The Role of Pocus in Acute Respiratory Failure: A Narrative Review on Airway and Breathing Assessment
Abstract
1. Introduction
2. Materials and Methods
- A.
- Papers concerning upper airway or intubation;
- B.
- Papers addressing respiratory function breathing-related impairment.
3. Results
4. Discussion
4.1. A: US & Airway
4.1.1. Endotracheal Tube (ETT) Positioning Assessment
4.1.2. Upper Airways Damage Identification and Procedures
4.1.3. Laryngeal Edema Assessment Pre-Extubation
4.2. B: US and Breathing
4.2.1. Protocols on Lung US
- -
- Abolition of lung sliding alone, sensitivity 100% specificity 78%;
- -
- Absent lung sliding plus the A-line sign, sensitivity 95% specificity 94%;
- -
- Lung point, sensitivity 79% specificity 100% [35].
Acute Respiratory Distress Syndrome (ARDS)
4.2.2. Diagnostic Accuracy
- -
- Pneumonia, standard, 0.74, ultrasound, 0.87;
- -
- Acute hemodynamic pulmonary edema standard, 0.79, ultrasound, 0.93);
- -
- Decompensated COPD standard, 0.8, ultrasound, 0.92;
- -
- Pulmonary embolism standard, 0.65, ultrasound, 0.81;
- -
- Pneumonia/consolidation 89–92% and 94–97%;
- -
- Heart failure/interstitial syndrome 90–95% and 91–93%;
- -
- Pleural effusion 95% and 99%;
- -
- COPD/asthma (A profile) 78% and 94%.
4.2.3. Time-to-Diagnosis Improvement
4.2.4. Diaphragm Ultra-Sound (DUS)
- Diaphragm thickening fraction (DTF), measurement of the difference in end-inspiratory and end-expiratory diaphragmatic thickness, expressed as a fraction;
5. Limitations
- Availability and settings: the lack of ultrasound machines in specific settings like pre-hospital or in limited-resource countries; furthermore, updated software and probes are needed to obtain more reliable images. Moreover, in case of intensive use from patient to patient and the lack of disinfection and cleanliness, the probes could be a vector of infection [88].
- Technical impairment: “air” in itself is a limitation to ultrasonic wave propagation and their interaction with body tissue and fluids generates artifacts that have to be recognized and correctly interpreted. Furthermore, the correct use of the different probes and the many settings allowed by the new ultrasound machine is mandatory to properly set up adequate images. Finally, the lack of standardization with specific protocols for upper and lower airway POCUS execution may limit replication and increase interobserver variability [89].
- Competences: education in the POCUS technique and an adequate level of experience are cardinal points to obtain a reliable POCUS assessment. Continuing US utilization in daily clinical practice, comparison with other gold standard imaging exams and support of senior team members are needed to avoid clinical errors and to improve personal skills. Specific ultrasound training programs should be implemented in the trainee core curriculum [90].
- Scientific level evidence: most of the published data about POCUS clinical utilization and effectiveness are based on observational studies. However, it is difficult to plan studies with strong levels of evidence such as TRIAL or prospective multicentric and interventional studies due to organizational and methodological impairments such as different ultrasound machines in different settings, interobserver variability, availability of ultrasonologists with the same level of competencies, contradictory outcomes identification and measurements.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. LUNG SAFE Investigators, & ESICM Trials Group Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.P.; Nathanson, R.; LoPresti, C.M.; Mader, M.J.; Haro, E.K.; Drum, B.; O’Brien, E.; Khosla, R.; Boyd, J.S.; Bales, B.; et al. Current use, training, and barriers in point-of-care ultrasound in hospital medicine: A national survey of VA hospitals. J. Hosp. Med. 2022, 17, 601–608. [Google Scholar] [CrossRef]
- Abrokwa, S.K.; Ruby, L.C.; Heuvelings, C.C.; Bélard, S. Task shifting for point of care ultrasound in primary healthcare in low- and middle-income countries—A systematic review. EClinicalMedicine 2022, 45, 101333. [Google Scholar] [CrossRef]
- Kreiser, M.A.; Hill, B.; Karki, D.; Wood, E.; Shelton, R.; Peterson, J.; Riccio, J.; Zapata, I.; Khalil, P.A.; Gubler, D.; et al. Point-of-Care Ultrasound Use by EMS Providers in Out-of-Hospital Cardiac Arrest. Prehosp. Disaster Med. 2022, 37, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Gullett, J.P.; Hill, H.F.; Fuller, D.; Westergaard, M.C.; Hosek, W.T.; Smith, J.A. Bedside ultrasound of the neck confirms endotracheal tube position in emergency intubations. Ultraschall Med. 2014, 35, 451–458. [Google Scholar] [CrossRef]
- Wojtczak, J.A.; Cattano, D. Laryngo-tracheal ultrasonography to confirm correct endotracheal tube and laryngeal mask airway placement. J. Ultrason. 2014, 14, 362–366. [Google Scholar] [CrossRef]
- Hossein-Nejad, H.; Mehrjerdi, M.S.; Abdollahi, A.; Loesche, M.A.; Schulwolf, S.; Ghadipasha, M.; Mohammadinejad, P.; Ataeinia, B.; Shokoohi, H. Ultrasound for Intubation Confirmation: A Randomized Controlled Study among Emergency Medicine Residents. Ultrasound Med. Biol. 2021, 47, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.S.; Lien, W.C.; Chou, H.C.; Chong, K.M.; Liu, S.H.; Wang, C.H.; Chen, S.Y.; Hsu, C.Y.; Yen, Z.S.; Chang, W.T.; et al. Ultrasonographic lung sliding sign in confirming proper endotracheal intubation during emergency intubation. Resuscitation 2012, 83, 307–312. [Google Scholar] [CrossRef]
- Schick, M.; Grether-Jones, K. Point-of-Care Sonographic Findings in Acute Upper Airway Edema. West. J. Emerg. Med. 2016, 17, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Adi, O.; Sum, K.M.; Ahmad, A.H.; Wahab, M.A.; Neri, L.; Panebianco, N. Novel role of focused airway ultrasound in early airway assessment of suspected laryngeal trauma. Ultrasound J. 2020, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.E.; Sweeney, T.W.; Ferre, R.M.; Strout, T.D. Bedside sonography by emergency physicians for the rapid identification of landmarks relevant to cricothyrotomy. Am. J. Emerg. Med. 2008, 26, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Adi, O.; Fong, C.P.; Sum, K.M.; Ahmad, A.H. Usage of airway ultrasound as an assessment and prediction tool of a difficult airway management. Am. J. Emerg. Med. 2021, 42, 263.e1–263.e4. [Google Scholar] [CrossRef] [PubMed]
- Iqhbal, M.; Noor, J.M.; Karim, N.A.; Ismail, I.; Sanib, H.; Mokhtar, M.A.; Salim, S.S.F. Point-of-Care Airway Ultrasonography Prior to an Emergency Cricothyroidotomy: Case Report. Sultan Qaboos Univ. Med. J. 2018, 18, e219–e222. [Google Scholar] [CrossRef] [PubMed]
- Sutherasan, Y.; Theerawit, P.; Hongphanut, T.; Kiatboonsri, C.; Kiatboonsri, S. Predicting laryngeal edema in intubated patients by portable intensive care unit ultrasound. J. Crit. Care 2013, 28, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Mikaeili, H.; Yazdchi, M.; Tarzamni, M.K.; Ansarin, K.; Ghasemzadeh, M. Laryngeal ultrasonography versus cuff leak test in predicting postextubation stridor. J. Cardiovasc. Thorac. Res. 2014, 6, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Kandil, E.; Deniwar, A.; Noureldine, S.I.; Hammad, A.Y.; Mohamed, H.; Al-Qurayshi, Z.; Tufano, R.P. Assessment of Vocal Fold Function Using Transcutaneous Laryngeal Ultrasonography and Flexible Laryngoscopy. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 74–78. [Google Scholar] [CrossRef]
- Wong, K.P.; Au, K.P.; Lam, S.; Lang, B.H. Lessons Learned After 1000 Cases of Transcutaneous Laryngeal Ultrasound (TLUSG) with Laryngoscopic Validation: Is There a Role of TLUSG in Patients Indicated for Laryngoscopic Examination Before Thyroidectomy? Thyroid Off. J. Am. Thyroid Assoc. 2017, 27, 88–94. [Google Scholar] [CrossRef]
- Noel, J.E.; Orloff, L.A.; Sung, K. Laryngeal Evaluation during the COVID-19 Pandemic: Transcervical Laryngeal Ultrasonography. Otolaryngol. Head Neck Surg. 2020, 163, 51–53. [Google Scholar] [CrossRef]
- Chenkin, J.; McCartney, C.J.; Jelic, T.; Romano, M.; Heslop, C.; Bandiera, G. Defining the learning curve of point-of-care ultrasound for confirming endotracheal tube placement by emergency physicians. Crit. Ultrasound J. 2015, 7, 14. [Google Scholar] [CrossRef]
- Tsai, W.W.; Hung, K.C.; Huang, Y.T.; Yu, C.H.; Lin, C.H.; Chen, I.W.; Sun, C.K. Diagnostic efficacy of sonographic measurement of laryngeal air column width difference for predicting the risk of post-extubation stridor: A meta-analysis of observational studies. Front. Med. 2023, 10, 1109681. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Mezière, G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest 2008, 134, 117–125. [Google Scholar] [CrossRef]
- Asmara, O.D.; Pitoyo, C.W.; Wulani, V.; Harimurti, K.; Araminta, A.P. Accuracy of Bedside Lung Ultrasound in Emergency (BLUE) Protocol to Diagnose the Cause of Acute Respiratory Distress Syndrome (ARDS): A Meta-Analysis. Acta Med. Indones. 2022, 54, 266–282. [Google Scholar]
- Neto, F.L.D.; De Andrade, J.M.S.; Raupp, A.C.T.; Townsend, R.D.S.; Beltrami, F.G.; Brisson, H.; Lu, Q.; Dalcin, P.D.T.R. Diagnostic accuracy of the Bedside Lung Ultrasound in Emergency protocol for the diagnosis of acute respiratory failure in spontaneously breathing patients. J. Bras. Pneumol. 2015, 41, 58–64. [Google Scholar] [CrossRef]
- Patel, C.J.; Bhatt, H.B.; Parikh, S.N.; Jhaveri, B.N.; Puranik, J.H. Bedside Lung Ultrasound in Emergency Protocol as a Diagnostic Tool in Patients of Acute Respiratory Distress Presenting to Emergency Department. J. Emerg. Trauma Shock 2018, 11, 125–129. [Google Scholar] [CrossRef]
- Chaitra, S.; Hattiholi, V.V. Diagnostic Accuracy of Bedside Lung Ultrasound in Emergency Protocol for the Diagnosis of Acute Respiratory Failure. J. Med. Ultrasound 2021, 30, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Arslan, B.; Sonmez, O. Diagnosis of a Ruptured Pulmonary Hydatid Cyst in a 26-Week Pregnant Female with Bedside Lung Ultrasound in Emergency (BLUE) Protocol: A Case Report. Cureus 2022, 14, e25431. [Google Scholar] [CrossRef] [PubMed]
- Haaksma, M.E.; Nossent, E.J.; Elbers, P.; Tuinman, P.R. Case Report: Pulmonary hemorrhage as a rare cause of lung ultrasound A/B-profile. F1000Research 2019, 8, 788. [Google Scholar] [CrossRef]
- Staub, L.J.; Mazzali Biscaro, R.R.; Kaszubowski, E.; Maurici, R. Lung Ultrasound for the Emergency Diagnosis of Pneumonia, Acute Heart Failure, and Exacerbations of Chronic Obstructive Pulmonary Disease/Asthma in Adults: A Systematic Review and Meta-analysis. J. Emerg. Med. 2019, 56, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.A.; Shams, N.; Ellington, L.E.; Naithani, N.; Gilman, R.H.; Steinhoff, M.C.; Santosham, M.; Black, R.E.; Price, C.; Gross, M.; et al. Lung ultrasound for the diagnosis of pneumonia in adults: A systematic review and meta-analysis. Respir. Res. 2014, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.A.; Al-Salamah, M.A.; Al-Madani, W.H.; Elbarbary, M.A. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit. Ultrasound J. 2017, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Grabala, J.; Grabala, M.; Onichimowski, D.; Grabala, P. Assessment of the applicability of transthoracic lung ultrasound for diagnosing purulent Lobar Pneumonia: A case study. Pol. Ann. Med. 2020, 27, 174–177. [Google Scholar] [CrossRef]
- Gardecki, J.; Patel, K.; Rowshan, O. Scan the lung: Point-of-care ultrasound of a pulmonary consolidation with loculated pleural effusion. Am. J. Emerg. Med. 2019, 37, 377.e1–377.e3. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.A.; Mezière, G.; Lascols, N.; Biderman, P.; Courret, J.P.; Gepner, A.; Goldstein, I.; Tenoudji-Cohen, M. Ultrasound diagnosis of occult pneumothorax. Crit. Care Med. 2005, 33, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Huang, X.Y.; Zhang, L. Ultrasound guiding the rapid diagnosis and treatment of perioperative pneumothorax: A case report. World J. Clin. Cases 2021, 9, 11043–11049. [Google Scholar] [CrossRef] [PubMed]
- Mallow, C.; Isakow, W. Risk Factors for Loss of Lung Sliding in a Medical Intensive Care Population with Acute Respiratory Failure. J. Bronchol. Interv. Pulmonol. 2019, 26, 102–107. [Google Scholar] [CrossRef]
- Aziz, S.G.; Patel, B.B.; Ie, S.R.; Rubio, E.R. The Lung Point Sign, not Pathognomonic of a Pneumothorax. Ultrasound Q. 2016, 32, 277–279. [Google Scholar] [CrossRef]
- Bass, C.M.; Sajed, D.R.; Adedipe, A.A.; West, T.E. Pulmonary ultrasound and pulse oximetry versus chest radiography and arterial blood gas analysis for the diagnosis of acute respiratory distress syndrome: A pilot study. Crit. Care 2015, 19, 282. [Google Scholar] [CrossRef]
- Todur, P.; Souvik Chaudhuri FNB Critical Care; Vedaghosh Amara FNB Critical Care; Srikant, N.; Prakash, P. Correlation of Oxygenation and Radiographic Assessment of Lung Edema (RALE) Score to Lung Ultrasound Score (LUS) in Acute Respiratory Distress Syndrome (ARDS) Patients in the Intensive Care Unit. Can. J. Respir. Ther. 2021, 57, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Jiang, L.; Xi, X.; Jiang, Q.; Zhu, B.; Wang, M.; Xing, J.; Zhang, D. Prognostic value of extravascular lung water assessed with lung ultrasound score by chest sonography in patients with acute respiratory distress syndrome. BMC Pulm. Med. 2015, 15, 98. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, S.; Mou, Z.; Wang, Y.; Li, X. Correlation Analysis between Mechanical Power and Lung Ultrasound Score and Their Evaluation of Severity and Prognosis in ARDS Patients. BioMed Res. Int. 2021, 2021, 4156162. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Qi, B.; Zhang, X.; Meng, L.; Wu, X. Prophetic values of lung ultrasound score on post-extubation distress in patients with acute respiratory distress syndrome. Eur. J. Med. Res. 2022, 27, 27. [Google Scholar] [CrossRef] [PubMed]
- See, K.C.; Ong, V.; Tan, Y.L.; Sahagun, J.; Taculod, J. Chest radiography versus lung ultrasound for identification of acute respiratory distress syndrome: A retrospective observational study. Crit. Care 2018, 22, 203. [Google Scholar] [CrossRef]
- Lv, W.; Wang, S.; Wang, L.; Wu, Z.; Jiang, Y.; Chen, X.; Gao, R. G994T polymorphism in exon 9 of plasma platelet-activating factor acetylhydrolase gene and lung ultrasound score as prognostic markers in evaluating the outcome of acute respiratory distress syndrome. Exp. Ther. Med. 2019, 17, 3174–3180. [Google Scholar] [CrossRef] [PubMed]
- Riishede, M.; Lassen, A.T.; Baatrup, G.; Pietersen, P.I.; Jacobsen, N.; Jeschke, K.N.; Laursen, C.B. Point-of-care ultrasound of the heart and lungs in patients with respiratory failure: A pragmatic randomized controlled multicenter trial. Scand. J. Trauma Resusc. Emerg. Med. 2021, 29, 60. [Google Scholar] [CrossRef] [PubMed]
- Mantuani, D.; Frazee, B.W.; Fahimi, J.; Nagdev, A. Point-of-Care Multi-Organ Ultrasound Improves Diagnostic Accuracy in Adults Presenting to the Emergency Department with Acute Dyspnea. West. J. Emerg. Med. 2016, 17, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Laursen, C.B.; Sloth, E.; Lambrechtsen, J.; Lassen, A.T.; Madsen, P.H.; Henriksen, D.P.; Davidsen, J.R.; Rasmussen, F. Focused sonography of the heart, lungs, and deep veins identifies missed life-threatening conditions in admitted patients with acute respiratory symptoms. Chest 2013, 144, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Zieleskiewicz, L.; Lagier, D.; Contargyris, C.; Bourgoin, A.; Gavage, L.; Martin, C.; Leone, M. Lung ultrasound-guided management of acute breathlessness during pregnancy. Anaesthesia 2013, 68, 97–101. [Google Scholar] [CrossRef]
- Barman, B.; Parihar, A.; Kohli, N.; Agarwal, A.; Dwivedi, D.K.; Kumari, G. Impact of Bedside Combined Cardiopulmonary Ultrasound on Etiological Diagnosis and Treatment of Acute Respiratory Failure in Critically Ill Patients. Indian J. Crit. Care Med. 2020, 24, 1062–1070. [Google Scholar] [CrossRef]
- Sen, S.; Acash, G.; Sarwar, A.; Lei, Y.; Dargin, J.M. Utility and diagnostic accuracy of bedside lung ultrasonography during medical emergency team (MET) activations for respiratory deterioration. J. Crit. Care 2017, 40, 58–62. [Google Scholar] [CrossRef]
- Silva, S.; Biendel, C.; Ruiz, J.; Olivier, M.; Bataille, B.; Geeraerts, T.; Mari, A.; Riu, B.; Fourcade, O.; Genestal, M. Usefulness of cardiothoracic chest ultrasound in the management of acute respiratory failure in critical care practice. Chest 2013, 144, 859–865. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, L.; Chang, W.; Wu, Z.; Huang, L.; Chao, Y.; Lu, X.; Xie, J.; Yang, Y.; Qiu, H. Diagnosis Accuracy of Lung Ultrasound for ARF in Critically Ill Patients: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 705960. [Google Scholar] [CrossRef] [PubMed]
- Smit, J.M.; Haaksma, M.E.; Winkler, M.H.; Heldeweg, M.L.A.; Arts, L.; Lust, E.J.; Elbers, P.W.G.; Meijboom, L.J.; Girbes, A.R.J.; Heunks, L.M.A.; et al. Lung ultrasound in a tertiary intensive care unit population: A diagnostic accuracy study. Crit. Care 2021, 25, 339. [Google Scholar] [CrossRef]
- Chiumello, D.; Umbrello, M.; Sferrazza Papa, G.F.; Angileri, A.; Gurgitano, M.; Formenti, P.; Coppola, S.; Froio, S.; Cammaroto, A.; Carrafiello, G. Global and Regional Diagnostic Accuracy of Lung Ultrasound Compared to CT in Patients with Acute Respiratory Distress Syndrome. Crit. Care Med. 2019, 47, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Hew, M.; Corcoran, J.P.; Harriss, E.K.; Rahman, N.M.; Mallett, S. The diagnostic accuracy of chest ultrasound for CT-detected radiographic consolidation in hospitalised adults with acute respiratory failure: A systematic review. BMJ Open 2015, 5, e007838. [Google Scholar] [CrossRef] [PubMed]
- Tierney, D.M.; Huelster, J.S.; Overgaard, J.D.; Plunkett, M.B.; Boland, L.L.; St Hill, C.A.; Agboto, V.K.; Smith, C.S.; Mikel, B.F.; Weise, B.E.; et al. Comparative Performance of Pulmonary Ultrasound, Chest Radiograph, and CT Among Patients with Acute Respiratory Failure. Crit. Care Med. 2020, 48, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Nazerian, P.; Volpicelli, G.; Vanni, S.; Gigli, C.; Betti, L.; Bartolucci, M.; Zanobetti, M.; Ermini, F.R.; Iannello, C.; Grifoni, S. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am. J. Emerg. Med. 2015, 33, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Gaber, H.R.; Mahmoud, M.I.; Carnell, J.; Rohra, A.; Wuhantu, J.; Williams, S.; Rafique, Z.; Peacock, W.F. Diagnostic accuracy and temporal impact of ultrasound in patients with dyspnea admitted to the emergency department. Clin. Exp. Emerg. Med. 2019, 6, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.A.; Bahmani, A.; Fathi, M.; Arefi, M.; Hossein Sarbazi, A.; Teimoori, M. Role of point-of-care ultrasound study in early disposition of patients with undifferentiated acute dyspnea in emergency department: A multi-center prospective study. J. Ultrasound 2022, 25, 443–449. [Google Scholar] [CrossRef]
- Baid, H.; Vempalli, N.; Kumar, S.; Arora, P.; Walia, R.; Chauhan, U.; Shukla, K.; Verma, A.; Chawang, H.; Agarwal, D. Point of care ultrasound as initial diagnostic tool in acute dyspnea patients in the emergency department of a tertiary care center: Diagnostic accuracy study. Int. J. Emerg. Med. 2022, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Kilaru, D.; Panebianco, N.; Baston, C. Diaphragm Ultrasound in Weaning from Mechanical Ventilation. Chest 2021, 159, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.H.; Saha, B.; Jones, D.M.; Beegle, S. Respiratory Failure Secondary to Diaphragmatic Paralysis from Acute Exacerbation of Dermatomyositis. Am. J. Med. Sci. 2021, 361, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Kalın, B.S.; Gürsel, G. Does it make difference to measure diaphragm function with M mode (MM) or B mode (BM)? J. Clin. Monit. Comput. 2020, 34, 1247–1257. [Google Scholar] [CrossRef]
- Chu, S.E.; Lu, J.X.; Chang, S.C.; Hsu, K.H.; Goh, Z.N.L.; Seak, C.K.; Seak, J.C.; Ng, C.J.; Seak, C.J. Point-of-care application of diaphragmatic ultrasonography in the emergency department for the prediction of development of respiratory failure in community-acquired pneumonia: A pilot study. Front. Med. 2022, 9, 960847. [Google Scholar] [CrossRef] [PubMed]
- Antenora, F.; Fantini, R.; Iattoni, A.; Castaniere, I.; Sdanganelli, A.; Livrieri, F.; Tonelli, R.; Zona, S.; Monelli, M.; Clini, E.M.; et al. Prevalence and outcomes of diaphragmatic dysfunction assessed by ultrasound technology during acute exacerbation of COPD: A pilot study. Respirology 2017, 22, 338–344. [Google Scholar] [CrossRef]
- Elsayed, A.A.; Neanaa, E.H.M.; Beshey, B.N. Diaphragmatic impairment as a predictor of invasive ventilation in acute exacerbation of chronic obstructive pulmonary disease patients. Egypt. J. Anaesth. 2022, 38, 334–341. [Google Scholar] [CrossRef]
- Marchioni, A.; Castaniere, I.; Tonelli, R.; Fantini, R.; Fontana, M.; Tabbì, L.; Viani, A.; Giaroni, F.; Ruggieri, V.; Cerri, S.; et al. Ultrasound-assessed diaphragmatic impairment is a predictor of outcomes in patients with acute exacerbation of chronic obstructive pulmonary disease undergoing noninvasive ventilation. Crit. Care 2018, 22, 109. [Google Scholar] [CrossRef]
- Cammarota, G.; Sguazzotti, I.; Zanoni, M.; Messina, A.; Colombo, D.; Vignazia, G.L.; Vetrugno, L.; Garofalo, E.; Bruni, A.; Navalesi, P.; et al. Diaphragmatic Ultrasound Assessment in Subjects with Acute Hypercapnic Respiratory Failure Admitted to the Emergency Department. Respir. Care 2019, 64, 1469–1477. [Google Scholar] [CrossRef]
- Barbariol, F.; Deana, C.; Guadagnin, G.M.; Cammarota, G.; Vetrugno, L.; Bassi, F. Ultrasound diaphragmatic excursion during non-invasive ventilation in ICU: A prospective observational study. Acta Bio-Med. Atenei Parm. 2021, 92, e2021269. [Google Scholar] [CrossRef]
- Laverdure, F.; Genty, T.; Rezaiguia-Delclaux, S.; Herve, P.; Stephan, F. Ultrasound Assessment of Respiratory Workload with High-Flow Nasal Oxygen Versus Other Noninvasive Methods After Chest Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3042–3047. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, G.S. Point-of-care ultrasonography and liberation from mechanical ventilation. Crit. Care 2017, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Khan, A.; Khalil, A.; Asghar, A. Diaphragmatic Excursion: Does it Predict Successful Weaning from Mechanical Ventilation? J. Coll. Physicians Surg. 2017, 27, 743–746. [Google Scholar]
- Pirompanich, P.; Romsaiyut, S. Use of diaphragm thickening fraction combined with rapid shallow breathing index for predicting success of weaning from mechanical ventilator in medical patients. J. Intensive Care 2018, 6, 6. [Google Scholar] [CrossRef]
- Tenza-Lozano, E.; Llamas-Alvarez, A.; Jaimez-Navarro, E.; Fernández-Sánchez, J. Lung and diaphragm ultrasound as predictors of success in weaning from mechanical ventilation. Crit. Ultrasound J. 2018, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Haaksma, M.E.; Smit, J.M.; Heldeweg, M.; Nooitgedacht, J.S.; Atmowihardjo, L.N.; Jonkman, A.H.; de Vries, H.J.; Lim, E.H.; Steenvoorden, T.; Lust, E.; et al. Holistic Ultrasound to Predict Extubation Failure in Clinical Practice. Respir. Care 2021, 66, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P.; McCarty, J.P.; Lazzara, A.A. Case Study of Phrenic Nerve Paralysis: “I Can’t Breathe!”. J. Emerg. Med. 2020, 58, e237–e241. [Google Scholar] [CrossRef]
- Yajima, W.; Yoshida, T.; Kondo, T.; Uzura, M. Respiratory failure due to diaphragm paralysis after brachial plexus injury diagnosed by point-of-care ultrasound. BMJ Case Rep. 2022, 15, e246923. [Google Scholar] [CrossRef]
- Shrestha, G.S. Bedside sonographic evaluation of the diaphragm in ventilator dependent patients with amyotrophic lateral sclerosis. A report of two cases. Nepal Med. Coll. J. 2014, 16, 95–98. [Google Scholar]
- Lichtenstein, D. Lung ultrasound in the critically ill. Curr. Opin. Crit. Care 2014, 20, 315–322. [Google Scholar] [CrossRef]
- Lichtenstein, D. Novel approaches to ultrasonography of the lung and pleural space: Where are we now? Breathe 2017, 13, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; van Hooland, S.; Elbers, P.; Malbrain, M.L. Ten good reasons to practice ultrasound in critical care. Anaesthesiol. Intensive Ther. 2014, 46, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Reissig, A.; Copetti, R. Lung ultrasound in community-acquired pneumonia and in interstitial lung diseases. Respiration 2014, 87, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Cereda, M.; Xin, Y.; Goffi, A.; Herrmann, J.; Kaczka, D.W.; Kavanagh, B.P.; Perchiazzi, G.; Yoshida, T.; Rizi, R.R. Imaging the Injured Lung: Mechanisms of Action and Clinical Use. Anesthesiology 2019, 131, 716–749. [Google Scholar] [CrossRef]
- Tuinman, P.R.; Jonkman, A.H.; Dres, M.; Shi, Z.H.; Goligher, E.C.; Goffi, A.; de Korte, C.; Demoule, A.; Heunks, L. Respiratory muscle ultrasonography: Methodology, basic and advanced principles and clinical applications in ICU and ED patients—A narrative review. Intensive Care Med. 2020, 46, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Haji, K.; Royse, A.; Green, C.; Botha, J.; Canty, D.; Royse, C. Interpreting diaphragmatic movement with bedside imaging, review article. J. Crit. Care 2016, 34, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, Q. Research Progress on Diaphragm Ultrasound in Chronic Obstructive Pulmonary Disease: A Narrative Review. Ultrasound Med. Biol. 2022, 48, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Westerway, S.C.; Basseal, J.M.; Brockway, A.; Hyett, J.A.; Carter, D.A. Potential infection control risks associated with ultrasound equipment—A bacterial perspective. Ultrasound Med. Biol. 2017, 43, 421–426. [Google Scholar] [CrossRef]
- Moore, C.L.; Wang, J.; Battisti, A.J.; Chen, A.; Fincke, J.; Wang, A.; Baloescu, C. Interobserver Agreement and Correlation of an Automated Algorithm for B-Line Identification and Quantification with Expert Sonologist Review in a Handheld Ultrasound Device. J. Ultrasound Med. 2022, 41, 2487–2495. [Google Scholar] [CrossRef]
- Kumar, A.; Kugler, J.; Jensen, T. Evaluation of trainee competency with point-of-care ultrasonography (POCUS): A conceptual framework and review of existing assessments. J. Gen. Intern. Med. 2019, 34, 1025–1031. [Google Scholar] [CrossRef]
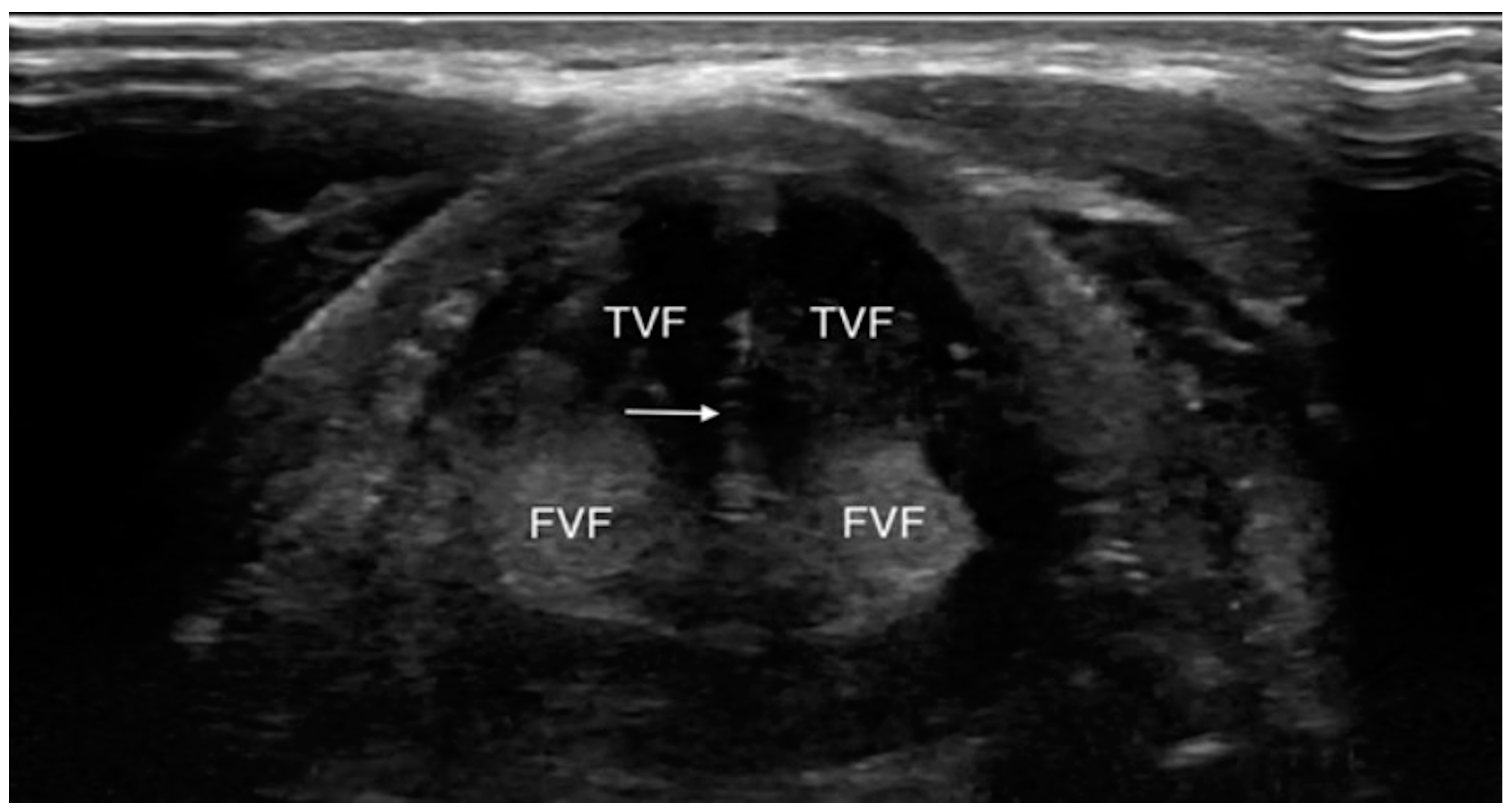
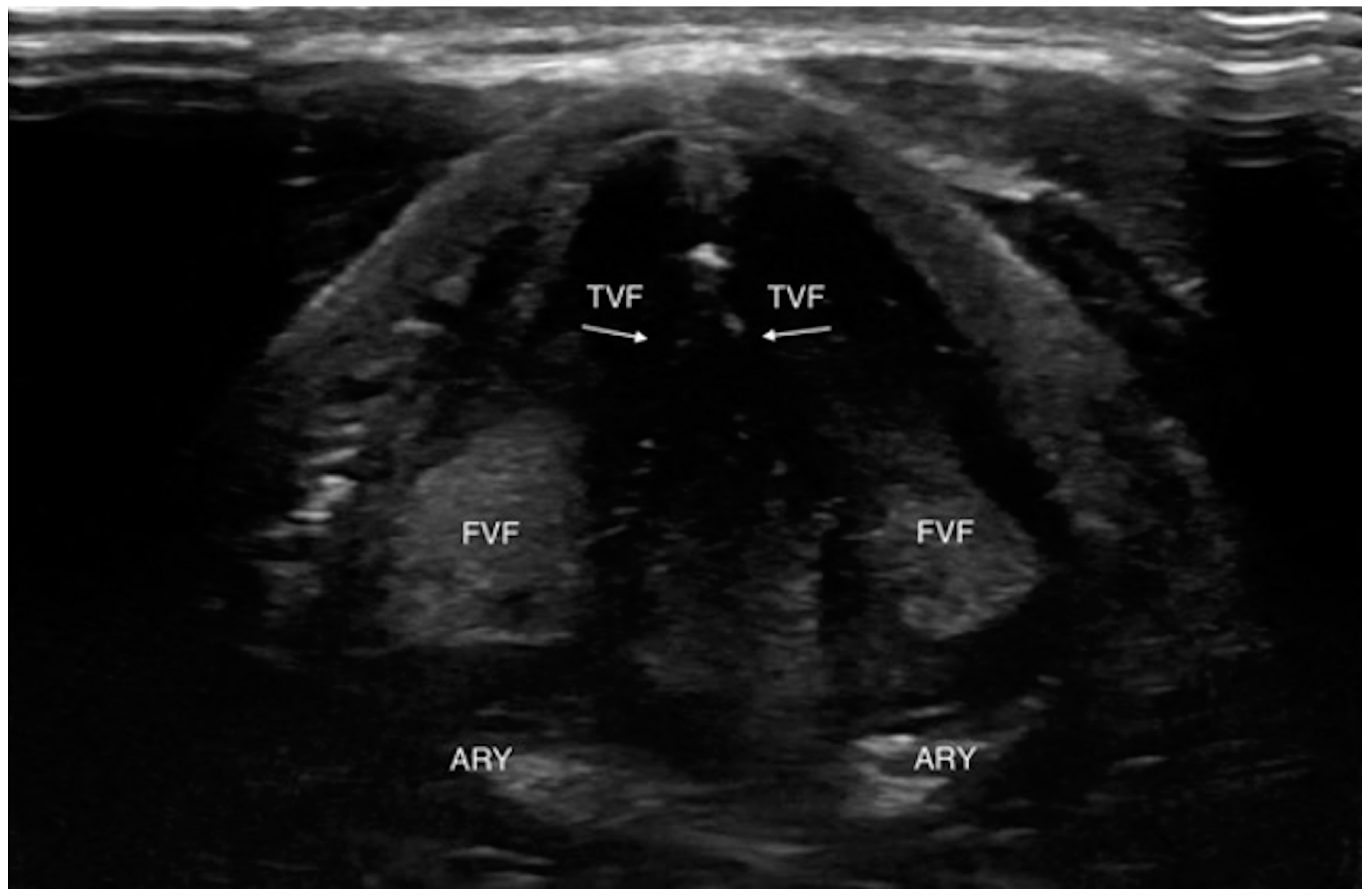
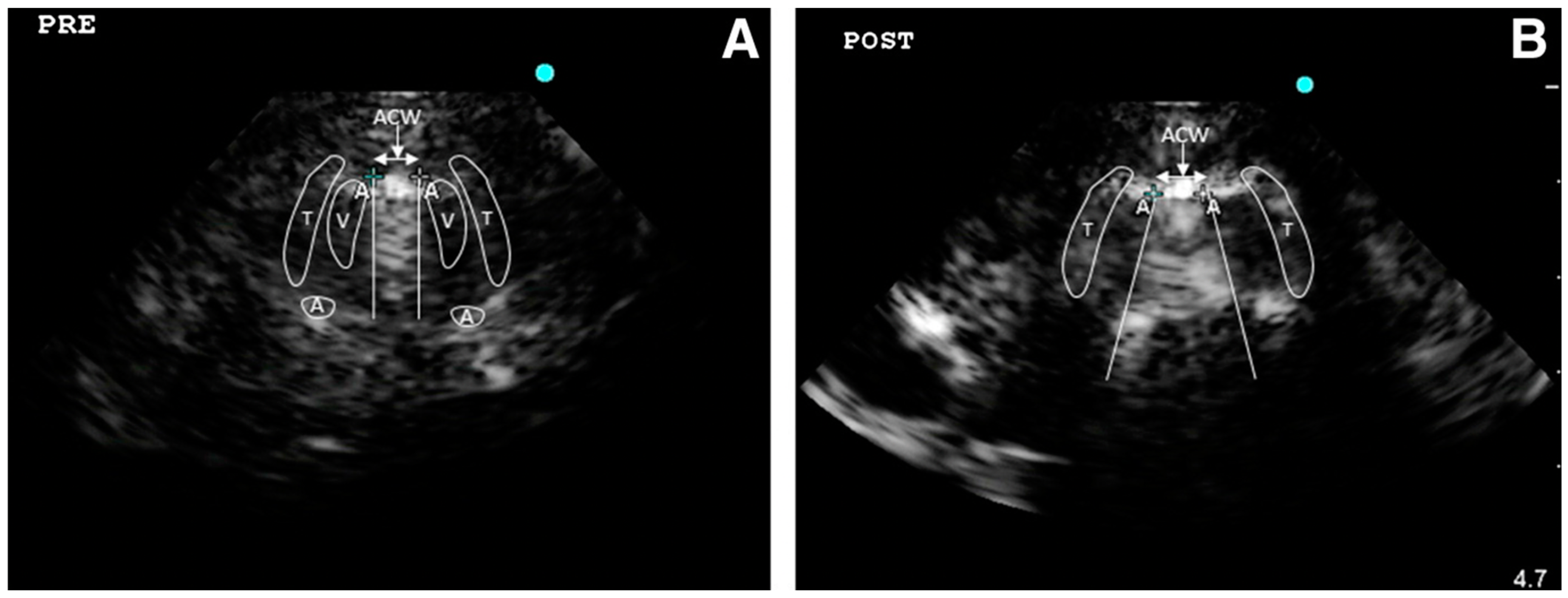

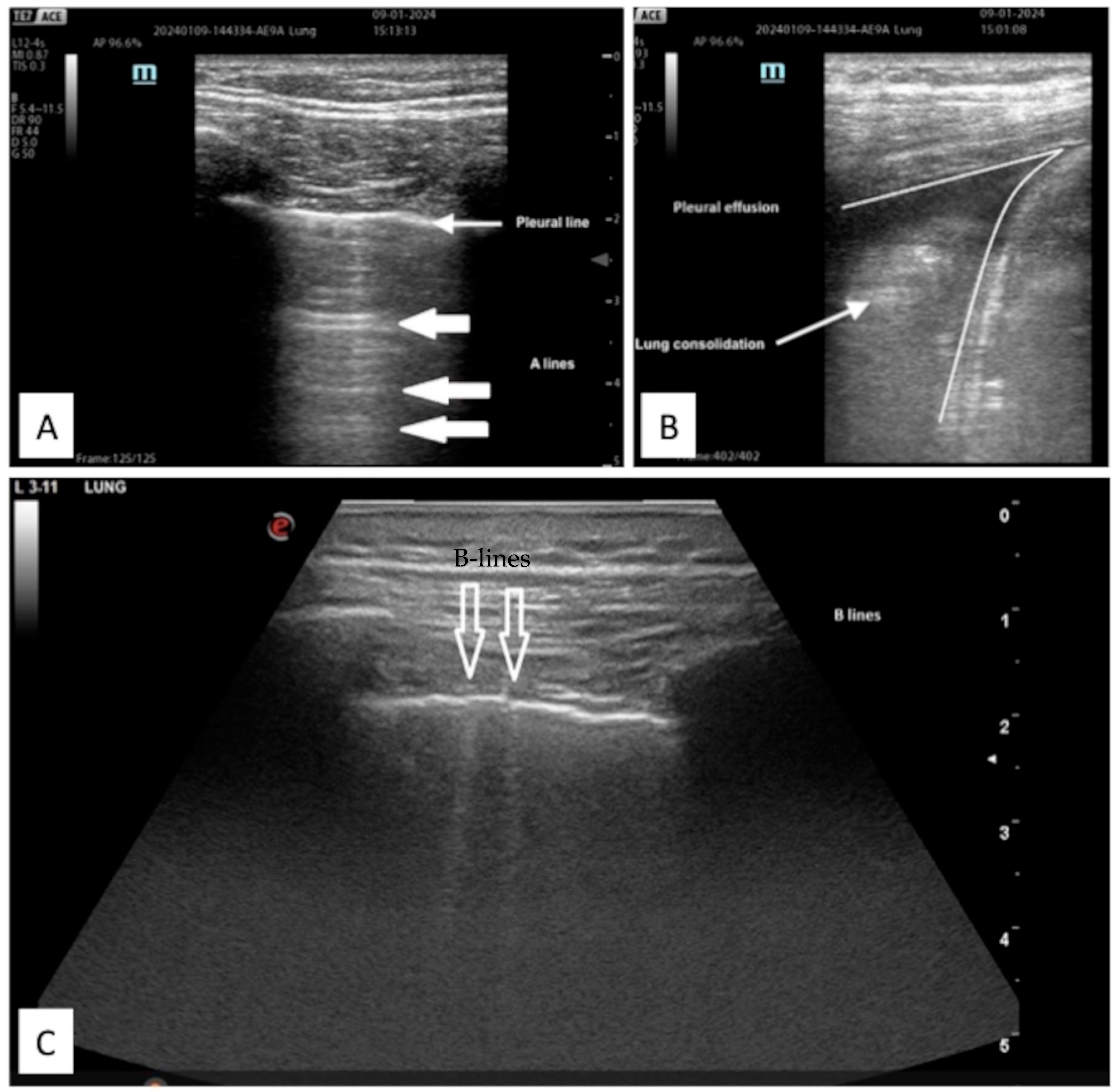

| First Author, Year | State | Kind of Study | N. Subject |
|---|---|---|---|
| Endotracheal tube (ETT) positioning assessment | |||
| Hoffmann B et al., 2014 [7] | USA | Observational study | 86 |
| Wojtczak JA et al., 2014 [8] | USA | Sperimental | |
| Hossein-Nejad, 2021 [9] | Iran | RCT | 16 students perform trial on 3 different cadavers |
| Sim SS et al., 2011 [10] | Taiwan | Observational study | 115 |
| Upper Airways damage identification | |||
| Schick M et al., 2016 [11] | USA | Case report | 1 |
| Adi O et al., 2020 [12] | Malaysia | Case series | 4 |
| Difficult airways management and post-trauma injury identification | |||
| Nicholls SE et al., 2008 [13] | USA | Quasi sperimental | 50 |
| Adi O et al., 2021 [14] | Malaysia | Case report | 1 |
| Iqhbal M et al., 2018 [15] | Malaysia | Case report | 1 |
| Laringeal edema assessment pre-extubation | |||
| Sutherasan Y et al., 2013 [16] | Thailand | Observational study | 101 |
| Mikaeili H 2014 [17] | Iran | Prospective study | 41 |
| Schaefer Classification | CT Scan Findings, Based on Schaefer Classification | Focused Airway Ultrasound Findings | Standard Management and Intervention |
|---|---|---|---|
| Group 1 | Minor endolaryngeal hematoma or laceration without detectable fracture | Endolaryngeal hematoma without detectable fracture | Supportive care including observation, antibiotics, humidified air, supplemental oxygen, anti-reflux medications, voice rest and early steroid administration |
| Group 2 | Edema, hematoma, minor mucosal disruption without exposed cartilage, nondisplaced fracture noted on CT | Edema, endolaryngeal hematoma, minor mucosal disruption without exposed cartilage, nondisplaced fracture, mucosal hematoma/edema, nondisplaced fracture of cartilage framework | Patients with Group 2 injuries should be serially examined, since the injuries may worsen or progress with time. Occasionally group 2 injuries may require a tracheotomy |
| Group 3 | Massive edema, mucosal tear, exposed cartilage, cord immobility, displaced fracture | Edema, cord immobility, displaced fracture, vocal fold immobility, obvious displaced fracture | Direct laryngoscopy, esophagoscopy and immediate open surgical repair are deemed necessary due to extension of injuries |
| Group 4 | Addition of more than two fracture lines or massive trauma to laryngeal mucosa | Addition of more than two fracture lines, comminuted fracture of laryngeal cartilage framework | |
| Group 5 | Complete laryngeal separation |
| First Author, Year | State | Kind of Study | N. Subject |
|---|---|---|---|
| Protocol on lung US | |||
| Lichtenstein DA and Mezier GA, 2008 [23] | France | Observational study | 301 |
| Asmara OD et al., 2022 [24] | Indonesia | Systematic review and meta-analysis | |
| Dexheimer Neto FL et al., 2015 [25] | Brazil | Observational study | 42 |
| Patel CJ et al., 2018 [26] | India | Observational study | 50 |
| Chaitra S and Hattiholi VV, 2022 [27] | India | Cross-sectional study | 130 |
| Arslan B and Sonmez O, 2022 [28] | Instanbul | Case report | 1 |
| Haaksma, ME et al., 2019 [29] | The Netherlands | Case report | 1 |
| Staub LJ et al., 2019 [30] | Brazil | Systematic review and meta-analysis | |
| Chavez MA et al., 2014 [31] | Perù | Systematic review and meta-analysis | |
| Alzahrani, S.A [32] | Saudi Arabia | Sistematic review | |
| Grabala J et al., 2020 [33] | Poland | Case study | 1 |
| Gardecki J et al., 2019 [34] | USA | Case study | 1 |
| Pneumothorax (PNX) | |||
| Lichtenstein DA et al., 2005 [35] | France | Observational study | 200 |
| Zhang G et al., 2021 [36] | China | Case report | 1 |
| Mallow C et al., 2019 [37] | USA | Observational study | 159 |
| Aziz SG et al., 2016 [38] | USA | Case report | 1 |
| ARDS | |||
| Bass CM et al., 2015 [39] | USA | Prospective comparative study | 77 |
| Todur P et al., 2021 [40] | India | Observational study | 37 |
| Zhao Z et al., 2015 [41] | China | Observational study | 21 |
| Xie Y et al., 2021 [42] | China | Prospective observational study | 121 |
| Wang R et al., 2022 [43] | China | Prospective observational study | 92 |
| See KC et al., 2018 [44] | Singapore | Retrospective observational study | 456 |
| Lv W et al., 2019 [45] | China | Prospective observational (?) | 112 |
| Diagnostic accuracy | |||
| Riishede M et al., 2021 [46] | Denmark | RCT | 211 |
| Mantuani D et al., 2016 [47] | UK | Observational study | 57 |
| Laursen CB et al., 2013 [48] | Denmark | Observational study | 139 |
| Zieleskiewicz L et al., 2013 [49] | France | Observational study | 165 |
| Barman B et al., 2020 [50] | India | Observational study | 108 |
| Sen S et al., 2017 [51] | USA | Prospective study | 50 |
| Silva S et al., 2013 [52] | France | Observational study | 78 |
| Yuan X et al., 2021 [53] | China | Systematic review and meta-analysis | |
| Smit JM et al., 2021 [54] | The Netherlands | Observational study | 87 |
| Chiumello et al., 2019 [55] | Italy | ERS statement | |
| Hew M et al., 2015 [56] | Singapore | Systematic review | |
| Tierney DM et al., 2020 [57] | USA | Cohort study | 67 |
| Nazerian P et al., 2015 [58] | Italy | Observational study | 285 |
| Time-to-diagnosis improvement | |||
| Lichtenstein DA and Mezier GA, 2008 [23] | France | Observational study | 301 |
| Gaber HR et al., 2019 [59] | Egypt/USA | RCT | 59 |
| Zare MA et al., 2022 [60] | Iran | Observational study | 103 |
| Baid H et al., 2022 [61] | India | Observational study | 237 |
| Riishede M et al., 2021 [46] | Denmark | RCT | 211 |
| Kilaru D et al., 2021 [62] | USA | Case report | 1 |
| Chong WH et al., 2021 [63] | USA | Case report | 1 |
| Kalın BS et al., 2020 [64] | Turkey | Observational study | 62 |
| Chu SE et al., 2022 [65] | Taiwan | Cohort study | 50 |
| Antenora F et al., 2017 [66] | Italy | Pilot study | 41 |
| Elsayed AA et al., 2022 [67] | Canada | Observational study | 15 |
| Marchioni A et al., 2018 [68] | Italy | Cohort study | 75 |
| Cammarota G et al., 2019 [69] | Italy | Evaluation study | 22 |
| Barbariol F et al., 2021 [70] | Italy | Observational study | 47 |
| Laverdure F et al., 2019 [71] | France | Clinical trial | 50 |
| Shrestha GS et al., 2017 [72] | Nepal | Letter to editor | |
| Hayat A et al., 2017 [73] | UK | Cross-sectional comparative study | 100 |
| Pirompanich P and Romsaiyut S, 2018 [74] | Thailand | Observational study | 34 |
| Tenza-Lozano E et al., 2018 [75] | Spain | Cross-sectional comparative study | 109 |
| Haaksma ME et al., 2021 [76] | UK | Case report | 1 |
| Doyle MP et al., 2020 [77] | USA | Case report | 1 |
| Yajima W et al., 2022 [78] | Japan | Case report | 1 |
| Shrestha GS et al., 2014 [79] | Nepal | Case report | 2 |
| Diagnostic Accuracy with Standard Care vs. POCUS | Appropriate Treatment with Standard Care vs. POCUS | ||
|---|---|---|---|
| Controlled multicenter study | Riishede M et al., 2021 [46] | 77.1–79.3% | 65.7–79.3% |
| Observational study | Mantuani D et al., 2016 [47] | 53–77% | |
| Randomized controlled study | Laursen CB et al., 2014 [48] | 63.7–88.0% | |
| Prospective observational study | Zieleskiewicz L et al., 2013 [49] | 80–94% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartini, S.; Ferrari, L.; Cutuli, O.; Castellani, L.; Bagnasco, M.; Moisio Corsello, L.; Bracco, C.; Cristina, M.L.; Arboscello, E.; Sartini, M. The Role of Pocus in Acute Respiratory Failure: A Narrative Review on Airway and Breathing Assessment. J. Clin. Med. 2024, 13, 750. https://doi.org/10.3390/jcm13030750
Sartini S, Ferrari L, Cutuli O, Castellani L, Bagnasco M, Moisio Corsello L, Bracco C, Cristina ML, Arboscello E, Sartini M. The Role of Pocus in Acute Respiratory Failure: A Narrative Review on Airway and Breathing Assessment. Journal of Clinical Medicine. 2024; 13(3):750. https://doi.org/10.3390/jcm13030750
Chicago/Turabian StyleSartini, Stefano, Lorenzo Ferrari, Ombretta Cutuli, Luca Castellani, Maddalena Bagnasco, Luca Moisio Corsello, Cristina Bracco, Maria Luisa Cristina, Eleonora Arboscello, and Marina Sartini. 2024. "The Role of Pocus in Acute Respiratory Failure: A Narrative Review on Airway and Breathing Assessment" Journal of Clinical Medicine 13, no. 3: 750. https://doi.org/10.3390/jcm13030750
APA StyleSartini, S., Ferrari, L., Cutuli, O., Castellani, L., Bagnasco, M., Moisio Corsello, L., Bracco, C., Cristina, M. L., Arboscello, E., & Sartini, M. (2024). The Role of Pocus in Acute Respiratory Failure: A Narrative Review on Airway and Breathing Assessment. Journal of Clinical Medicine, 13(3), 750. https://doi.org/10.3390/jcm13030750







