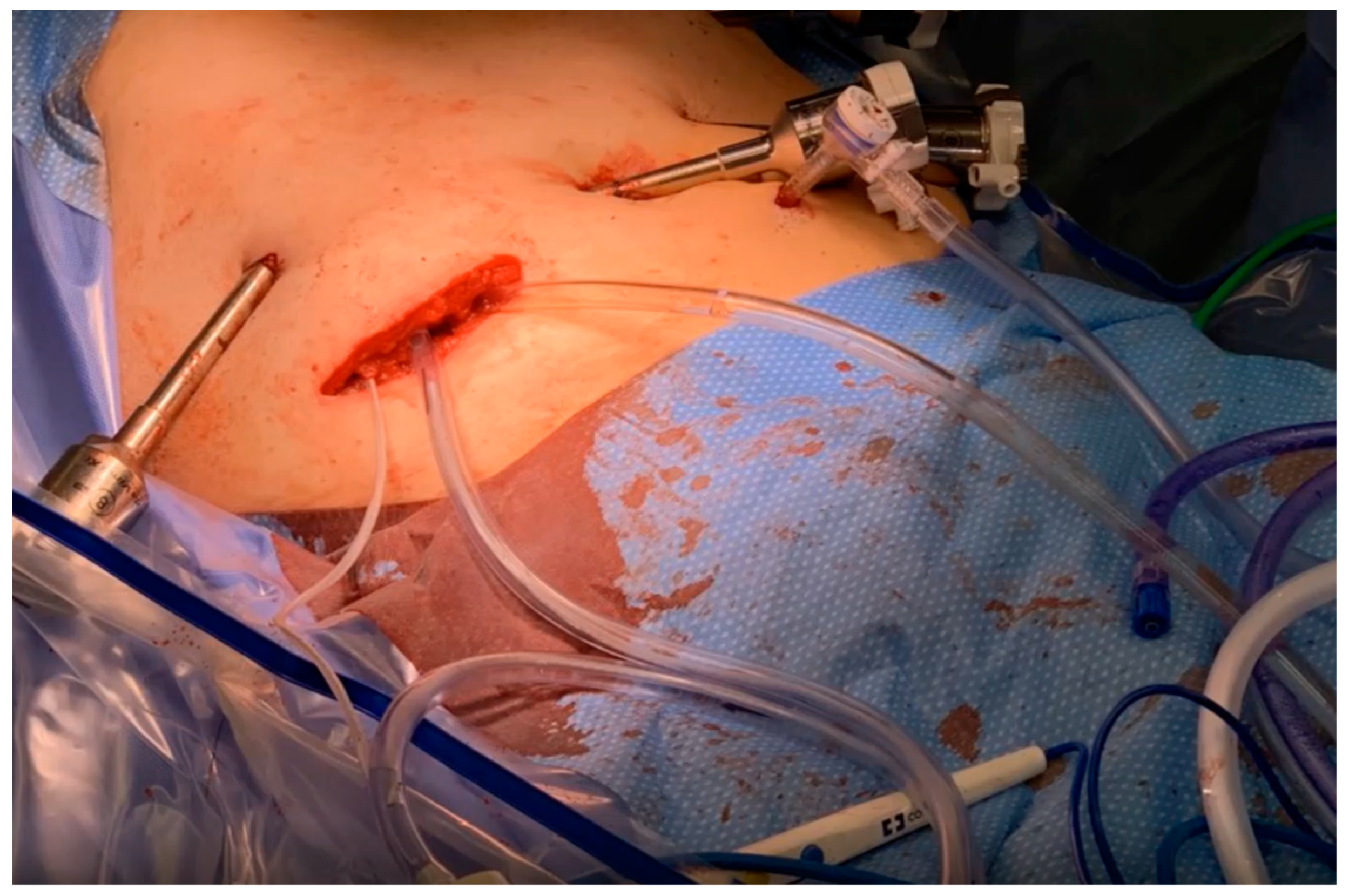
2.4. Cytoreductive Surgery and HIPEC for Peritoneal Metastases from Gallbladder Cancer
2.5. Rationale for Prophylactic HIPEC
2.6. Prospective Clinical Trial of Prophylactic HIPEC in Gallbladder Cancer
2.7. Future Directions
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Noone, A.M.; Howlader, N.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2015; National Cancer Institute: Bethesda, MD, USA, 2018. [Google Scholar]
- Graziosi, L.; Cantarella, F.; Mingrone, E.; Gunnellini, M.; Cavazzoni, E.; Liberati, M.; Donini, A. Preliminary results of prophylactic HIPEC in patients with locally advanced gastric cancer. Ann. Ital. Chir. 2013, 84, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Beeharry, M.K.; Zhu, Z.L.; Liu, W.T.; Yao, X.X.; Yan, M.; Zhu, Z.G. Prophylactic HIPEC with radical D2 gastrectomy improves survival and peritoneal recurrence rates for locally advanced gastric cancer: Personal experience from a randomized case control study. BMC Cancer 2019, 19, 932. [Google Scholar]
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; Bremers, A.J.A.; Burger, J.W.A.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770. [Google Scholar] [CrossRef]
- Tuvin, D.; Berger, Y.; Aycart, S.N.; Shtilbans, T.; Hiotis, S.; Labow, D.M.; Sarpel, U. Prophylactic hyperthermic intraperitoneal chemotherapy in patients with epithelial appendiceal neoplasms. Int. J. Hyperth. 2016, 32, 311–315. [Google Scholar] [CrossRef]
- Goéré, D.; Glehen, O.; Quenet, F.; Guilloit, J.M.; Bereder, J.M.; Lorimier, G.; Thibaudeau, E.; Ghouti, L.; Pinto, A.; Tuech, J.J.; et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): A randomised, phase 3 study. Lancet Oncol. 2020, 21, 1147–1154. [Google Scholar] [CrossRef]
- Arjona-Sánchez, A.; Espinosa-Redondo, E.; Gutiérrez-Calvo, A.; Segura-Sampedro, J.J.; Pérez-Viejo, E.; Concepción-Martín, V.; Sánchez-García, S.; García-Fadrique, A.; Prieto-Nieto, I.; Barrios-Sanchez, P.; et al. Efficacy and Safety of Intraoperative Hyperthermic Intraperitoneal Chemotherapy for Locally Advanced Colon Cancer: A Phase 3 Randomized Clinical Trial. JAMA Surg. 2023, 158, 683–691. [Google Scholar] [CrossRef]
- Amblard, I.; Mercier, F.; Bartlett, D.L.; Ahrendt, S.A.; Lee, K.W.; Zeh, H.J.; Levine, E.A.; Baratti, D.; Deraco, M.; Piso, P.; et al. Cytoreductive surgery and HIPEC improve survival compared to palliative chemotherapy for biliary carcinoma with peritoneal metastasis: A multi-institutional cohort from PSOGI and BIG RENAPE groups. Eur. J. Surg. Oncol. 2018, 44, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.T.; Talwalkar, J.A.; Rosen, C.B.; Smyrk, T.C.; Abraham, S.C. Prevalence and risk factors for gallbladder neoplasia in patients with primary sclerosing cholangitis: Evidence for a metaplasia-dysplasia-carcinoma sequence. Am. J. Surg. Pathol. 2007, 31, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.E.; Berger, D.L. Carcinoma in the porcelain gallbladder: A relationship revisited. Surgery 2001, 129, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Okamoto, H.; Kitahara, F.; Kobayashi, K.; Karikome, K.; Miura, K.; Matsumoto, Y.; Fujino, M.A. Ultrasonographic evidence of association of polyps and stones with gallbladder cancer. Am. J. Gastroenterol. 1999, 94, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Matsukura, N.; Yokomuro, S.; Yamada, S.; Tajiri, T.; Sundo, T.; Hadama, T.; Kamiya, S.; Naito, Z.; Fox, J.G. Association between Helicobacter bilis in bile and biliary tract malignancies: H. bilis in bile from Japanese and Thai patients with benign and malignant diseases in the biliary tract. Jpn. J. Cancer Res. 2002, 93, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Dutta, U.; Garg, P.K.; Kumar, R.; Tandon, R.K. Typhoid carriers among patients with gallstones are at increased risk for carcinoma of the gallbladder. Am. J. Gastroenterol. 2000, 95, 784–787. [Google Scholar] [CrossRef]
- Voyles, C.R.; Smadja, C.; Shands, W.C.; Blumgart, L.H. Carcinoma in choledochal cysts. Age-related incidence. Arch. Surg. 1983, 118, 986–988. [Google Scholar] [CrossRef]
- Goldberg, M.S.; Thériault, G. Retrospective cohort study of workers of a synthetic textiles plant in Quebec: I. General mortality. Am. J. Ind. Med. 1994, 25, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Gao, Y.T.; Dean, M.; Egner, P.; Nepal, C.; Jones, K.; Wang, B.; Rashid, A.; Luo, W.; Van Dyke, A.L.; et al. Association of Aflatoxin and Gallbladder Cancer. Gastroenterology 2017, 153, 488–494.e1. [Google Scholar] [CrossRef]
- Goussous, N.; Maqsood, H.; Patel, K.; Ferdosi, H.; Muhammad, N.; Sill, A.M.; Kowdley, G.C.; Cunningham, S.C. Clues to predict incidental gallbladder cancer. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 149–154. [Google Scholar] [CrossRef]
- Duffy, A.; Capanu, M.; Abou-Alfa, G.K.; Huitzil, D.; Jarnagin, W.; Fong, Y.; D’Angelica, M.; Dematteo, R.P.; Blumgart, L.H.; O’Reilly, E.M. Gallbladder cancer (GBC): 10-Year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J. Surg. Oncol. 2008, 98, 485–489. [Google Scholar] [CrossRef]
- Shih, S.P.; Schulick, R.D.; Cameron, J.L.; Lillemoe, K.D.; Pitt, H.A.; Choti, M.A.; Campbell, K.A.; Yeo, C.J.; Talamini, M.A. Gallbladder cancer: The role of laparoscopy and radical resection. Ann. Surg. 2007, 245, 893–901. [Google Scholar] [CrossRef]
- Vega, E.A.; Vinuela, E.; Okuno, M.; Joechle, K.; Sanhueza, M.; Diaz, C.; Jarufe, N.; Martinez, J.; Troncoso, A.; Diaz, A.; et al. Incidental versus non-incidental gallbladder cancer: Index cholecystectomy before oncologic re-resection negatively impacts survival in T2b tumors. HPB 2019, 21, 1046–1056. [Google Scholar] [CrossRef]
- Melillo, A.; Linden, K.; Spitz, F.; Atabek, U.; Gaughan, J.; Hong, Y.K. Disparities in Treatment for Gallbladder Carcinoma: Does Treatment Site Matter? J. Gastrointest. Surg. 2020, 24, 1071–1076. [Google Scholar] [CrossRef]
- Aloia, T.A.; Járufe, N.; Javle, M.; Maithel, S.K.; Roa, J.C.; Adsay, V.; Coimbra, F.J.; Jarnagin, W.R. Gallbladder cancer: Expert consensus statement. HPB 2015, 17, 681–690. [Google Scholar] [CrossRef]
- Lamarca, A.; Barriuso, J.; Chander, A.; McNamara, M.G.; Hubner, R.A.; ÓReilly, D.; Manoharan, P.; Valle, J.W. (18)F-fluorodeoxyglucose positron emission tomography ((18)FDG-PET) for patients with biliary tract cancer: Systematic review and meta-analysis. J. Hepatol. 2019, 71, 115–129. [Google Scholar] [CrossRef]
- Yang, Y.; Tu, Z.; Ye, C.; Cai, H.; Yang, S.; Chen, X.; Tu, J. Site-specific metastases of gallbladder adenocarcinoma and their prognostic value for survival: A SEER-based study. BMC Surg. 2021, 21, 59. [Google Scholar] [CrossRef]
- Nishio, H.; Nagino, M.; Ebata, T.; Yokoyama, Y.; Igami, T.; Nimura, Y. Aggressive surgery for stage IV gallbladder carcinoma; what are the contraindications? J. Hepatobiliary Pancreat. Surg. 2007, 14, 351–357. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Ruo, L.; Little, S.A.; Klimstra, D.; D’Angelica, M.; DeMatteo, R.P.; Wagman, R.; Blumgart, L.H.; Fong, Y. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: Implications for adjuvant therapeutic strategies. Cancer 2003, 98, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Wibbenmeyer, L.A.; Wade, T.P.; Chen, R.C.; Meyer, R.C.; Turgeon, R.P.; Andrus, C.H. Laparoscopic cholecystectomy can disseminate in situ carcinoma of the gallbladder. J. Am. Coll. Surg. 1995, 181, 504–510. [Google Scholar] [PubMed]
- Brockmann, J.G.; Kocher, T.; Senninger, N.J.; Schürmann, G.M. Complications due to gallstones lost during laparoscopic cholecystectomy. Surg. Endosc. Other Interv. Tech. 2002, 16, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.C.; Memon, M.A.; Jamison, R.L.; Agnessi, T.; Ilstrup, D.; Bannon, M.B.; Farnell, M.B.; Grant, C.S.; Sarr, M.G.; Thompson, G.B.; et al. Long-Term Consequences of Intraoperative Spillage of Bile and Gallstones during Laparoscopic Cholecystectomy. J. Gastrointest. Surg. 1997, 1, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Suter, C.; Klaiber, C.; Wehrli, H.; Frei, E.; Krähenbühl, L. Spilled gallstones after laparoscopic cholecystectomy: A relevant problem? A retrospective analysis of 10,174 laparoscopic cholecystectomies. Surg. Endosc. 1998, 12, 305–309. [Google Scholar] [CrossRef]
- Sarli, L.; Pietra, N.; Costi, R.; Grattarola, M. Gallbladder perforation during laparoscopic cholecystectomy. World J. Surg. 1999, 23, 1186–1190. [Google Scholar] [CrossRef]
- Ouchi, K.; Mikuni, J.; Kakugawa, Y. Laparoscopic cholecystectomy for gallbladder carcinoma: Results of a Japanese survey of 498 patients. J. Hepatobiliary Pancreat. Surg. 2002, 9, 256–260. [Google Scholar] [CrossRef]
- Weiland, S.T.; Mahvi, D.M.; Niederhuber, J.E.; Heisey, D.M.; Chicks, D.S.; Rikkers, L.F. Should suspected early gallbladder cancer be treated laparoscopically? J. Gastrointest. Surg. 2002, 6, 50–56, discussion 56–57. [Google Scholar] [CrossRef]
- Shindoh, J.; Aretxabala, X.D.; Aloia, T.A.; Roa, J.C.; Zimmitti, G.; Javle, M.; Conrad, C.; Maru, D.M.; Aoki, T.; Vigano, L.; et al. Tumor location is a strong predictor of tumor progression and survival in T2 gallbladder cancer: An international multicenter study. Ann. Surg. 2015, 261, 733. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, D. The 8th Edition American Joint Committee on Cancer Staging for Hepato-pancreato-biliary Cancer: A Review and Update. Arch. Pathol. Lab. Med. 2021, 145, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liao, Z.; Sun, C.; Chen, Z. Impact of Primary Tumor Resection on Survival of Patients with Metastatic Gallbladder Carcinoma: A Population-Based, Propensity-Matched Study. Med. Sci. Monit. 2022, 28, e934447. [Google Scholar] [CrossRef] [PubMed]
- Kuga, D.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Mizuno, T.; Yamaguchi, J.; Nagino, M. Long-term survival after multidisciplinary therapy for residual gallbladder cancer with peritoneal dissemination: A case report. Surg. Case Rep. 2017, 3, 76. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Takano, K.; Shimazu, M.; Okihara, M.; Sano, T.; Chiba, N.; Kawachi, S. Long-term survival of a recurrent gallbladder carcinoma patient with lymph node and peritoneal metastases after multidisciplinary treatments: A case report. Surg. Case Rep. 2016, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.D.; Deraco, M.; Baratti, D.; Kusamura, S.; Elias, D.; Glehen, O.; Gilly, F.N.; Levine, E.A.; Shen, P.; Mohamed, F.; et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: Multi-institutional experience. J. Clin. Oncol. 2009, 27, 6237–6242. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.C.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Glehen, O.; Gilly, F.N.; Baratti, D.; Deraco, M.; Elias, D.; Sardi, A.; et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Clin. Oncol. 2012, 30, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.B.; Napoli, R.D. Hyperthermic Intraperitoneal Chemotherapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570563/ (accessed on 20 January 2024).
- da Silva, R.G.; Sugarbaker, P.H. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J. Am. Coll. Surg. 2006, 203, 878–886. [Google Scholar] [CrossRef]
- Huang, C.Q.; Min, Y.; Wang, S.Y.; Yang, X.J.; Liu, Y.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for peritoneal carcinomatosis from colorectal cancer: A systematic review and meta-analysis of current evidence. Oncotarget 2017, 8, 55657–55683. [Google Scholar] [CrossRef]
- Elias, D.; Raynard, B.; Boige, V.; Laplanche, A.; Estphan, G.; Malka, D.; Pocard, M. Impact of the extent and duration of cytoreductive surgery on postoperative hematological toxicity after intraperitoneal chemohyperthermia for peritoneal carcinomatosis. J. Surg. Oncol. 2005, 90, 220–225. [Google Scholar] [CrossRef]
- Piso, P.; Nedelcut, S.D.; Rau, B.; Königsrainer, A.; Glockzin, G.; Ströhlein, M.A.; Hörbelt, R.; Pelz, J. Morbidity and Mortality Following Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Data from the DGAV StuDoQ Registry with 2149 Consecutive Patients. Ann. Surg. Oncol. 2019, 26, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Randle, R.W.; Levine, E.A.; Clark, C.J.; Stewart, J.H.; Shen, P.; Votanopoulos, K.I. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for gallbladder cancer: A retrospective review. Am. Surg. 2014, 80, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.J.; Daniel, S.K.; Lee, B. The Role of Prophylactic and Adjuvant Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Prevention of Peritoneal Metastases in Advanced Colorectal Cancer. J. Clin. Med. 2023, 12, 6443. [Google Scholar] [CrossRef] [PubMed]
- Nagourney, R.A.; Evans, S.; Tran, P.H.; Nagourney, A.J.; Sugarbaker, P.H. Colorectal cancer cells from patients treated with FOLFOX or CAPOX are resistant to oxaliplatin. Eur. J. Surg. Oncol. 2021, 47, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhong, Z.; Yi, W.; Yu, Z.; Zhang, Z.; Xia, G.; Jiang, B.; Song, Y.; Peng, C. Effect of Hyperthermic Intraperitoneal Perfusion Chemotherapy Combined with Radical Surgery and Capecitabine on Stage III Gallbladder Cancer. Can. J. Gastroenterol. Hepatol. 2021, 2021, 4006786. [Google Scholar] [CrossRef] [PubMed]
- Smibert, O.C.; Slavin, M.A.; Teh, B.; Heriot, A.G.; Penno, J.; Ismail, H.; Thursky, K.A.; Worth, L.J. Epidemiology and risks for infection following cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. Support. Care Cancer 2020, 28, 2745–2752. [Google Scholar] [CrossRef]
- Network, N.C.C. Pancreatic Adenocarcinoma (Version 2.2023). Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 20 January 2024).
- Zhou, Y.; Chen, S.; Wu, Y.; Li, L.; Lou, Q.; Chen, Y.; Xu, S. Multi-clinical index classifier combined with AI algorithm model to predict the prognosis of gallbladder cancer. Front. Oncol. 2023, 13, 1171837. [Google Scholar] [CrossRef]
- Sestito, M.; Pratt, H.; Schmidt, C.; Thomay, A. Recent advances for treatment of upper gastrointestinal malignancy. J. Surg. Oncol. 2024, 129, 48–62. [Google Scholar] [CrossRef]
- Shah, M.A.; Shitara, K.; Ajani, J.A.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Lordick, F.; Van Cutsem, E.; Gallego Plazas, J.; Huang, J.; et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: The randomized, phase 3 GLOW trial. Nat. Med. 2023, 29, 2133–2141. [Google Scholar] [CrossRef]
- Shitara, K.; Lordick, F.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.H.; Aprile, G.; Xu, J.; et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2023, 401, 1655–1668. [Google Scholar] [CrossRef]
- Blay, J.Y.; Serrano, C.; Heinrich, M.C.; Zalcberg, J.; Bauer, S.; Gelderblom, H.; Schöffski, P.; Jones, R.L.; Attia, S.; D’Amato, G.; et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 923–934. [Google Scholar] [CrossRef]
- Bauer, S.; Jones, R.L.; Blay, J.Y.; Gelderblom, H.; George, S.; Schöffski, P.; von Mehren, M.; Zalcberg, J.R.; Kang, Y.K.; Razak, A.A.; et al. Ripretinib Versus Sunitinib in Patients With Advanced Gastrointestinal Stromal Tumor After Treatment With Imatinib (INTRIGUE): A Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2022, 40, 3918–3928. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Kawazoe, A.; Bai, Y.; Xu, J.; Lonardi, S.; Metges, J.P.; Yanez, P.; Wyrwicz, L.S.; Shen, L.; Ostapenko, Y.; et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: Interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet 2023, 402, 2197–2208. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.O.; Hochhauser, D.; Arnold, D.; Oh, D.Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cao, Y.; Yang, X.; Li, G.; Shi, Y.; Wang, D.; Long, J.; Song, Y.; Mao, J.; Xie, F.; et al. Mutational spectrum and precision oncology for biliary tract carcinoma. Theranostics 2021, 11, 4585–4598. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crum, A.E.; Sestito, M.; Garland-Kledzik, M.; Boone, B.A. Prophylactic Hyperthermic Intraperitoneal Chemotherapy for Patients at High Risk of Developing Gallbladder Cancer Peritoneal Metastases: Case Report and Rationale for a Prospective Clinical Trial. J. Clin. Med. 2024, 13, 768. https://doi.org/10.3390/jcm13030768
Crum AE, Sestito M, Garland-Kledzik M, Boone BA. Prophylactic Hyperthermic Intraperitoneal Chemotherapy for Patients at High Risk of Developing Gallbladder Cancer Peritoneal Metastases: Case Report and Rationale for a Prospective Clinical Trial. Journal of Clinical Medicine. 2024; 13(3):768. https://doi.org/10.3390/jcm13030768
Chicago/Turabian StyleCrum, Alexander E., Michael Sestito, Mary Garland-Kledzik, and Brian A. Boone. 2024. "Prophylactic Hyperthermic Intraperitoneal Chemotherapy for Patients at High Risk of Developing Gallbladder Cancer Peritoneal Metastases: Case Report and Rationale for a Prospective Clinical Trial" Journal of Clinical Medicine 13, no. 3: 768. https://doi.org/10.3390/jcm13030768
APA StyleCrum, A. E., Sestito, M., Garland-Kledzik, M., & Boone, B. A. (2024). Prophylactic Hyperthermic Intraperitoneal Chemotherapy for Patients at High Risk of Developing Gallbladder Cancer Peritoneal Metastases: Case Report and Rationale for a Prospective Clinical Trial. Journal of Clinical Medicine, 13(3), 768. https://doi.org/10.3390/jcm13030768








