To Be Frail or Not to Be Frail: This Is the Question—A Critical Narrative Review of Frailty
Abstract
1. Introduction
1.1. What Does Frailty Mean and Who Are the Frail Elderly?
1.2. Frailty as a Syndrome with Impairments of Multiple Systems
1.3. Frailty in the Era of COVID-19 Pandemic
1.4. Aim of This Narrative Review
2. Definition of Frailty in History
2.1. The Science of Frailty
2.2. Multiple Areas and Domains of Frailty
3. The Concept of Frailty in the Pandemics
3.1. The Black Death
3.2. The Russian Flu Pandemic of 1889
3.3. Pandemics of the 20th Century
3.3.1. The Spanish Flu
3.3.2. The Asian Flu
3.3.3. The Hong Kong Flu
3.4. HIV/AIDS
3.5. Pandemics of the 21st Century, the Century of the Coronavirus
3.5.1. Severe Acute Respiratory Syndrome (SARS)
3.5.2. Middle East Respiratory Syndrome (MERS)
3.5.3. Coronavirus Disease 2019 (COVID-19)
4. Prevalence and Incidence of Frailty
5. Risk Factors for Frailty
- Skills for Health, NHS England, and the Health Education England Frailty Framework of Core Capabilities [116]
- NHS England Practical guide to healthy aging [117]
- Age UK advice on keeping active and aging well [118]
- NHS Choices provides advice on how physical activity and exercise can help people stay healthy, energetic, and independent as they grow older [119]
- NICE supporting guidance on healthy aging [120]
- Public Health England guidance on productive healthy aging and musculoskeletal health [121]
- The Academy of Medical Royal Colleges has published “Exercise: The miracle cure and the role of the doctor in promoting it” [122]
- CDC’s National Center for Chronic Disease Prevention and Health Promotion [123]
- Italian National Center for Disease Prevention and Health Promotion [124]
- Gaining Health: Making Healthy Choices Easier Italy [125]
6. Various Pathogenic Mechanisms of Frailty
6.1. Relevance of Genetic Predisposition to Frailty
6.2. Frailty and Socioeconomic and Demographic Factors
6.3. The Role of Aging
6.4. Frailty and Subjective Perception of Aging
6.5. The Importance of “Inflammaging”
6.6. Frailty and Aging of the Brain
6.7. Frailty and Nutritional State
6.8. Frailty and Energy Metabolism (the Role of Mitochondria)
6.9. The Accumulation of Morbidities and Frailty
7. Frailty and Diseases
7.1. Frailty and Chronic Diseases
7.2. Frailty and Cardiovascular Diseases
7.3. Frailty and Cancer
7.4. Frailty and Neurocognitive System
7.5. Frailty and Chronic Pain
7.6. Frailty and Mental Health
7.6.1. Frailty and Stress
7.6.2. Frailty and Psychosocial Factors
7.6.3. Frailty and Depression
7.6.4. Frailty and Anxiety
7.6.5. Frailty and Suicide
7.6.6. Frailty and Sleep Disorders
7.6.7. Frailty and Dementia
7.6.8. Frailty and Delirium
7.6.9. Frailty and Severe Mental Illness (SMI)
7.7. Frailty and the Endocrine System
Frailty and the Low T3 Syndrome
8. Frailty and Vaccines
9. Biomarkers of Frailty
10. Methods to Assess Frailty
10.1. Physical Frailty Phenotype (PFP)
10.2. The Clinical Frailty Scale (CFS)
10.3. The Cardiovascular Health Study (CHS) Criteria
10.4. The Edmonton Frail Scale (EFS)
10.5. The Five-Item Frailty Screening Index
10.6. The Frailty Index (FI)
10.7. The Comprehensive Geriatric Assessment (CGA)
11. The European Guidelines
12. Interventions to Reduce Frailty
12.1. Vitamin D Supplementation
12.2. Weight Loss
12.3. Polypharmacy Reduction
12.4. Increase in Physical Activity
13. Frailty and Implications for Policy and Practice
14. Frailty and Digital Health
15. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AADL | Advanced Activities of Daily Living |
| AARC | Awareness of Age-Related Changes |
| AC | Acute Care |
| AD | Alzheimer’s Disease |
| ADL | Activity of Daily Living |
| AHA | American Heart Association |
| ALT | Alanine Aminotransferase |
| ATP | Adenosine TriPhosphate |
| BIA | Bioelectrical Impedance Analysis |
| BMI | Body Mass Index |
| Brain2ICV | Brain-to-IntraCranial-Volume |
| CABG | coronary artery bypass graft |
| cART | combination AntiRetroviral Therapy |
| CFS | Clinical Frailty Scale |
| CGA | Comprehensive Geriatric Assessment |
| CGAST | Comprehensive Geriatric Assessment Screening Tests |
| CHS | Cardiovascular Health Study |
| COVID-19 | COronaVirus Disease-2019 |
| CRP | C-Reactive Protein |
| CSF | CerebroSpinal Fluid |
| CSHA | Canadian Study of Health and Aging |
| CVD | CardioVascular Disease |
| DEXA | Dual-energy X-ray Absorptiometry |
| DHEA-S | DeHydroEpiAndrosterone Sulfate |
| EC | European Commission |
| EFS | Edmonton Frail Scale |
| ELSA | English Longitudinal Study of Ageing |
| EPCRC | European Palliative Care Research Collaborative |
| EuGMS | European Geriatric Medicine Society |
| FFP | Frailty Physical Phenotype |
| FI | Frailty Index |
| FI40 | 40-item Frailty Index |
| FS | Frail Scale |
| FSS | Frailty Staging System |
| FTSS | Five-Times-Sit-to-Stand |
| G8 | G-8 Geriatric Screening Tool |
| GFI | Groningen Frailty Indicator |
| GVAP | Global Vaccine Action Plan |
| GWAS | Genome Wide Association Studies |
| HHS | Health and Human Services |
| HPA | Hypothalamic-Pituitary-Adrenal |
| HRCA | Hebrew Rehabilitation Center for Aged Vulnerability Index |
| I.A.G.G. | International Association of Gerontology and Geriatrics |
| I.A.N.A | International Academy on Nutrition and Aging |
| IADL | Intermediate Activities of Daily Living |
| ICU | Intensive Care Unit |
| interRAI | international Resident Assessment Instrument |
| ISTAT | “Istituto Nazionale di Statistica” |
| KMBI | Korean Modified Barthel Index |
| LAC | Latin America and the Caribbean |
| LCFA | Long Chain Fatty Acid |
| LTC | Long-Term Care |
| LTL | Leukocyte Telomere Length |
| MCI | Mild Cognitive Impairment |
| MFRTA | Mitochondrial Free Radical Theory of Ageing |
| MI | myocardial infarction |
| MRI | Magnetic Resonance Imaging |
| mtROS | Mitochondrial Reactive Oxygen Species |
| NCHS | National Center for Health Statistics |
| NGAL | Neutrophil Gelatinase-Associated Lipocalin |
| NGEU | Next Generation EU |
| NITS | NonThyroidal Illness Syndrome |
| NLTCS | Long-Term Care Survey Frailty Index |
| NRRP | National Recovery and Resilience Plan (Piano Nazionale di Ripresa e Resilienza) |
| NSAID | Non-Steroid Anti-Inflammatory Drug |
| NUTRIONCO | NUTRItional status at first medical oncology visit ON Clinical Outcomes |
| OA | OsteoArthritis |
| OARS | Older Americans Resources and Services |
| PCI | percutaneous coronary intervention |
| PFI | Physical Frailty Index |
| PFP | Physical Frailty Phenotype |
| PhA | Phase Angle |
| PHF | Phenotype of Frailty |
| PLWH | People Living With HIV |
| PreMiO | Prevalence of Malnutrition in Oncology |
| ROS | Reactive Oxygen Species |
| SARS | Severe Acute Respiratory Syndrome |
| SHCFS | Canadian Study of Health and Aging Clinical Frailty Scale |
| SMI | Severe Mental Illness |
| TAVR | Trans-cutaneous Aortic Valve Replacement |
| TUG | Timed Up and Go |
| VaD | Vascular Dementia |
| VAS | Visual Analogue Scale |
| VE13S | Vulnerable Elders Survey |
| WHRH | World Health organization assessment of functional capacity and self-Reported Health |
| WMH | White Matter Hyperintensity |
References
- Vaupel, J.W.; Manton, K.G.; Stallard, E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography 1979, 16, 439–454. [Google Scholar] [CrossRef]
- Woodhouse, K.W.; Wynne, H.; Baillie, S.; James, O.F.; Rawlins, M.D. Who are the frail elderly? Q. J. Med. 1988, 68, 505–506. [Google Scholar]
- Winograd, C.H.; Gerety, M.B.; Brown, E.; Kolodny, V. Targeting the hospitalized elderly for geriatric consultation. J. Am. Geriatr. Soc. 1988, 36, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, S.R. The social construction of frailty: An anthropological perspective. J. Ageing Stud. 1994, 8, 45–58. [Google Scholar] [CrossRef]
- Walston, J.; Buta, B.; Xue, Q.L. Frailty screening and interventions: Considerations for clinical practice. Clin. Geriatr. Med. 2018, 34, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Langlois, F.; Vu, T.T.; Kergoat, M.J.; Chassé, K.; Dupuis, G.; Bherer, L. The multiple dimensions of frailty: Physical capacity, cognition, and quality of life. Int. Psychogeriatr. 2012, 24, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Gabrovec, B.; Antoniadou, E.; Soleymani, D.; Kadalska, E.; Carriazo, A.M.; Samaniego, L.L.; Csizmadia, P.; Hendry, A.; Bacaicoa, O.A.; Jelenc, M.; et al. Need for comprehensive management of frailty at an individual level: European perspective from the Advantage joint action on frailty. J. Rehabil. Med. 2020, 52, jrm00075. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.B.; MacKnight, C.; Bergman, H. Models, definitions, and criteria of frailty. Ageing Clin. Exp. Res. 2003, 15 (Suppl. 3), 1–29. [Google Scholar]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Aburto, J.M.; Schöley, J.; Kashnitsky, I.; Zhang, L.; Rahal, C.; Missov, T.I.; Mills MCDowd, J.B.; Kashyap, R. Quantifying impacts of the COVID-19 pandemic through life-expectancy losses: A population-level study of 29 countries. Int. J. Epidemiol. 2022, 51, 63–74. [Google Scholar] [CrossRef]
- Silvestris, N.; Belleudi, V.; Addis, A.; Pimpinelli, F.; Morrone, A.; Sciacchitano, S.; Mancini, R.; Garrisi, V.M.; Costantini, M.; Ciliberto, G.; et al. Development of Approaches and Metrics to Measure the Impact and Improve the Clinical Outcomes of Patients With Frailty in the Era of COVID-19. The COMETA Italian Protocol. Front. Oncol. 2022, 12, 828660. [Google Scholar] [CrossRef] [PubMed]
- Stamford, B.A. Physiological effects of training upon institutionalized geriatric men. J. Gerontol. 1972, 27, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.V.; Wykle, M.H.; Cowling, W.R., 3rd. Failure to thrive in older persons: A concept derived. Gerontologist 1988, 28, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Theou, O.; Cann, L.; Blodgett, J.; Wallace, L.M.; Brothers, T.D.; Rockwood, K. Modifications to the frailty phenotype criteria: Systematic review of the current literature and investigation of 262 frailty phenotypes in the survey of health, ageing, and retirement in Europe. Ageing Res. Rev. 2015, 21, 78–94. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). World Report on Ageing and Health. 2015. Available online: https://www.who.int/publications/i/item/9789241565042 (accessed on 2 November 2023).
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Abellan van Kan, G.; Rolland, Y.; Bergman, H.; Morley, J.E.; Kritchevsky, S.B.; Vellas, B. The IANA task force on frailty assessment of older people in clinical practice. J. Nutr. Health Ageing 2008, 12, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.L. The frailty syndrome: Definition and natural history. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef]
- Gobbens, R.J.; Luijkx, K.G.; Wijnen-Sponselee, M.T.; Schols, J.M. In search of an integral conceptual definition of frailty: Opinions of experts. J. Am. Med. Dir. Assoc. 2010, 11, 338–343. [Google Scholar] [CrossRef]
- Rodríguez-Mañas, L.; Féart, C.; Mann, G.; Viña, J.; Chatterji, S.; Chodzko-Zajko, W.; Gonzalez-Colaço Harmand, M.; Bergman, H.; Carcaillon, L.; Nicholson, C.; et al. Searching for an operational definition of frailty: A Delphi method-based consensus statement: The frailty operative definition-consensus conference project. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 62–67. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Integrated Care for Older People: Recommendations on Interventions to Manage Declining Physical and Mental Capacities in Older People at Community Level. 2017. Available online: https://iris.who.int/bitstream/handle/10665/258981/9789241550109-eng.pdf?sequence=1 (accessed on 2 November 2023).
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Cesari, M. Frailty: What Is It? Adv. Exp. Med. Biol. 2020, 1216, 1–7. [Google Scholar] [PubMed]
- Kinney, J.M. Nutritional frailty, sarcopenia and falls in the elderly. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Collins’ Teams of Language Experts. Definition of ‘Frail’. Available online: https://www.collinsdictionary.com/dictionary/english/frail (accessed on 2 November 2023).
- Piret, J.; Boivin, G. Pandemics Throughout History. Front. Microbiol. 2021, 11, 631736, Erratum in Front. Microbiol. 2022, 13, 988058. [Google Scholar] [CrossRef] [PubMed]
- Alfani, G.; Murphy, T.E. Plague and lethal epidemics in the pre-industrial world. J. Econ. Hist. 2017, 77, 314–343. [Google Scholar] [CrossRef]
- Mora, C.; McKenzie, T.; Gaw, I.M.; Dean, J.M.; von Hammerstein, H.; Knudson, T.A.; Setter, R.O.; Smith, C.Z.; Webster, K.M.; Patz, J.A.; et al. Over half of known human pathogenic diseases can be aggravated by climate change. Nat. Clim. Chang. 2022, 12, 869–875. [Google Scholar] [CrossRef]
- Walsh, B. The World Is Not Ready for the Next Pandemic. Time, 4 May 2017. Available online: https://time.com/magazine/us/4766607/may-15th-2017-vol-189-no-18-u-s/ (accessed on 2 November 2023).
- Potter, P. Of tidal waves and human frailty. Emerg. Infect. Dis. 2005, 11, 1653–1654. [Google Scholar] [CrossRef]
- Available online: https://en.wikipedia.org/wiki/File:The_Great_Wave_off_Kanagawa.jpg (accessed on 2 November 2023).
- Zedda, N.; Rinaldo, N.; Gualdi-Russo, E.; Bramanti, B. Overall frailty gauged in victims of the Italian plague (Imola, 1630–1632): Was plague an indiscriminate killer? Archaeol. Anthropol. Sci. 2022, 14, 199. [Google Scholar] [CrossRef]
- Boldsen, J.L. Early childhood stress and adult age mortality—A study of dental enamel hypoplasia in the medieval Danish village of Tirup. Am. J. Phys. Anthropol. 2007, 132, 59–66. [Google Scholar] [CrossRef]
- DeWitte, S.N. Sex differentials in frailty in medieval England. Am. J. Phys. Anthropol. 2010, 143, 285–297. [Google Scholar] [CrossRef] [PubMed]
- DeWitte, S.N. Sex differences in periodontal disease in catastrophic and attritional assemblages from medieval London. Am. J. Phys. Anthropol. 2012, 149, 405–416. [Google Scholar] [CrossRef] [PubMed]
- DeWitte, S.N. Differential survival among individuals with active and healed periosteal new bone formation. Int. J. Paleopathol. 2014, 7, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Marklein, K.E.; Leahy, R.E.; Crews, D.E. In sickness and in death: Assessing frailty in human skeletal remains. Am. J. Phys. Anthropol. 2016, 161, 208–225. [Google Scholar] [CrossRef] [PubMed]
- Kyle, B.; Reitsema, L.J.; Tyler, J.; Fabbri, P.F.; Vassallo, S. Examining the osteological paradox: Skeletal stress in mass graves versus civilians at the Greek colony of Himera (Sicily). Am. J. Phys. Anthropol. 2018, 167, 161–172. [Google Scholar] [CrossRef]
- DeWitte, S.N.; Wood, J.W. Selectivity of Black Death mortality with respect to preexisting health. Proc. Natl. Acad. Sci. USA 2008, 105, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- DeWitte, S.N.; Hughes-Morey, G. Stature and frailty during the Black Death: The effect of stature on risks of epidemic mortality in London, A.D. 1348–1350. J. Archaeol. Sci. 2012, 39, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- DeWitte, S.N. Age patterns of mortality during the Black Death in London, A.D. 1349–1350. J. Archaeol. Sci. 2010, 37, 3394–3400. [Google Scholar] [CrossRef]
- Godde, K.; Pasillas, V.; Sanchez, A. Survival analysis of the Black Death: Social inequality of women and the perils of life and death in Medieval London. Am. J. Phys. Anthropol. 2020, 173, 168–178. [Google Scholar] [CrossRef]
- Klunk, J.; Vilgalys, T.P.; Demeure, C.E.; Cheng, X.; Shiratori, M.; Madej, J.; Beau, R.; Elli, D.; Patino, M.I.; Redfern, R.; et al. Evolution of immune genes is associated with the Black Death. Nature 2022, 611, 312–319. [Google Scholar] [CrossRef]
- Kempińska-Mirosławska, B.; Woźniak-Kosek, A. The influenza epidemic of 1889–90 in selected European cities—A picture based on the reports of two Poznań daily newspapers from the second half of the nineteenth century. Med. Sci. Monit. 2013, 19, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Berche, P. The enigma of the 1889 Russian flu pandemic: A coronavirus? Presse Med. 2022, 51, 104111. [Google Scholar] [CrossRef] [PubMed]
- Bresalier, M. ‘A most protean disease’: Aligning medical knowledge of modern influenza, 1890–1914. Med. Hist. 2012, 56, 481–510. [Google Scholar] [CrossRef] [PubMed]
- Valtat, S.; Cori, A.; Carrat, F.; Valleron, A.J. Age distribution of cases and deaths during the 1889 influenza pandemic. Vaccine 2011, 29 (Suppl. 2), B6–B10. [Google Scholar] [CrossRef]
- Ramiro, D.; Garcia, S.; Casado, Y.; Cilek, L.; Chowell, G. Age-specific excess mortality patterns and transmissibility during the 1889–1890 influenza pandemic in Madrid, Spain. Ann. Epidemiol. 2018, 28, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Forum on Microbial Threats. Summary and Assessment. In The Impact of Globalization on Infectious Disease Emergence and Control: Exploring the Consequences and Opportunities: Workshop Summary; Knobler, S., Mahmoud, A., Lemon, S., Pray, L., Eds.; National Academies Press: Washington, DC, USA, 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK56579/ (accessed on 2 November 2023).
- Saunders-Hastings, P.R.; Krewski, D. Reviewing the History of Pandemic Influenza: Understanding Patterns of Emergence and Transmission. Pathogens 2016, 5, 66. [Google Scholar] [CrossRef]
- CDC. History of 1918 Flu Pandemic. Pandemic Influenza [Flu]. 2019. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/flu/pandemic-resources/1918-commemoration/1918-pandemic-history.htm (accessed on 2 November 2023).
- Institute of Medicine (US) Forum on Microbial Threats. The Story of Influenza. In The Threat of Pandemic Influenza: Are We Ready? Workshop Summary; Knobler, S.L., Mack, A., Mahmoud, A., Lemon, S.M., Eds.; National Academies Press: Washington, DC, USA, 2005. Available online: https://www.ncbi.nlm.nih.gov/books/NBK22148/ (accessed on 2 November 2023).
- Taubenberger, J.K. The origin and virulence of the 1918 “Spanish” influenza virus. Proc. Am. Philos. Soc. 2006, 150, 86–112. [Google Scholar]
- The 1918 Influenza Pandemic. 1918. Available online: https://virus.stanford.edu/uda/ (accessed on 2 November 2023).
- Patterson, K.D.; Pyle, G.F. The geography and mortality of the 1918 influenza pandemic. Bull. Hist. Med. 1991, 65, 4–21. [Google Scholar]
- Chandra, S.; Christensen, J. RE: Reassessing the global mortality burden of the 1918 influenza pandemic. Am. J. Epidemiol. 2019, 188, 1404–1406. [Google Scholar] [CrossRef]
- Morens, D.M.; Taubenberger, J.K. The mother of all pandemics is 100 years old (and going strong)! Am. J. Public Health 2018, 108, 1449–1454. [Google Scholar] [CrossRef]
- Viboud, C.; Eisenstein, J.; Reid, A.H.; Janczewski, T.A.; Morens, D.M.; Taubenberger, J.K. Age- and sex-specific mortality associated with the 1918–1919 influenza pandemic in Kentucky. J. Infect. Dis. 2013, 207, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Wissler, A.; Gauthier, N. The Frailty-Mortality Paradox: Insights from the Spanish Flu Pandemic of 1918. Available online: https://nick-gauthier.github.io/talk/saa-2019a/ (accessed on 2 November 2023).
- Erkoreka, A. The Spanish influenza pandemic in occidental Europe (1918–1920) and victim age. Influenza Other Respir. Viruses 2010, 4, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Woo, G. Age-dependence of the 1918 pandemic. Br. Actuar. J. 2019, 24, e3. [Google Scholar] [CrossRef]
- World Health Organization. Influenza Pandemic Plan. The Role of WHO and Guidelines for National and Regional Planning. April 1999. pp. 38–41. Available online: https://web.archive.org/web/20201203084733/https://www.who.int/csr/resources/publications/influenza/whocdscsredc991.pdf (accessed on 2 November 2023).
- Michaelis, M.; Doerr, H.W.; Cinatl, J. Novel swine-origin influenza A virus in humans: Another pandemic knocking at the door. Med. Microbiol. Immunol. 2009, 198, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Mermel, L.A. Swine-origin influenza virus in young age groups. Lancet 2009, 373, 2108–2109. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.M. Some aspects of the epidemiology of the 1957 influenza pandemic. Proc. R. Soc. Med. 1958, 51, 1009–1015. [Google Scholar]
- Viboud, C.; Simonsen, L.; Fuentes, R.; Flores, J.; Miller, M.A.; Chowell, G. Global mortality impact of the 1957–1959 influenza pandemic. J. Infect. Dis. 2016, 213, 738–745. [Google Scholar] [CrossRef]
- Cockburn, W.C.; Delon, P.J.; Ferreira, W. Origin and progress of the 1968–69 Hong Kong influenza epidemic. Bull. World Health Organ. 1969, 41, 345–348. [Google Scholar]
- Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 2 November 2023).
- Kehler, D.S.; Milic, J.; Guaraldi, G.; Fulop, T.; Falutz, J. Frailty in older people living with HIV: Current status and clinical management. BMC Geriatr. 2022, 22, 919. [Google Scholar] [CrossRef]
- Terzian, A.S.; Holman, S.; Nathwani, N.; Robison, E.; Weber, K.; Young, M.; Greenblatt, R.M.; Gange, S.J.; Women’s Interagency HIV Study. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. J. Womens Health 2009, 18, 1965–1974. [Google Scholar] [CrossRef]
- Gustafson, D.R.; Shi, Q.; Thurn, M.; Holman, S.; Minkoff, H.; Cohen, M.; Plankey, M.W.; Havlik, R.; Sharma, A.; Gange, S.; et al. Frailty and Constellations of Factors in Ageing HIV-infected and Uninfected Women—The Women’s Interagency HIV Study. J. Frailty Ageing 2016, 5, 43–48. [Google Scholar]
- Cohen, M.H.; Hotton, A.L.; Hershow, R.C.; Levine, A.; Bacchetti, P.; Golub, E.T.; Anastos, K.; Young, M.; Gustafson, D.; Weber, K.M. Gender-Related Risk Factors Improve Mortality Predictive Ability of VACS Index Among HIV-Infected Women. J. Acquir. Immune Defic. Syndr. 2015, 70, 538–544. [Google Scholar] [CrossRef]
- Sciacchitano, S.; Giovagnoli, S.; Amodeo, R.; Santino, I.; Simmaco, M.; Anibaldi, P.; French, D.; Mancini, R.; De Vitis, C.; D’Ascanio, M.; et al. AIDS and COVID-19 are two diseases separated by a common lymphocytopenia. Fortune J. Health Sci. 2023, 6, 200–209. [Google Scholar] [CrossRef]
- Centers for Disease Prevention and Control. HIV Among People Aged 50 and Over; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2016.
- Piggott, D.A.; Erlandson, K.M.; Yarasheski, K.E. Frailty in HIV: Epidemiology, biology, measurement, interventions, and research needs. Curr. HIV/AIDS Rep. 2016, 13, 340–348. [Google Scholar] [CrossRef]
- Guaraldi, G.; Malagoli, A.; Theou, O.; Brothers, T.D.; Wallace, L.; Torelli, R.; Mussini, C.; Sartini, S.; Kirkland, S.A.; Rockwood, K. Correlates of frailty phenotype and frailty index and their associations with clinical outcomes. HIV Med. 2017, 18, 764–771. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Das, A.; Sengupta, P.; Dutta, S.; Roychoudhury, S.; Choudhury, A.P.; Ahmed, A.B.F.; Bhattacharjee, S.; Slama, P. Viral Pandemics of the Last Four Decades: Pathophysiology, Health Impacts and Perspectives. Int. J. Environ. Res. Public Health 2020, 17, 9411. [Google Scholar] [CrossRef]
- Khabbaz, R.; Bell, B.P.; Schuchat, A.; Ostroff, S.M.; Moseley, R.; Levitt, A.; Hughes, J.M. Emerging and Reemerging Infectious Disease Threats. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier Saunders: Philadelphia, PA, USA, 2015; pp. 158–177.e6. [Google Scholar]
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H.; et al. Bats are natural reservoirs of SARS-like coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef]
- Guan, Y.; Zheng, B.J.; He, Y.Q.; Liu, X.L.; Zhuang, Z.X.; Cheung, C.L.; Luo, S.W.; Li, P.H.; Zhang, L.J.; Guan, Y.J.; et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 2003, 302, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Tong, S. Update on SARS research and other possibly zoonotic coronaviruses. Int. J. Antimicrob. Agents 2010, 36, S21–S25. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.S.C.; Chan, P.K.S. Severe acute respiratory syndrome and coronavirus. Infect. Dis. Clin. N. Am. 2010, 24, 619–638. [Google Scholar] [CrossRef] [PubMed]
- Cleri, D.J.; Ricketti, A.J.; Vernaleo, J.R. Severe acute respiratory syndrome (SARS). Infect. Dis. Clin. N. Am. 2010, 24, 175–202. [Google Scholar] [CrossRef]
- Conzade, R.; Grant, R.; Malik, M.R.; Elkholy, A.; Elhakim, M.; Samhouri, D.; Ben Embarek, P.K.; Van Kerkhove, M.D. Reported direct and indirect contact with dromedary camels among laboratory-confirmed MERS-CoV cases. Viruses 2018, 10, 425. [Google Scholar] [CrossRef]
- World Health Organization (WHO). MERS Situation Update, January 2020. Available online: http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html (accessed on 2 November 2023).
- In Hunt for COVID-19 Origin, Patient Zero Points to Second Wuhan Market—The Man with the First Confirmed Infection of the New Coronavirus Told the WHO Team That His Parents Had Shopped There. The Wall Street Journal, 26 February 2021.
- Gill, V. Covid Origin Studies Say Evidence Points to Wuhan Market. BBC News. 26 July 2022. Available online: https://www.bbc.com/news/science-environment-62307383 (accessed on 2 November 2023).
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 2 November 2023).
- WHO Coronavirus (COVID-19) Dashboard Overview. Available online: https://covid19.who.int (accessed on 28 June 2023).
- Islam, N.; Jdanov, D.A.; Shkolnikov, V.M.; Khunti, K.; Kawachi, I.; White, M.; Lewington, S.; Lacey, B. Effects of COVID-19 pandemic on life expectancy and premature mortality in 2020: Time series analysis in 37 countries. BMJ 2021, 375, e066768. [Google Scholar] [CrossRef]
- Parasher, A. COVID-19: Current understanding of its pathophysiology, clinical presentation and treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.L.; Pereira, R.M.R. Frailty in the context of COVID-19 pandemic: A life-threatening condition. Front. Med. 2022, 9, 965562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Jiao, J.; Cao, J.; Huo, X.P.; Zhu, C.; Wu, X.J.; Xie, X.H. Frailty as a predictor of mortality among patients with COVID-19: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 186. [Google Scholar] [CrossRef] [PubMed]
- Sablerolles, R.S.G.; Lafeber, M.; van Kempen, J.A.L.; van de Loo, B.P.A.; Boersma, E.; Rietdijk, W.J.R.; Polinder-Bos, H.A.; Mooijaart, S.P.; van der Kuy, H.; Versmissen, J.; et al. Association between clinical frailty scale score and hospital mortality in adult patients with COVID-19 (COMET): An international, multicentre, retrospective, observational cohort study. Lancet Healthy Longev. 2021, 2, e163–e170. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, F.; Branje, K.E.; Hladkowicz, E.S.; Lalu, M.; McIsaac, D.I. Association of frailty with outcomes in individuals with COVID-19: A living review and meta-analysis. J. Am. Geriatr. Soc. 2021, 69, 2419–2429. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A.; Shekar, K.; Afroz, A.; Ashwin, S.; Billah, B.; Brown, H.; Kundi, H.; Lim, Z.J.; Ponnapa Reddy, M.; Curtis, J.R. Frailty and mortality associations in patients with COVID-19: A systematic review and meta-analysis. Intern. Med. J. 2022, 52, 724–739. [Google Scholar] [CrossRef]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Ofori-Asenso, R.; Chin, K.L.; Mazidi, M.; Zomer, E.; Ilomaki, J.; Zullo, A.R.; Gasevic, D.; Ademi, Z.; Korhonen, M.J.; LoGiudice, D.; et al. Global Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults. A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e198398. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G. Prevalence of Frailty in Nursing Homes: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2015, 16, 940–945. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Goodkind, D.; Kowal, P. An Ageing World: 2015; U.S. Census Bureau, International Population Reports, P95/16-1; U.S. Government Publishing Office: Washington, DC, USA, 2016.
- Xue, Q.L.; Buta, B.; Varadhan, R. Frailty and Geriatric Syndromes. In Ageing, Place, and Health: A Global Perspective; Satariano, W.A., Maus, M., Eds.; Jones & Bartlett Learning: Burlington, MA, USA, 2017; pp. 191–230. [Google Scholar]
- Gill, T.M.; Gahbauer, E.A.; Allore, H.G.; Han, L. Transitions between frailty states among community-living older persons. Arch. Intern. Med. 2006, 166, 418–423. [Google Scholar] [CrossRef]
- Semba, R.D.; Blaum, C.S.; Bartali, B.; Xue, Q.L.; Ricks, M.O.; Guralnik, J.M.; Fried, L.P. Denture use, malnutrition, frailty, and mortality among older women living in the community. J. Nutr. Health Ageing 2006, 10, 161–167. [Google Scholar]
- O’Caoimh, R.; Galluzzo, L.; Rodríguez-Laso, Á.; Van der Heyden, J.; Ranhoff, A.H.; Lamprini-Koula, M.; Ciutan, M.; López-Samaniego, L.; Carcaillon-Bentata, L.; Kennelly, S.; et al. Prevalence of frailty at population level in European ADVANTAGE Joint Action Member States: A systematic review and meta-analysis. Ann. Ist. Super. Sanita 2018, 54, 226–238. [Google Scholar] [PubMed]
- Mata, F.A.F.d.; Pereira, P.P.d.S.; Andrade, K.R.C.d.; Figueiredo, A.C.M.G.; Silva, M.T.; Pereira, M.G. Prevalence of frailty in Latin America and the Caribbean: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0160019. [Google Scholar] [CrossRef]
- Bandeen-Roche, K.; Seplaki, C.L.; Huang, J.; Buta, B.; Kalyani, R.R.; Varadhan, R.; Xue, Q.L.; Walston, J.D.; Kasper, J.D. Frailty in Older Adults: A Nationally Representative Profile in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1427–1434. [Google Scholar] [CrossRef]
- Kalaiselvan, M.S.; Yadav, A.; Kaur, R.; Menon, A.; Wasnik, S. Prevalence of Frailty in ICU and its Impact on Patients’ Outcomes. Indian J. Crit. Care Med. 2023, 27, 335–341. [Google Scholar] [CrossRef]
- Feng, Z.; Lugtenberg, M.; Franse, C.; Fang, X.; Hu, S.; Jin, C.; Raat, H. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PLoS ONE 2017, 12, e0178383. [Google Scholar] [CrossRef]
- Vina, J.; Borras, C.; Gomez-Cabrera, M.C. A free radical theory of frailty. Free Radic. Biol. Med. 2018, 124, 358–363. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Geisler, C.; Braun, W.; Pourhassan, M.; Schweitzer, L.; Gluer, C.C.; Bosy-Westphal, A.; Müller, M.J. Gender-specific associations in age-related changes in resting energy expenditure (REE) and MRI measured body composition in healthy Caucasians. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Berry, P.; Schnitter, R. (Eds.) Health of Canadians in a Changing Climate: Advancing our Knowledge for Action; Government of Canada: Ottawa, ON, Canada, 2022; Available online: https://changingclimate.ca/site/assets/uploads/sites/5/2022/02/CCHA-REPORT-EN.pdf (accessed on 2 November 2023).
- Reres, A.; Hou, S. Identifying resources for promoting healthy ageing in community. Int. J. Popul. Stud. 2022, 8, 79–88. [Google Scholar] [CrossRef]
- NHS England and Health Education England Frailty Framework of Core Capabilities. Available online: http://www.skillsforhealth.org.uk/services/item/607-frailty-core-capabilities-framework (accessed on 2 November 2023).
- NHS England Practical Guide to Healthy Ageing. Available online: https://www.england.nhs.uk/ourwork/clinical-policy/older-people/healthy-ageing-caring/ (accessed on 2 November 2023).
- Age UK Advice on Keeping Active and Ageing Well. Available online: https://www.ageuk.org.uk/information-advice/health-wellbeing/ (accessed on 2 November 2023).
- NHS Choices Provides Advice on How Physical Activity and Exercise Can Help People Stay Healthy, Energetic, and Independent as They Get Older. Available online: https://www.england.nhs.uk/ourwork/clinical-policy/older-people/frailty/preventing-frailty/ (accessed on 2 November 2023).
- NICE Supporting Guidance on Healthy Ageing. Available online: https://www.nice.org.uk/search?q=Healthy+ageing (accessed on 2 November 2023).
- Public Health England Guidance on Productive Healthy Ageing and Musculoskeletal Health. Available online: https://www.gov.uk/government/publications/productive-healthy-ageing-and-musculoskeletal-health/productive-healthy-ageing-and-musculoskeletal-msk-health (accessed on 2 November 2023).
- Academy of Medical Royal Colleges. Exercise: The Miracle Cure and the Role of the Doctor in Promoting It. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwia2I71s_WDAxXjhP0HHTDHAWEQFnoECBUQAQ&url=https%3A%2F%2Fwww.aomrc.org.uk%2Fwp-content%2Fuploads%2F2016%2F03%2FExercise_the_Miracle_Cure_0215.pdf&usg=AOvVaw3xE0LLt3BWjNLM__p7t0jh&opi=89978449 (accessed on 2 November 2023).
- National Center for Chronic Disease Prevention and Health Promotion. Available online: https://www.cdc.gov/chronicdisease/resources/publications/factsheets/promoting-health-for-older-adults.htm (accessed on 2 November 2023).
- Italian National Center for Disease Prevention and Health Promotion. Available online: https://www.iss.it/web/iss-en/disease-prevention-and-health-promotion (accessed on 2 November 2023).
- Gaining Health: Making Healthy Choices Easier Italy. Available online: http://chrodis.eu/wp-content/uploads/2017/03/gaining-health-making-health-choices-easier.pdf (accessed on 2 November 2023).
- Buchner, D.M.; Wagner, E.H. Preventing frail health. Clin. Geriatr. Med. 1992, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P. Conference on the physiologic basis of frailty. April 28, 1992, Baltimore, Maryland, U.S.A. Introduction. Ageing 1992, 4, 251–252. [Google Scholar]
- Fried, L.P.; Walston, J. Frailty and failure to thrive. In Principles of Geriatric Medicine and Gerontology; Hazzard, W.R., Blass, J.P., Ettinger, W.H., Jr., Halter, J.B., Ouslander, J., Eds.; McGraw Hill: New York, NY, USA, 1998; Volume 4, pp. 1387–1402. [Google Scholar]
- Bortz, W.M., 2nd. The physics of frailty. J. Am. Geriatr. Soc. 1993, 41, 1004–1008. [Google Scholar]
- Lipsitz, L.A. Dynamics of stability: The physiologic basis of functional health and frailty. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B115–B125. [Google Scholar] [CrossRef]
- Varadhan, R.; Seplaki, C.L.; Xue, Q.L.; Bandeen-Roche, K.; Fried, L.P. Stimulus-response paradigm for characterizing the loss of resilience in homeostatic regulation associated with frailty. Mech. Ageing Dev. 2008, 129, 666–670. [Google Scholar] [CrossRef]
- Ferrucci, L.; Windham, B.G.; Fried, L. Frailty in Older Persons. Genus 2005, 61, 39–53. [Google Scholar]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of ageing. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Landi, F.; Calvani, R.; Cherubini, A.; Di Bari, M.; Kortebein, P.; Del Signore, S.; Le Lain, R.; Vellas, B.; Pahor, M.; et al. Rationale for a preliminary operational definition of physical frailty and sarcopenia in the SPRINTT trial. Ageing Clin. Exp. Res. 2017, 29, 81–88. [Google Scholar] [CrossRef]
- Harman, D. A theory based on free radical and radical chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Harman, D. The biological clock: The mitochondria? J. Am. Geriatr. Soc. 1972, 20, 99–117. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Sánchez-Flores, M.; Marcos-Pérez, D.; Lorenzo-López, L.; Maseda, A.; Millán-Calenti, J.C.; Pásaro, E.; Laffon, B. Exploring Genetic Outcomes as Frailty Biomarkers. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 168–175. [Google Scholar] [CrossRef]
- Skytthe, A.; Pedersen, N.L.; Kaprio, J.; Stazi, M.A.; Hjelmborg, J.V.; Iachine, I.; Vaupel, J.W.; Christensen, K. Longevity studies in GenomEUtwin. Twin Res. 2003, 6, 448–454. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dato, S.; Montesanto, A.; Lagani, V.; Jeune, B.; Christensen, K.; Passarino, G. Frailty phenotypes in the elderly based on cluster analysis: A longitudinal study of two Danish cohorts. Evidence for a genetic influence on frailty. Age 2012, 34, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Ni Lochlainn, M.; Malkin, I.; Bowyer, R.; Verdi, S.; Steves, C.J.; Williams, F.M.K. Shared genetic influence on frailty and chronic widespread pain: A study from TwinsUK. Age Ageing 2018, 47, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.L.; Fernandes, A.; Aguilar-Pimentel, J.A.; de Angelis, M.H.; Guedes, J.R.; Brito, M.A.; Ortolano, S.; Pani, G.; Athanasopoulou, S.; Gonos, E.S.; et al. Towards frailty biomarkers: Candidates from genes and pathways regulated in ageing and age-related diseases. Ageing Res. Rev. 2018, 47, 214–277. [Google Scholar] [CrossRef] [PubMed]
- Yashin, A.I.; Iachine, I.A. Genetic analysis of durations: Correlated frailty model applied to survival of Danish twins. Genetic Epidemiology 1995, 12, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Young, A.C.M.; Glaser, K.; Spector, T.D.; Steves, C.J. The Identification of Hereditary and Environmental Determinants of Frailty in a Cohort of UK Twins. Twin Research and Human Genetics. 2016, 19, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Murabito, J.M.; Yuan, R.; Lunetta, K.L. The search for longevity and healthy ageing genes: Insights from epidemiological studies and samples of long-lived individuals. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 470–479. [Google Scholar] [CrossRef]
- Mekli, K.; Stevens, A.; Marshall, A.D.; Arpawong, T.E.; Phillips, D.F.; Tampubolon, G.; Lee, J.; Prescott, C.A.; Nazroo, J.Y.; Pendleton, N. Frailty Index associates with GRIN2B in two representative samples from the United States and the United Kingdom. PLoS ONE 2018, 13, e0207824. [Google Scholar] [CrossRef]
- Sathyan, S.; Verghese, J. Genetics of frailty: A longevity perspective. Transl. Res. 2020, 221, 83–96. [Google Scholar] [CrossRef]
- Gurinovich, A.; Andersen, S.L.; Puca, A.; Atzmon, G.; Barzilai, N.; Sebastiani, P. Varying Effects of APOE Alleles on Extreme Longevity in European Ethnicities. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, S45–S51. [Google Scholar] [CrossRef]
- Sebastiani, P.; Gurinovich, A.; Nygaard, M.; Sasaki, T.; Sweigart, B.; Bae, H.; Andersen, S.L.; Villa, F.; Atzmon, G.; Christensen, K.; et al. APOE alleles and extreme human longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 74, 44–51. [Google Scholar] [CrossRef]
- Willcox, B.J.; Donlon, T.A.; He, Q.; Chen, R.; Grove, J.S.; Yano, K.; Masaki, K.H.; Willcox, D.C.; Rodriguez, B.; Curb, J.D. FOXO3A genotype is strongly associated with human longevity. Proc. Natl. Acad. Sci. USA 2008, 105, 13987–13992. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.J.; Willcox, D.C.; Donlon, T.A.; Willcox, B.J. FOXO3: A major gene for human longevity-a mini-review. Gerontology 2015, 61, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Noche, R.B.; Szejko, N.; Both, C.P.; Acosta, J.N.; Leasure, A.C.; Brown, S.C.; Sheth, K.N.; Gill, T.M.; Zhao, H.; et al. A genome-wide association study of frailty identifies significant genetic correlation with neuropsychiatric, cardiovascular, and inflammation pathways. Geroscience 2023, 45, 2511–2523. [Google Scholar] [CrossRef] [PubMed]
- Severe COVID-19 GWAS Group; Ellinghaus, D.; Degenhardt, F.; Bujanda, L.; Buti, M.; Albillos, A.; Fernandez, J.; Fernandez, J.; Prati, D.; Baselli, G.; et al. Genomewide Association Study of Severe COVID-19 with Respiratory Failure. N. Engl. J. Med. 2020, 383, 1522–1534. [Google Scholar] [PubMed]
- Breno, M.; Noris, M.; Rubis, N.; Parvanova, A.I.; Martinetti, D.; Gamba, S.; Liguori, L.; Mele, C.; Piras, R.; Orisio, S.; et al. A GWAS in the pandemic epicenter highlights the severe COVID-19 risk locus introgressed by Neanderthals. iScience 2023, 26, 107629. [Google Scholar] [CrossRef] [PubMed]
- Jagoda, E.; Marnetto, D.; Senevirathne, G.; Gonzalez, V.; Baid, K.; Montinaro, F.; Richard, D.; Falzarano, D.; LeBlanc, E.V.; Colpitts, C.C.; et al. Regulatory dissection of the severe COVID-19 risk locus introgressed by Neanderthals. eLife 2023, 12, e71235. [Google Scholar] [CrossRef]
- Siriwardhana, D.D.; Hardoon, S.; Rait, G.; Weerasinghe, M.C.; Walters, K.R. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries: A systematic review and meta-analysis. BMJ Open 2018, 8, e018195. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155,722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Majid, Z.; Welch, C.; Davies, J.; Jackson, T. Global frailty: The role of ethnicity, migration and socioeconomic factors. Maturitas 2020, 139, 33–41. [Google Scholar] [CrossRef]
- Periyakoil, V.S. Building a culturally competent workforce to care for diverse older adults: Scope of the problem and potential solutions. J. Am. Geriatr. Soc. 2019, 67, S423–S432. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs, Population Division (2020). World Population Ageing 2020 HIGHLIGHTS: Living Arrangements of Older Persons (ST/ESA/SER.A/451). Available online: https://digitallibrary.un.org/record/3898412/files/undesa_pd-2020_world_population_ageing_highlights.pdf (accessed on 2 November 2023).
- United Nations Department of Economic and Social Affairs. World Social Report 2023: Leaving No One Behind in an Ageing World; United Nations Publication ST/ESA/379; United Nations: New York, NY, USA, 2023; ISBN 978-92-1-130458-9/978-92-1-001968-2. Available online: https://www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2023/01/2023wsr-fullreport.pdf (accessed on 2 November 2023).
- Ng, K. BBC News Singapore. Available online: https://www.bbc.com/news/world-asia-66850943 (accessed on 2 November 2023).
- Trentesima Edizione del Rapporto Annuale 2022—La Situazione del Paese; Italian National Institute of Statistics: Rome, Italy, 2022; ISBN 978-88-458-2080-9. Available online: www.istat.it (accessed on 2 November 2023).
- Fulop, T.; Larbi, A.; Witkowski, J.M.; McElhaney, J.; Loeb, M.; Mitnitski, A.; Pawelec, G. Ageing, frailty and age-related diseases. Biogerontology 2010, 11, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Ahn, A.; Kim, S.; Won, C.W. Global prevalence of physical frailty by Fried’s criteria in community-dwelling elderly with national population-based surveys. J. Am. Med. Dir. Assoc. 2015, 16, 548–550. [Google Scholar] [CrossRef]
- Austad, S.N. The Geroscience Hypothesis: Is It Possible to Change the Rate of Ageing? In Advances in Geroscience; Sierra, F., Kohanski, R., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty measurement in research and clinical practice: A review. Eur. J. Intern. Med. 2016, 31, 3–10. [Google Scholar] [CrossRef]
- Alves, S.; Teixeira, L.; Ribeiro, O.; Paúl, C. Examining Frailty Phenotype Dimensions in the Oldest Old. Front. Psychol. 2020, 11, 434. [Google Scholar] [CrossRef]
- Ribeiro, O.; Duarte, N.; Teixeira, L.; Paúl, C. Frailty and depression in centenarians. Int. Psychogeriatr. 2018, 30, 115–124. [Google Scholar] [CrossRef]
- Stephan, Y.; Sutin, A.R.; Terracciano, A. Physical activity and subjective age across adulthood in four samples. Eur. J. Ageing 2019, 17, 469–476. [Google Scholar] [CrossRef]
- Hwang, Y.; Hong, G.S. Predictors of subjective age in community-dwelling older adults in Korea. Geriatr. Nurs. 2019, 40, 314–319. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Vacante, M.; Frazzetto, P.M.; Motta, M. What is the frailty in elderly? Value and significance of the multidimensional assessments. Arch. Gerontol. Geriatr. 2013, 56, 23–26. [Google Scholar] [CrossRef]
- Rudolph, C.W.; Kunze, F.; Zacher, H. Getting Objective About Subjective Age: Introduction to a Special Issue. Work Ageing Retire. 2019, 5, 265–272. [Google Scholar] [CrossRef]
- Rubin, D.C.; Berntsen, D. People over forty feel 20% younger than their age: Subjective age across the lifespan. Psychon. Bull. Rev. 2006, 13, 776–780. [Google Scholar] [CrossRef]
- Kotter-Grühn, D.; Neupert, S.D.; Stephan, Y. Feeling old today? Daily health, stressors, and affect explain day-to-day variability in subjective age. Psychol. Health 2015, 30, 1470–1485. [Google Scholar] [CrossRef] [PubMed]
- Stephan, Y.; Sutin, A.R.; Terracciano, A. How old do you feel? The role of age discrimination and biological ageing in subjective age. PLoS ONE 2015, 10, e0119293. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, M.; Miyawaki, C.E.; Sun, X.; Hou, T.; Tang, S.; Szanton, S.L. Bidirectional relationship between subjective age and frailty: A prospective cohort study. BMC Geriatr. 2021, 21, 395. [Google Scholar] [CrossRef] [PubMed]
- Armenta, B.M.; Stroebe, K.; Scheibe, S.; Postmes, T.; Van Yperen, N.W. Feeling younger and identifying with older adults: Testing two routes to maintaining well-being in the face of age discrimination. PLoS ONE 2017, 12, e0187805. [Google Scholar] [CrossRef]
- Stephan, Y.; Chalabaev, A.; Kotter-Grühn, D.; Jaconelli, A. “Feeling younger, being stronger”: An experimental study of subjective age and physical functioning among older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2013, 68, 1–7. [Google Scholar] [CrossRef]
- Fried, L.P.; Kronma, l.R.A.; Newman, A.B.; Bild, D.E.; Mittelmark, M.B.; Polak, J.F.; Robbins, J.A.; Gardin, J.M. Risk factors for 5-year mortality in older adults: The Cardiovascular Health Study. JAMA 1998, 279, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.; McBurnie, M.A.; Newman, A.; Tracy, R.P.; Kop, W.J.; Hirsch, C.H.; Gottdiener, J.; Fried, L.P. Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the Cardiovascular Health Study. Arch. Intern. Med. 2002, 162, 2333–2341. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Knopp, P.; Miles, A.; Webb, T.E.; Mcloughlin, B.C.; Mannan, I.; Raja, N.; Wan, B.; Davis, D. Presenting features of COVID-19 in older people: Relationships with frailty, inflammation and mortality. Eur. Geriatr. Med. 2020, 11, 1089–1094. [Google Scholar] [CrossRef]
- Diehl, M.K.; Wahl, H.W. Awareness of age-related change: Examination of a (Mostly) unexplored concept. J. Gerontol. B Psychol. Sci. Soc. Sci. 2010, 65B, 340–350. [Google Scholar] [CrossRef]
- Kwak, S.; Kim, H.; Chey, J.; Youm, Y. Feeling How Old I Am: Subjective Age Is Associated With Estimated Brain Age. Front. Ageing Neurosci. 2018, 10, 168. [Google Scholar] [CrossRef]
- Jernigan, T.L.; Archibald, S.L.; Berhow, M.T.; Sowell, E.R.; Foster, D.S.; Hesselink, J.R. Cerebral structure on MRI, Part I: Localization of age-related changes. Biol. Psychiatry 1991, 29, 55–67. [Google Scholar] [CrossRef]
- Raz, N.; Gunning, F.M.; Head, D.; Dupuis, J.H.; McQuain, J.; Briggs, S.D.; Loken, W.J.; Thornton, A.E.; Acker, J.D. Selective ageing of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cereb. Cortex 1997, 7, 268–282. [Google Scholar] [CrossRef]
- Resnick, S.M.; Goldszal, A.F.; Davatzikos, C.; Golski, S.; Kraut, M.A.; Metter, E.J.; Bryan, R.N.; Zonderman, A.B. One-year age changes in MRI brain volumes in older adults. Cereb. Cortex 2000, 10, 464–472. [Google Scholar] [CrossRef]
- Tisserand, D.J.; Pruessner, J.C.; Sanz Arigita, E.J.; van Boxtel, M.P.; Evans, A.C.; Jolles, J.; Uylings, H.B. Regional frontal cortical volumes decrease differentially in ageing: An MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage 2002, 17, 657–669. [Google Scholar] [CrossRef]
- Resnick, S.M.; Pham, D.L.; Kraut, M.A.; Zonderman, A.B.; Davatzikos, C. Longitudinal magnetic resonance imageing studies of older adults: A shrinking brain. J. Neurosci. 2003, 23, 3295–3301. [Google Scholar] [CrossRef]
- Raz, N.; Gunning-Dixon, F.; Head, D.; Rodrigue, K.M.; Williamson, A.; Acker, J.D. Ageing, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol. Ageing 2004, 25, 377–396. [Google Scholar] [CrossRef]
- Salat, D.H.; Buckner, R.L.; Snyder, A.Z.; Greve, D.N.; Desikan, R.S.; Busa, E.; Morris, J.C.; Dale, A.M.; Fischl, B. Thinning of the cerebral cortex in ageing. Cereb. Cortex 2004, 14, 721–730. [Google Scholar] [CrossRef]
- Allen, J.S.; Bruss, J.; Brown, C.K.; Damasio, H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiol. Ageing 2005, 26, 1245–1260, discussion 1279–1282. [Google Scholar] [CrossRef]
- Grieve, S.M.; Clark, C.R.; Williams, L.M.; Peduto, A.J.; Gordon, E. Preservation of limbic and paralimbic structures in ageing. Hum. Brain Mapp. 2005, 25, 391–401. [Google Scholar] [CrossRef]
- Raz, N.; Lindenberger, U.; Rodrigue, K.M.; Kennedy, K.M.; Head, D.; Williamson, A.; Dahle, C.; Gerstorf, D.; Acker, J.D. Regional brain changes in ageing healthy adults: General trends, individual differences and modifiers. Cereb. Cortex 2005, 15, 1676–1689. [Google Scholar] [CrossRef]
- Raz, N. Ageing of the brain and its impact on cognitive performance: Integration of structural and functional findings. In The Handbook of Aging and Cognition; Erlbaum: London, UK, 2000. [Google Scholar]
- Davis, S.W.; Dennis, N.A.; Buchler, N.G.; White, L.E.; Madden, D.J.; Cabeza, R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage 2009, 46, 530–541. [Google Scholar] [CrossRef]
- Fjell, A.M.; Westlye, L.T.; Amlien, I.; Espeseth, T.; Reinvang, I.; Raz, N.; Agartz, I.; Salat, D.H.; Greve, D.N.; Fischl, B.; et al. High consistency of regional cortical thinning in ageing across multiple samples. Cereb. Cortex 2009, 19, 2001–2012. [Google Scholar] [CrossRef]
- McGinnis, S.M.; Brickhouse, M.; Pascual, B.; Dickerson, B.C. Age-related changes in the thickness of cortical zones in humans. Brain Topogr. 2011, 24, 279–291. [Google Scholar] [CrossRef]
- Lindenberger, U.; Baltes, P.B. Sensory functioning and intelligence in old age: A strong connection. Psychol. Ageing 1994, 9, 339–355. [Google Scholar] [CrossRef]
- Baltes, P.B.; Lindenberger, U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive ageing? Psychol. Ageing 1997, 12, 12–21. [Google Scholar] [CrossRef]
- Garo-Pascual, M.; Gaser, C.; Zhang, L.; Tohka, J.; Medina, M.; Strange, B.A. Brain structure and phenotypic profile of superagers compared with age-matched older adults: A longitudinal analysis from the Vallecas Project. Lancet Healthy Longev. 2023, 4, e374–e385. [Google Scholar] [CrossRef]
- Bartali, B.; Frongillo, E.A.; Bandinelli, S.; Lauretani, F.; Semba, R.D.; Fried, L.P.; Ferrucci, L. Low nutrient intake is an essential component of frailty in older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, C.O.; Whiting, S.J.; Zello, G.A. Nutrient inadequacies among elderly residents of long-term care facilities. Can. J. Diet. Pract. Res. 2008, 69, 82–88. [Google Scholar] [CrossRef]
- Wikby, K.; Ek, A.C.; Christensson, L. Nutritional status in elderly people admitted to community residential homes: Comparisons between two cohorts. J. Nutr. Health Ageing 2006, 10, 232–238. [Google Scholar]
- Kulnik, D.; Elmadfa, I. Assessment of the nutritional situation of elderly nursing home residents in Vienna. Ann. Nutr. Metab. 2008, 52 (Suppl. 1), 51–53. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, C.; Alix, E.; Boirie, Y.; Berrut, G.; Ritz, P. Are elderly hospitalized patients getting enough protein? J. Am. Geriatr. Soc. 2008, 56, 1045–1049. [Google Scholar] [CrossRef]
- Peterson, M.J.; Giuliani, C.; Morey, M.C.; Pieper, C.F.; Evenson, K.R.; Mercer, V.; Cohen, H.J.; Visser, M.; Brach, J.S.; Kritchevsky, S.B.; et al. Physical activity as a preventative factor for frailty: The health, ageing, and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 61–68. [Google Scholar] [CrossRef]
- Villareal, D.T.; Banks, M.; Siener, C.; Sinacore, D.R.; Klein, S. Physical frailty and body composition in obese elderly men and women. Obes. Res. 2004, 12, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.J.; Cho, J.; Choi, S.M.; Park, Y.S.; Lee, C.H.; Lee, S.M.; Yoo, C.G.; Kim, Y.W.; Lee, J. Phase angle and frailty are important prognostic factors in critically ill medical patients: A prospective cohort study. J. Nutr. Health Ageing 2021, 25, 218–223. [Google Scholar] [CrossRef]
- Jung, H.Y.; Park, B.K.; Shin, H.S.; Kang, Y.K.; Pyun, S.B.; Paik, N.J.; Kim, S.-H.; Kim, T.-H.; Han, T.-R. Development of the Korean Version of Modified Barthel Index (K-MBI): Multi-center Study for Subjects with Stroke. J. Korean Acad. Rehabil. Med. 2007, 31, 283–297. [Google Scholar]
- Tanaka, S.; Ando, K.; Kobayashi, K.; Seki, T.; Hamada, T.; Machino, M.; Ota, K.; Morozumi, M.; Kanbara, S.; Ito, S.; et al. Low Bioelectrical Impedance Phase Angle Is a Significant Risk Factor for Frailty. BiomMed Res. Int. 2019, 10, 6283153. [Google Scholar] [CrossRef]
- Saitoh, M.; Ogawa, M.; Kondo, H.; Suga, K.; Takahashi, T.; Itoh, H.; Tabata, Y. Bioelectrical impedance analysis-derived phase angle as a determinant of protein energy wasting and frailty in maintenance hemodialysis patients: Retrospective cohort study. BMC Nephrol. 2020, 21, 438. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bandinelli, S.; Lunenfeld, B. Frailty and the role of nutrition in older people. A review of the current literature. Acta Biomed. 2010, 81 (Suppl. 1), 37–45. [Google Scholar]
- Rodriguez-Mañas, L.; Fried, L.P. Frailty in the clinical scenario. Lancet 2015, 385, e7–e9. [Google Scholar] [CrossRef]
- Walston, J.; Fried, L.P. Frailty and the older man. Med. Clin. N. Am. 1999, 83, 1173–1194. [Google Scholar] [CrossRef]
- Walston, J. Frailty-the search for underlying causes. Sci. Ageing Knowl. Environ. 2004, 2004, pe4. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Svensson, Y.; Sohal, B.H.; Brunk, U.T. Superoxide anion radical production in different species. Mech. Ageing Dev. 1989, 49, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.H.; Brunk, U.T.; Sohal, R.S. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Radic. Biol. Med. 1993, 15, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Boudoulas, K.; Borer, D.; Boudoulas, J.S. Heart Rate, Life Expectancy and the Cardiovascular System: Therapeutic Considerations. Cardiology 2015, 132, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, M.A. Heart rate and longevity. Cardiorespir. Physiother. Crit. Care Rehabil. 2021, 1, e42591. [Google Scholar] [CrossRef]
- Hernández-Vicente, A.; Hernando, D.; Santos-Lozano, A.; Rodríguez-Romo, G.; Vicente-Rodríguez, G.; Pueyo, E.; Bailón, R.; Garatachea, N. Heart Rate Variability and Exceptional Longevity. Front. Physiol. 2020, 11, 566399. [Google Scholar] [CrossRef] [PubMed]
- Naffah de Souza Breda, C.; Davanzo, G.G.; Basso, P.J.; Saraiva Câmara, N.O.; Moraes-Vieira, P.M.M. Mitochondria as central hub of the immune system. Redox Biol. 2019, 26, 101255. [Google Scholar]
- Faasa, M.M.; de Vosa, P. Mitochondrial function in immune cells in health and disease. BBA Mol. Basis Dis. 2020, 1866, 165845. [Google Scholar] [CrossRef]
- Rattray, N.J.W.; Trivedi, D.K.; Xu, Y.; Chandola, T.; Johnson, C.H.; Marshall, A.D.; Mekli, K.; Rattray, Z.; Tampubolon, G.; Vanhoutte, B.; et al. Metabolic dysregulation in vitamin E and carnitine shuttle energy mechanisms associate with human frailty. Nat. Commun. 2019, 10, 5027. [Google Scholar] [CrossRef]
- Ashar, F.N.; Moes, A.; Moore, A.Z.; Grove, M.L.; Chaves, P.H.M.; Coresh, J.; Newman, A.B.; Matteini, A.M.; Bandeen-Roche, K.; Boerwinkle, E.; et al. Association of mitochondrial DNA levels with frailty and all-cause mortality. J. Mol. Med. 2015, 93, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Tomkova, K.; Pathak, S.; Abbasciano, R.; Wozniak, M.; Murphy, G.J. A systematic review and meta-analysis of studies that have evaluated the role of mitochondrial function and iron metabolism in frailty. Clin. Transl. Sci. 2021, 14, 2370–2378. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of ageing: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Fried, L.P.; Wallace, R.B. The complexity of chronic illness in the elderly. In The Epidemiologic Study of the Elderly; Wallace, R.B., Woolson, R.F., Eds.; Oxford University Press: New York, NY, USA, 1992. [Google Scholar]
- Boersma, P.; Black, L.I.; Ward, B.W. Prevalence of Multiple Chronic Conditions Among US Adults, 2018. Prev. Chronic Dis. 2020, 17, 200130. [Google Scholar] [CrossRef]
- Salive, M.E. Multimorbidity in Older Adults. Epidemiol. Rev. 2013, 35, 75–83. [Google Scholar] [CrossRef]
- Fried, L.P.; Xue, Q.L.; Cappola, A.R.; Ferrucci, L.; Chaves, P.; Varadhan, R.; Guralnik, J.M.; Leng, S.X.; Semba, R.D.; Walston, J.D.; et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Fillenbaum, G.G.; Pieper, C.F.; Cohen, H.J.; Cornoni-Huntley, J.C.; Guralnik, J.M. Comorbidity of five chronic health conditions in elderly community residents: Determinants and impact on mortality. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M84–M89. [Google Scholar] [PubMed]
- D’ascanio, M.; Innammorato, M.; Pasquariello, L.; Pizzirusso, D.; Guerrieri, G.; Castelli, S.; Pezzuto, A.; De Vitis, C.; Anibaldi, P.; Marcolongo, A.; et al. Age is not the only risk factor in COVID-19: The role of comorbidities and of long staying in residential care homes. BMC Geriatr. 2021, 21, 63. [Google Scholar] [CrossRef] [PubMed]
- Risk Factors for COVID-19 Mortality among Privately Insured Patients; FAIR Health White Paper by West Health Institute and Johns Hopkins University School of Medicine; FAIR Health, Inc.: New York, NY, USA, 2020.
- Woolford, S.J.; D’Angelo, S.; Curtis, E.M.; Parsons, C.M.; Ward, K.A.; Dennison, E.M.; Patel, H.P.; Cooper, C.; Harvey, N.C. COVID-19 and associations with frailty and multimorbidity: A prospective analysis of UK Biobank participants. Aging Clin Exp Res. 2020, 32, 1897–1905. [Google Scholar] [CrossRef]
- Canevelli, M.; Raganato, R.; Remiddi, F.; Quarata, F.; Valletta, M.; Bruno, G.; Cesari, M. Counting deficits or diseases? The agreement between frailty and multimorbidity in subjects with cognitive disturbances. Aging Clin Exp Res. 2020, 32, 179–182. [Google Scholar] [CrossRef]
- Inouye, S.K.; Studenski, S.; Tinetti, M.E.; Kuchel, G.A. Geriatric syndromes: Clinical, research, and policy implications of a core geriatric concept. J. Am. Geriatr. Soc. 2007, 55, 780–791. [Google Scholar] [CrossRef]
- Youn, H.; Gastner, M.T.; Jeong, H. Price of anarchy in transportation networks: Efficiency and optimality control. Phys. Rev. Lett. 2008, 101, 128701. [Google Scholar] [CrossRef]
- Navar, L.G. Physiology: Hemodynamics, endothelial function, renin-angiotensin-aldosterone system, sympathetic nervous system. J. Am. Soc. Hypertens. 2014, 8, 519–524. [Google Scholar] [CrossRef]
- Weiss, C.O. Frailty and Chronic Diseases in Older Adults. Clin. Geriatr. Med. 2011, 27, 39–52. [Google Scholar] [CrossRef]
- Afilalo, J. Frailty in patients with cardiovascular disease: Why, when, and how to measure. Curr. Cardiovasc. Risk Rep. 2011, 5, 467–472. [Google Scholar] [CrossRef]
- Veronese, N.; Cereda, E.; Stubbs, B.; Solmi, M.; Luchini, C.; Manzato, E.; Sergi, G.; Manu, P.; Harris, T.; Fontana, L.; et al. Risk of cardiovascular disease morbidity and mortality in frail and pre-frail older adults: Results from a meta-analysis and exploratory meta-regression analysis. Ageing Res. Rev. 2017, 35, 63–73. [Google Scholar] [CrossRef]
- Veronese, N. Frailty as Cardiovascular Risk Factor (and vice versa). Adv. Exp. Med. Biol. 2020, 1216, 51–54. [Google Scholar]
- Burke, G.; Fried, L.; Gottdiener, J.; Klein, R.; Kronmal, R.; Kuller, L.; Oleary, D.; Robbins, J.; Tracy, R.; Yousem, D. Cardiovascular Health Study (CHS), ClinicalTrials.gov Identifier: NCT00005133. Available online: https://chs-nhlbi.org/ (accessed on 30 October 2023).
- Ensrud, K.E.; Ewing, S.K.; Cawthon, P.M.; Fink, H.A.; Taylor, B.C.; Cauley, J.A.; Dam, T.T.; Marshall, L.M.; Orwoll, E.S.; Cummings, S.R. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J. Am. Geriatr. Soc. 2009, 57, 492–498. [Google Scholar] [CrossRef]
- von Haehling, S.; Anker, S.D.; Doehner, W.; Morley, J.E.; Vellas, B. Frailty and heart disease. Int. J. Cardiol. 2013, 168, 1745–1747. [Google Scholar] [CrossRef]
- Khan, H.; Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Newman, A.B.; Harris, T.B.; Rodondi, N.; Bauer, D.C.; Kritchevsky, S.B.; Butler, J. Frailty and risk for heart failure in older adults: The health, ageing, and body composition study. Am. Heart J. 2013, 166, 887–894. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure/A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Joglekar, S.; Asghar, A.; Mott, S.L.; Johnson, B.E.; Button, A.M.; Clark, E.; Mezhir, J.J. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J. Surg. Oncol. 2015, 111, 771–775. [Google Scholar] [CrossRef] [PubMed]
- de Jong, C.; Chargi, N.; Herder, G.J.M.; van Haarlem, S.W.A.; van der Meer, F.; van Lindert, A.S.R.; Ten Heuvel, A.; Brouwer, J.; de Jong, P.A.; Devriese, L.A.; et al. The association between skeletal muscle measures and chemotherapy- induced toxicity in non-small cell lung cancer patients. J. Cachexia Sarcopenia Muscle 2022, 13, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Bajestani, S.M.; Mazurak, V.C.; Baracos, V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin. Cell Dev. Biol. 2016, 54, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.M.; Prado, C.M.; Sullivan, E.S.; Power, D.G.; Daly, L.E. Effects of weight loss and sarcopenia on response to chemotherapy, quality of life, and survival. Nutrition 2019, 67–68, 110539. [Google Scholar] [CrossRef]
- van der Meij, B.S.; Teleni, L.; McCarthy, A.L.; Isenring, E.A. Cancer Cachexia: An Overview of Diagnostic Criteria and Therapeutic Approaches for the Accredited Practicing Dietitian. J. Hum. Nutr. Diet. 2020, 34, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhang, L. Cancer Cachexia: Definition, Stageing, and Emerging Treatments. Cancer Manag. Res. 2020, 12, 5597–5605. [Google Scholar] [CrossRef]
- Letilovic, T.; Perkov, S.; Mestric, Z.F.; Vrhovac, R. Differences in routine laboratory parameters related to cachexia between patients with hematological diseases and patients with solid tumors or heart failure—Is there only one cachexia? Nutr. J. 2013, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.M.; Power, D.G.; Daly, L.; Cushen, S.J.; Ní Bhuachalla, E.; Prado, C.M. Cancer-associated malnutrition, cachexia and sarcopenia: The skeleton in the hospital closet 40 years later. Proc. Nutr. Soc. 2016, 75, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Lucia, S.; Farcomeni, A.; Lorusso, V.; Saracino, V.; Barone, C.; Plastino, F.; Gori, S.; Magarotto, R.; Carteni, G.; et al. Prevalence of malnutrition in patients at first medical oncology visit: The PreMiO study. Oncotarget 2017, 8, 79884–79896. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Modena, A.; Valerio, M.; Marchetti, P.; Magarotto, R.; Quadrini, S.; Narducci, F.; Tonini, G.; Grassani, T.; Cavanna, L.; et al. The Impact of NUTRItional Status at First Medical Oncology Visit on Clinical Outcomes: The NUTRIONCO Study. Cancers 2023, 15, 3206. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Arai, H.; Sakurai, T. An update on cognitive frailty: Its definition, impact, associated factors and underlying mechanisms, and interventions. Geriatr Gerontol Int. 2022, 22, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.O.; Knopman, D.S. Classification and Epidemiology of MCI. Clin. Geriatr. Med. 2013, 29, 753–772. [Google Scholar] [CrossRef]
- Batty, D.G.; Deary, I.J.; Zaninotto, P. Association of Cognitive Function with Cause-Specific Mortality in Middle and Older Age: Follow-up of Participants in the English Longitudinal Study of Ageing. Am. J. Epidemiol. 2016, 183, 183–190. [Google Scholar] [CrossRef]
- Kaufman, Y.; Anaki, D.; Binns, M.; Freedman, M. Cognitive decline in Alzheimer disease: Impact of spirituality, religiosity, and QOL. Neurology 2007, 68, 1509–1514. [Google Scholar] [CrossRef]
- Sui, S.X.; Williams, L.J.; Holloway-Kew, K.L.; Hyde, N.K.; Pasco, J.A. Skeletal Muscle Health and Cognitive Function: A Narrative Review. Int. J. Mol. Sci. 2020, 22, 255. [Google Scholar] [CrossRef]
- Subra, J.; Gillette-Guyonnet, S.; Cesari, M.; Oustric, S.; Vellas, B.; Platform, T. The integration of frailty into clinical practice: Preliminary results from the Gerontopole. J. Nutr. Health Aging 2012, 16, 714–720. [Google Scholar] [CrossRef]
- Rosano, C.; Newman, A.; Santanasto, A.; Zhu, X.; Goodpaster, B.; Miljkovic, I. Increase in skeletal muscular adiposity and cognitive decline in a biracial cohort of older men and women. J. Am. Geriatr. Soc. 2023, 71, 2759–2768. [Google Scholar] [CrossRef] [PubMed]
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic obesity: Definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, K.; Liu, Q.; Wu, J. The Relationship Between Sarcopenia, Cognitive Impairment, and Cerebral White Matter Hyperintensity in the Elderly. Clin. Interv. Ageing 2023, 18, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.X.; Balanta-Melo, J.; Pasco, J.A.; Plotkin, L.I. Musculoskeletal Deficits and Cognitive Impairment: Epidemiological Evidence and Biological Mechanisms. Curr. Osteoporos. Rep. 2022, 20, 260–272. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Ito, T.; Lee, S.; Park, H.; et al. Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J. Am. Med. Dir. Assoc. 2013, 14, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Ní Mhaoláin, A.M.; Gallagher, D.; Crosby, L.; Ryan, D.; Lacey, L.; Coen, R.; Bruce, I.; Walsh, J.B.; Cunningham, C.; Lawlor, B.A. Correlates of frailty in Alzheimer’s disease and mild cognitive impairment. Age Ageing 2011, 40, 630–633. [Google Scholar] [CrossRef][Green Version]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J. Am. Geriatr. Soc. 2010, 58, 248–255. [Google Scholar] [CrossRef]
- Hao, Q.; Dong, B.; Yang, M.; Dong, B.; Wei, Y. Frailty and Cognitive Impairment in Predicting Mortality Among Oldest-Old People. Front. Ageing Neurosci. 2018, 10, 295. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, J.; Chon, D.; Lee, K.E.; Kim, J.H.; Myeong, S.; Kim, S. The effects of frailty and cognitive impairment on 3-year mortality in older adults. Maturitas 2018, 107, 50–55. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Doi, T.; Park, H.; Tsutsumimoto, K.; Verghese, J.; Suzuki, T. Effects of Combined Physical and Cognitive Exercises on Cognition and Mobility in Patients With Mild Cognitive Impairment: A Randomized Clinical Trial. J. Am. Med. Dir. Assoc. 2018, 19, 584–591. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Custodero, C.; Lozupone, M.; Imbimbo, B.P.; Valiani, V.; Agosti, P.; Schilardi, A.; D’Introno, A.; La Montagna, M.; Calvani, M.; et al. Relationships of Dietary Patterns, Foods, and Micro- and Macronutrients with Alzheimer’s Disease and Late-Life Cognitive Disorders: A Systematic Review. J. Alzheimers Dis. 2017, 59, 815–849. [Google Scholar] [CrossRef]
- Butler, A.; Gallagher, D.; Gillespie, P.; Crosby, L.; Ryan, D.; Lacey, L.; Coen, R.; O’Shea, E.; Lawlor, B. Frailty: A costly phenomenon in caring for elders with cognitive impairment. Int. J. Geriatr. Psychiatry 2016, 31, 161–168. [Google Scholar] [CrossRef]
- O’Hanlon, S.; Rechner, J. Optimising pre-operative assessment for older people. Anaesthesia 2018, 73, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; van Kan, G.A.; Ousset, P.J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive frailty: Rational and definition from an (IANA/IAGG) international consensus group. J. Nutr. Health Ageing 2013, 17, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Li, G.; Wang, X.; Zheng, L.; Wang, C.; Wang, C.; Chen, L. Prevalence of cognitive frailty among community-dwelling older adults: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2022, 125, 104112. [Google Scholar] [CrossRef] [PubMed]
- Bu, Z.; Huang, A.; Xue, M.; Li, Q.; Bai, Y.; Xu, G. Cognitive frailty as a predictor of adverse outcomes among older adults: A systematic review and meta-analysis. Brain Behav. 2021, 11, e01926. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Guindon, J.; Mody, P.H.; Ashworth, G.; Kopel, J.; Chilakapati, S.; Adogwa, O.; Neugebauer, V.; Burton, M.D. Pain and ageing: A unique challenge in neuroinflammation and behavior. Mol. Pain. 2023, 19, 17448069231203090. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhao, Y.; Xia, X.; Ge, N.; Yue, J. Association between frailty and chronic pain among older adults: A systematic review and meta-analysis. Eur. Geriatr. Med. 2020, 11, 945–959. [Google Scholar] [CrossRef] [PubMed]
- D’Agnelli, S.; Amodeo, G.; Franchi, S.; Verduci, B.; Baciarello, M.; Panerai, A.E.; Bignami, E.G.; Sacerdote, P. Frailty and pain, human studies and animal models. Ageing Res. Rev. 2022, 73, 101515. [Google Scholar] [CrossRef] [PubMed]
- Coluzzi, F.; Mattia, C. Mechanism-based treatment in chronic neuropathic pain: The role of antidepressants. Curr. Pharm. Des. 2005, 11, 2945–2960. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflammageing: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Matsuda, M.; Huh, Y.; Ji, R.R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Guida, F.; Rocco, M.; Luongo, L.; Persiani, P.; Vulpiani, M.C.; Nusca, S.M.; Maione, S.; Coluzzi, F. Targeting Neuroinflammation in Osteoarthritis with Intra-Articular Adelmidrol. Biomolecules 2022, 12, 1453. [Google Scholar] [CrossRef]
- O’Brien, M.S.; McDougall, J.J. Age and frailty as risk factors for the development of osteoarthritis. Mech. Ageing Dev. 2019, 180, 21–28. [Google Scholar] [CrossRef]
- Chaplin, W.J.; McWilliams, D.F.; Millar, B.S.; Gladman, J.R.F.; Walsh, D.A. The bidirectional relationship between chronic joint pain and frailty: Data from the Investigating Musculoskeletal Health and Wellbeing cohort. BMC Geriatr. 2023, 23, 273. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Morlion, B.; Vowles, K.E.; Bannister, K.; Buchser, E.; Casale, R.; Chenot, J.F.; Chumbley, G.; Drewes, A.M.; Dom, G.; et al. European clinical practice recommendations on opioids for chronic noncancer pain—Part 1: Role of opioids in the management of chronic noncancer pain. Eur. J. Pain. 2021, 25, 949–968. [Google Scholar] [CrossRef] [PubMed]
- Abete, P.; Basile, C.; Bulli, G.; Curcio, F.; Liguori, I.; Della-Morte, D.; Gargiulo, G.; Langellotto, A.; Testa, G.; Galizia, G.; et al. The Italian version of the “frailty index” based on deficits in health: A validation study. Ageing Clin. Exp. Res. 2017, 29, 913–926. [Google Scholar] [CrossRef]
- Ip, H.Y.; Abrishami, A.; Peng, P.W.; Wong, J.; Chung, F. Predictors of postoperative pain and analgesic consumption: A qualitative systematic review. Anesthesiology 2009, 111, 657–677. [Google Scholar] [CrossRef] [PubMed]
- Varrassi, G.; Coluzzi, F.; Fornasari, D.; Fusco, F.; Gianni, W.; Guardamagna, V.A.; Puntillo, F.; Sotgiu, G. New Perspectives on the Adverse Effects of NSAIDs in Cancer Pain: An Italian Delphi Study from the Rational Use of Analgesics (RUA) Group. J. Clin. Med. 2022, 11, 7451. [Google Scholar] [CrossRef]
- Coluzzi, F. Assessing and Treating Chronic Pain in Patients with End-Stage Renal Disease. Drugs 2018, 78, 1459–1479. [Google Scholar] [CrossRef] [PubMed]
- Cravello, L.; Di Santo, S.; Varrassi, G.; Benincasa, D.; Marchettini, P.; de Tommaso, M.; Shofany, J.; Assogna, F.; Perotta, D.; Palmer, K.; et al. Chronic Pain in the Elderly with Cognitive Decline: A Narrative Review. Pain. Ther. 2019, 8, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.J.; Abbott, T.E.F.; Prowle, J.; Pearse, R.M. Age of patients undergoing surgery. Br. J. Surg. 2019, 106, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Zhang, M.; Liao, G.; Karkache, W.; Montroy, J.; Fergusson, D.A.; Khadaroo, R.G.; Tran, D.T.T.; McIsaac, D.I.; Lalu, M.M. A Systematic Review and Meta-analysis Examining the Impact of Age on Perioperative Inflammatory Biomarkers. Anesth. Analg. 2022, 134, 751–764. [Google Scholar] [CrossRef]
- White, S.M.; Altermatt, F.; Barry, J.; Ben-David, B.; Coburn, M.; Coluzzi, F.; Degoli, M.; Dillane, D.; Foss, N.B.; Gelmanas, A.; et al. International Fragility Fracture Network Delphi consensus statement on the principles of anaesthesia for patients with hip fracture. Anaesthesia 2018, 73, 863–874. [Google Scholar] [CrossRef]
- Deiner, S.; Fleisher, L.A.; Leung, J.M.; Peden, C.; Miller, T.; Neuman, M.D.; ASA Committee on Geriatric Anesthesia the ASAPerioperative Brain Health Initiative. Adherence to recommended practices for perioperative anesthesia care for older adults among US anesthesiologists: Results from the ASA Committee on Geriatric Anesthesia-Perioperative Brain Health Initiative ASA member survey. Perioper. Med. 2020, 9, 6. [Google Scholar] [CrossRef]
- Coluzzi, F.; Pergolizzi, J.; Raffa, R.B.; Mattia, C. The unsolved case of “bone-impairing analgesics”: The endocrine effects of opioids on bone metabolism. Ther. Clin. Risk Manag. 2015, 11, 515–523. [Google Scholar] [CrossRef]
- Coluzzi, F.; Scerpa, M.S.; Centanni, M. The Effect of Opiates on Bone Formation and Bone Healing. Curr. Osteoporos. Rep. 2020, 18, 325–335. [Google Scholar] [CrossRef]
- White, A.E.; Henry, J.K.; Dziadosz, D. The Effect of Nonsteroidal Anti-inflammatory Drugs and Selective COX-2 Inhibitors on Bone Healing. HSS J. 2021, 17, 231–234. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Healthy Ageing and Functional Ability. 2020. Available online: https://www.who.int/news-room/questions-and-answers/item/healthy-ageing-and-functional-ability (accessed on 2 November 2023).
- Kornadt, A.E.; Kessler, E.M.; Wurm, S.; Bowen, C.E.; Gabrian, M.; Klusmann, V. Views on ageing: A lifespan perspective. Eur. J. Ageing 2019, 17, 387–401, Erratum in Eur. J. Ageing 2021, 18, 289. [Google Scholar] [CrossRef]
- Lu, W.; Pikhart, H.; Sacker, A. Domains and Measurements of Healthy Ageing in Epidemiological Studies: A Review. Gerontologist 2019, 59, e294–e310. [Google Scholar] [CrossRef]
- Diehl, M.; Smyer, M.A.; Mehrotra, C.M. Optimizing ageing: A call for a new narrative. Am. Psychol. 2020, 75, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Somagutta, M.R.; Uday, U.; Bathula, N.R.; Pendyala, S.; Mahadevaiah, A.; Jain, M.S.; Mahmutaj, G.; Gad, M.; Jean Baptiste, J. Diagnosing Frailty in Primary Care Practice. Cureus 2022, 14, e23329. [Google Scholar] [CrossRef] [PubMed]
- Durepos, P.; Sakamoto, M.; Alsbury, K.; Hewston, P.; Borges, J.; Takaoka, A. Older Adults’ Perceptions of Frailty Language: A Scoping Review. Can. J. Aging 2022, 41, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Fedarko, N.S. The biology of ageing and frailty. Clin. Geriatr. Med. 2011, 27, 27–37. [Google Scholar] [CrossRef]
- Aprahamian, I.; Landowski, A.; Ahn, F.O.; Neves, B.A.; Rocha, J.T.; Strauss, J.; Borges, M.K.; Morley, J.E.; Oude Voshaar, R.C. Frailty in geriatric psychiatry inpatients: A retrospective cohort study. Int. Psychogeriatr. 2022, 34, 981–989. [Google Scholar] [CrossRef]
- Panayi, A.C.; Orkaby, A.R.; Sakthivel, D.; Endo, Y.; Varon, D.; Roh, D.; Orgill, D.P.; Neppl, R.L.; Javedan, H.; Bhasin, S.; et al. Impact of frailty on outcomes in surgical patients: A systematic review and meta-analysis. Am. J. Surg. 2019, 218, 393–400. [Google Scholar] [CrossRef]
- Adja, K.Y.C.; Lenzi, J.; Sezgin, D.; O’Caoimh, R.; Morini, M.; Damiani, G.; Buja, A.; Fantini, M.P. The Importance of Taking a Patient-Centered, Community-Based Approach to Preventing and Manageing Frailty: A Public Health Perspective. Front. Public Health 2020, 8, 599170. [Google Scholar] [CrossRef] [PubMed]
- Crooms, R.C.; Gelfman, L.P. Palliative Care and End-of-Life Considerations for the Frail Patient. Anesth. Analg. 2020, 130, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, E.; Jang, I.Y. Frailty and Comprehensive Geriatric Assessment. J. Korean Med. Sci. 2020, 35, e16. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Rezaei-Shahsavarloo, Z.; Atashzadeh-Shoorideh, F.; Gobbens, R.J.J.; Ebadi, A.; Ghaedamini Harouni, G. The impact of interventions on management of frailty in hospitalized frail older adults: A systematic review and meta-analysis. BMC Geriatr. 2020, 20, 526. [Google Scholar] [CrossRef] [PubMed]
- Delli Zotti, G.B.; Citterio, L.; Farinone, S.; Concas, M.P.; Brioni, E.; Zagato, L.; Messaggio, E.; Faienza, S.; Simonini, M.; Napoli, A.; et al. Association between Perceived Health-Related Quality of Life and Depression with Frailty in the FRASNET Study. Int. J. Environ. Res. Public Health 2022, 19, 16776. [Google Scholar] [CrossRef] [PubMed]
- Warren, N.; Leske, S.; Arnautovska, U.; Northwood, K.; Kisely, S.; Siskind, D. Prevalence of frailty in severe mental illness: Findings from the UK Biobank. BJPsych Open. 2023, 9, e185. [Google Scholar] [CrossRef]
- Junius-Walker, U.; Onder, G.; Soleymani, D.; Wiese, B.; Albaina, O.; Bernabei, R.; Marzetti, E.; ADVANTAGE JA WP4 Group. The essence of frailty: A systematic review and qualitative synthesis on frailty concepts and definitions. Eur. J. Intern. Med. 2018, 56, 3–10. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Sardone, R.; Battista, P.; Piccininni, M.; Dibello, V.; La Montagna, M.; Stallone, R.; Venezia, P.; Liguori, A.; et al. Sensorial frailty: Age-related hearing loss and the risk of cognitive impairment and dementia in later life. Ther. Adv. Chronic Dis. 2018, 10, 2040622318811000. [Google Scholar] [CrossRef]
- Apóstolo, J.; Cooke, R.; Bobrowicz-Campos, E.; Santana, S.; Marcucci, M.; Cano, A.; Vollenbroek-Hutten, M.; Germini, F.; D’Avanzo, B.; Gwyther, H.; et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: A systematic review. JBI Database System. Rev. Implement. Rep. 2018, 16, 140–232, Erratum in JBI Database System. Rev. Implement. Rep. 2018, 16, 1282–1283. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Custodero, C.; Maggi, S.; Polidori, M.C.; Veronese, N.; Ferrucci, L. A multidimensional approach to frailty in older people. Ageing Res. Rev. 2020, 60, 101047. [Google Scholar] [CrossRef] [PubMed]
- Benraad, C.E.M.; Disselhorst, L.; Laurenssen, N.C.W.; Hilderink, P.H.; Melis, R.J.F.; Spijker, J.; Olde Rikkert, M.G.M. Frailty, multimorbidity and functional status as predictors for health outcomes of acute psychiatric hospitalisation in older adults. Ageing Ment. Health 2020, 24, 119–128. [Google Scholar] [CrossRef]
- Mutz, J.; Choudhury, U.; Zhao, J.; Dregan, A. Frailty in individuals with depression, bipolar disorder and anxiety disorders: Longitudinal analyses of all-cause mortality. BMC Med. 2022, 20, 274. [Google Scholar] [CrossRef] [PubMed]
- Puts, M.T.E.; Toubasi, S.; Andrew, M.K.; Ashe, M.C.; Ploeg, J.; Atkinson, E.; Ayala, A.P.; Roy, A.; Rodríguez Monforte, M.; Bergman, H.; et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: A scoping review of the literature and international policies. Age Ageing 2017, 46, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Ringer, T.; Hazzan, A.A.; Agarwal, A.; Mutsaers, A.; Papaioannou, A. Relationship between family caregiver burden and physical frailty in older adults without dementia: A systematic review. Syst. Rev. 2017, 6, 55. [Google Scholar] [CrossRef]
- American Psychological Association (APA). APA Dictionary of Psychology “stress”. Available online: https://dictionary.apa.org/stress (accessed on 28 February 2023).
- Agorastos, A.; Pervanidou, P.; Chrousos, G.P.; Baker, D.G. Developmental Trajectories of Early Life Stress and Trauma: A Narrative Review on Neurobiological Aspects Beyond Stress System Dysregulation. Front. Psychiatry 2019, 10, 118. [Google Scholar] [CrossRef]
- Bottaccioli, A.G.; Bottaccioli, F.; Minelli, A. Stress and the psyche-brain-immune network in psychiatric diseases based on psychoneuroendocrineimmunology: A concise review. Ann. N. Y. Acad. Sci. 2019, 1437, 31–42. [Google Scholar] [CrossRef]
- Matheson, K.; Asokumar, A.; Anisman, H. Resilience: Safety in the Aftermath of Traumatic Stressor Experiences. Front. Behav. Neurosci. 2020, 14, 596919. [Google Scholar] [CrossRef]
- Ridout, K.K.; Khan, M.; Ridout, S.J. Adverse Childhood Experiences Run Deep: Toxic Early Life Stress, Telomeres, and Mitochondrial DNA Copy Number, the Biological Markers of Cumulative Stress. Bioessays 2018, 40, e1800077. [Google Scholar] [CrossRef]
- Wang, J.; Maxwell, C.A.; Yu, F. Biological Processes and Biomarkers Related to Frailty in Older Adults: A State-of-the-Science Literature Review. Biol. Res. Nurs. 2019, 21, 80–106. [Google Scholar] [CrossRef]
- Shah, J.; Kandil, O.A.; Mortagy, M.; Abdelhameed, A.; Shah, A.; Kuron, M.; Abdellatif, Y.O. Frailty and Suicidality in Older Adults: A Mini-Review and Synthesis. Gerontology 2022, 68, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Arts, M.H.L.; van den Berg, K.S.; Marijnissen, R.M.; de Jonge, L.; Hegeman, A.J.M.; Collard, R.M.; Comijs, H.C.; Aprahamian, I.; Naarding, P.; Oude Voshaar, R.C. Frailty as a Predictor of Mortality in Late-Life Depression: A Prospective Clinical Cohort Study. J. Clin. Psychiatry 2021, 82, 20m13277. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, B.R.; Taylor, W.D.; Brown, P.J.; Sneed, J.R.; Roose, S.P. Biological Ageing and the Future of Geriatric Psychiatry. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Bickford, D.; Morin, R.T.; Woodworth, C.; Verduzco, E.; Khan, M.; Burns, E.; Nelson, J.C.; Mackin, R.S. The relationship of frailty and disability with suicidal ideation in late life depression. Ageing Ment. Health 2021, 25, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Veronese, N.; Thompson, T.; Kahl, K.G.; Fernandes, B.S.; Prina, A.M.; Solmi, M.; Schofield, P.; Koyanagi, A.; Tseng, P.T.; et al. Relationship between depression and frailty in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 36, 78–87. [Google Scholar] [CrossRef]
- Brown, P.J.; Rutherford, B.R.; Yaffe, K.; Tandler, J.M.; Ray, J.L.; Pott, E.; Chung, S.; Roose, S.P. The Depressed Frail Phenotype: The Clinical Manifestation of Increased Biological Ageing. Am. J. Geriatr. Psychiatry 2016, 24, 1084–1094. [Google Scholar] [CrossRef]
- Borges, M.K.; Jeuring, H.W.; Marijnissen, R.M.; van Munster, B.C.; Aprahamian, I.; van den Brink, R.H.S.; Hoogendijk, E.O.; Oude Voshaar, R.C. Frailty and affective disorders throughout adult life: A 5-year follow-up of the Lifelines Cohort Study. J. Am. Geriatr. Soc. 2022, 70, 3424–3435. [Google Scholar] [CrossRef]
- Aprahamian, I.; Borges, M.K.; Hanssen, D.J.C.; Jeuring, H.W.; Oude Voshaar, R.C. The Frail Depressed Patient: A Narrative Review on Treatment Challenges. Clin. Interv. Ageing 2022, 17, 979–990. [Google Scholar] [CrossRef]
- Gritti, D.; Delvecchio, G.; Ferro, A.; Bressi, C.; Brambilla, P. Neuroinflammation in Major Depressive Disorder: A Review of PET Imageing Studies Examining the 18-kDa Translocator Protein. J. Affect. Disord. 2021, 292, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, N.A.L.; Del Ángel, D.S.; Brizuela, N.O.; Peraza, A.V.; Olguín, H.J.; Soto, M.P.; Guzmán, D.C. Inflammatory Process and Immune System in Major Depressive Disorder. Int. J. Neuropsychopharmacol. 2022, 25, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Mac Giollabhui, N.; Ng, T.H.; Ellman, L.M.; Alloy, L.B. The longitudinal associations of inflammatory biomarkers and depression revisited: Systematic review, meta-analysis, and meta-regression. Mol. Psychiatry 2021, 26, 3302–3314. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, N.V.; Frandsen, B.H.; Orlovska-Waast, S.; Buus, T.B.; Ødum, N.; Christensen, R.H.; Benros, M.E. Immune cell composition in unipolar depression: A comprehensive systematic review and meta-analysis. Mol. Psychiatry 2023, 28, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Mousten, I.V.; Sørensen, N.V.; Christensen, R.H.B.; Benros, M.E. Cerebrospinal Fluid Biomarkers in Patients With Unipolar Depression Compared With Healthy Control Individuals: A Systematic Review and Meta-analysis. JAMA Psychiatry 2022, 79, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Zorn, J.V.; Schür, R.R.; Boks, M.P.; Kahn, R.S.; Joëls, M.; Vinkers, C.H. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 77, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Strawbridge, R.; Vives, A.H.; Cleare, A.J. Cortisol as a predictor of psychological therapy response in depressive disorders: Systematic review and meta-analysis. Br. J. Psychiatry 2017, 210, 105–109. [Google Scholar] [CrossRef]
- Bazaz, M.R.; Balasubramanian, R.; Monroy-Jaramillo, N.; Dandekar, M.P. Linking the Triad of Telomere Length, Inflammation, and Gut Dysbiosis in the Manifestation of Depression. ACS Chem. Neurosci. 2021, 12, 3516–3526. [Google Scholar] [CrossRef]
- Manoliu, A.; Bosch, O.G.; Brakowski, J.; Brühl, A.B.; Seifritz, E. The potential impact of biochemical mediators on telomere attrition in major depressive disorder and implications for future study designs: A narrative review. J. Affect. Disord. 2018, 225, 630–646. [Google Scholar] [CrossRef]
- Pousa, P.A.; Souza, R.M.; Melo, P.H.M.; Correa, B.H.M.; Mendonça, T.S.C.; Simões-E-Silva, A.C.; Miranda, D.M. Telomere Shortening and Psychiatric Disorders: A Systematic Review. Cells 2021, 10, 1423. [Google Scholar] [CrossRef]
- Bernal-López, C.; Potvin, O.; Avila-Funes, J.A. Frailty is associated with anxiety in community-dwelling elderly adults. J. Am. Geriatr. Soc. 2012, 60, 2373–2374. [Google Scholar] [CrossRef]
- Tan, M.; Bhanu, C.; Frost, R. The association between frailty and anxiety: A systematic review. Int. J. Geriatr. Psychiatry 2023, 38, e5918. [Google Scholar] [CrossRef]
- Riera-Serra, P.; Gili, M.; Navarra-Ventura, G.; Riera-López Del Amo, A.; Montaño, J.J.; Coronado-Simsic, V.; Castro, A.; Roca, M. Longitudinal associations between executive function impairments and suicide risk in patients with major depressive disorder: A 1-year follow-up study. Psychiatry Res. 2023, 325, 115235. [Google Scholar] [CrossRef]
- Gaspar, L.S.; Santos-Carvalho, A.; Santos, B.; Carvalhas-Almeida, C.; Barros-Viegas, A.T.; Oliveiros, B.; Donato, H.; Santos, C.; Moita, J.; Cavadas, C.; et al. Peripheral biomarkers to diagnose obstructive sleep apnea in adults: A systematic review and meta-analysis. Sleep. Med. Rev. 2022, 64, 101659. [Google Scholar] [CrossRef]
- Carvalhas-Almeida, C.; Cavadas, C.; Álvaro, A.R. The impact of insomnia on frailty and the hallmarks of ageing. Ageing Clin. Exp. Res. 2023, 35, 253–269. [Google Scholar] [CrossRef]
- Wen, Q.; Yan, X.; Ren, Z.; Wang, B.; Liu, Y.; Jin, X. Association between insomnia and frailty in older population: A meta-analytic evaluation of the observational studies. Brain Behav. 2023, 13, e2793. [Google Scholar] [CrossRef]
- Chagas, J.M.; Santos, E.H.R.; Ronchi, C.F.; Biagini, A.P. Sleep and Life Quality in Frail Elderly—A Narrative Review. Sleep. Sci. 2023, 16, 102–116. [Google Scholar] [CrossRef]
- Gooneratne, N.S.; Vitiello, M.V. Sleep in older adults: Normative changes, sleep disorders, and treatment options. Clin. Geriatr. Med. 2014, 30, 591–627. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, Y.; Sato, S.; Kitabatake, Y.; Nakamura, M.; Takeda, N.; Maruo, K.; Arao, T. Bidirectional relationship between insomnia and frailty in older adults: A 2-year longitudinal study. Arch. Gerontol. Geriatr. 2021, 97, 104519. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.S.; Canevelli, M.; Cesari, M. Editorial: Dementia, Frailty and Ageing. Front Med. 2018, 5, 168. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.; Morley, J.E. Editorial: Dysphagia, Dementia and Frailty. J. Nutr. Health Ageing 2018, 22, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Lozupone, M.; Logroscino, G. Understanding frailty to predict and prevent dementia. Lancet Neurol. 2019, 18, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Taniguchi, Y.; Iliffe, S.; Walters, K. Frailty as a Predictor of Alzheimer Disease, Vascular Dementia, and All Dementia Among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 881–888. [Google Scholar] [CrossRef]
- Kojima, G.; Liljas, A.; Iliffe, S.; Walters, K. Prevalence of Frailty in Mild to Moderate Alzheimer’s Disease: A Systematic Review and Meta-analysis. Curr. Alzheimer Res. 2017, 14, 1256–1263. [Google Scholar] [CrossRef]
- Kulmala, J.; Nykänen, I.; Mänty, M.; Hartikainen, S. Association between frailty and dementia: A population-based study. Gerontology 2014, 60, 16–21. [Google Scholar] [CrossRef]
- Avila-Funes, J.A.; Carcaillon, L.; Helmer, C.; Carrière, I.; Ritchie, K.; Rouaud, O.; Tzourio, C.; Dartigues, J.F.; Amieva, H. Is frailty a prodromal stage of vascular dementia? Results from the Three-City Study. J. Am. Geriatr. Soc. 2012, 60, 1708–1712. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Scafato, E.; Frisardi, V.; Seripa, D.; Logroscino, G.; Maggi, S.; Imbimbo, B.P.; Galluzzo, L.; Baldereschi, M.; Gandin, C.; et al. Frailty syndrome and the risk of vascular dementia: The Italian Longitudinal Study on Ageing. Alzheimers Dement. 2013, 9, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.; Theou, O.; Rockwood, K.; Andrew, M.K. Relationship between frailty and Alzheimer’s disease biomarkers: A scoping review. Alzheimers Dement. 2018, 10, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.M.K.; Theou, O.; Godin, J.; Ward, D.D.; Andrew, M.K.; Bennett, D.A.; Rockwood, K. 10-year frailty trajectory is associated with Alzheimer’s dementia after considering neuropathological burden. Ageing Med. 2021, 4, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, M.; Piovesan, C.; Di Battista, M.E. Associations between the Frailty Index and Brain Atrophy: The Treviso Dementia (TREDEM) Registry. J. Alzheimers Dis. 2018, 62, 1623–1634. [Google Scholar] [CrossRef]
- Lin, H.R.; Tsuji, T.; Kondo, K.; Imanaka, Y. Development of a risk score for the prediction of incident dementia in older adults using a frailty index and health checkup data: The JAGES longitudinal study. Prev. Med. 2018, 112, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Boyle, P.A.; Wilson, R.S.; Tang, Y.; Bennett, D.A. Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom. Med. 2007, 69, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Scafato, E.; Seripa, D.; Lozupone, M.; Imbimbo, B.P.; D’Amato, A.; Tortelli, R.; Schilardi, A.; Galluzzo, L.; Gandin, C.; et al. Reversible Cognitive Frailty, Dementia, and All-Cause Mortality. The Italian Longitudinal Study on Ageing. J. Am. Med. Dir. Assoc. 2017, 18, 89.e1–89.e8. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ji, X.; Wu, X.; Tang, Z.; Zhang, X.; Guan, S.; Liu, H.; Fang, X. Frailty in Relation to the Risk of Alzheimer’s Disease, Dementia, and Death in Older Chinese Adults: A Seven-Year Prospective Study. J. Nutr. Health Ageing 2017, 21, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, M.; Grassi, A.; Focella, L.; Grassivaro, F.; Da Ronch, C.; Gallucci, M.; Marzetti, E. Association between the frailty index and vascular brain damage: The Treviso Dementia (TREDEM) registry. Exp. Gerontol. 2022, 167, 111894. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N.T.; Steptoe, A.; Cadar, D. Frailty is an independent predictor of incident dementia: Evidence from the English Longitudinal Study of Ageing. Sci. Rep. 2017, 7, 15746. [Google Scholar] [CrossRef] [PubMed]
- Abreu, W.; Tolson, D.; Jackson, G.A.; Staines, H.; Costa, N. The relationship between frailty, functional dependence, and healthcare needs among community-dwelling people with moderate to severe dementia. Health Soc. Care Community 2019, 27, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Abreu, W.; Tolson, D.; Jackson, G.A.; Costa, N. A cross-sectional study of family caregiver burden and psychological distress linked to frailty and functional dependency of a relative with advanced dementia. Dementia 2020, 19, 301–318. [Google Scholar] [CrossRef]
- Sugimoto, T.; Ono, R.; Kimura, A.; Saji, N.; Niida, S.; Toba, K.; Sakurai, T. Physical Frailty Correlates With Behavioral and Psychological Symptoms of Dementia and Caregiver Burden in Alzheimer’s Disease. J. Clin. Psychiatry 2018, 79, 17m11991. [Google Scholar] [CrossRef]
- Searle, S.D.; Rockwood, K. Frailty and the risk of cognitive impairment. Alzheimers Res. Ther. 2015, 7, 54. [Google Scholar] [CrossRef]
- Verloo, H.; Goulet, C.; Morin, D.; von Gunten, A. Association between frailty and delirium in older adult patients discharged from hospital. Clin. Interv. Aging 2016, 11, 55–63. [Google Scholar] [CrossRef]
- Chew, J.; Lim, W.S.; Chong, M.S.; Ding, Y.Y.; Tay, L. Impact of frailty and residual subsyndromal delirium on 1-year functional recovery: A prospective cohort study. Geriatr. Gerontol. Int. 2017, 17, 2472–2478. [Google Scholar] [CrossRef]
- Hubbard, R.E.; Peel, N.M.; Samanta, M.; Gray, L.C.; Mitnitski, A.; Rockwood, K. Frailty status at admission to hospital predicts multiple adverse outcomes. Age Ageing 2017, 46, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Bellelli, G.; Moresco, R.; Panina-Bordignon, P.; Arosio, B.; Gelfi, C.; Morandi, A.; Cesari, M. Is Delirium the Cognitive Harbinger of Frailty in Older Adults? A Review about the Existing Evidence. Front. Med. 2017, 4, 188. [Google Scholar] [CrossRef] [PubMed]
- Gracie, T.J.; Caufield-Noll, C.; Wang, N.Y.; Sieber, F.E. The Association of Preoperative Frailty and Postoperative Delirium: A Meta-analysis. Anesth. Analg. 2021, 133, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Jiao, J.; Xie, X.H.; Wu, X.J. The Association Between Frailty and Delirium Among Hospitalized Patients: An Updated Meta-Analysis. J. Am. Med. Dir. Assoc. 2021, 22, 527–534. [Google Scholar] [CrossRef]
- Cechinel, C.; Lenardt, M.H.; Rodrigues, J.A.M.; Binotto, M.A.; Aristides, M.M.; Kraus, R. Frailty and delirium in hospitalized older adults: A systematic review with meta-analysis. Rev. Lat. Am. Enfermagem 2022, 30, e3687. [Google Scholar]
- Fu, D.; Tan, X.; Zhang, M.; Chen, L.; Yang, J. Association between frailty and postoperative delirium: A meta-analysis of cohort study. Ageing Clin. Exp. Res. 2022, 34, 25–37. [Google Scholar] [CrossRef]
- Persico, I.; Cesari, M.; Morandi, A.; Haas, J.; Mazzola, P.; Zambon, A.; Annoni, G.; Bellelli, G. Frailty and Delirium in Older Adults: A Systematic Review and Meta-Analysis of the Literature. J. Am. Geriatr. Soc. 2018, 66, 2022–2030. [Google Scholar] [CrossRef]
- Dani, M.; Owen, L.H.; Jackson, T.A.; Rockwood, K.; Sampson, E.L.; Davis, D. Delirium, Frailty, and Mortality: Interactions in a Prospective Study of Hospitalized Older People. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 415–418. [Google Scholar] [CrossRef]
- Eeles, E.M.; White, S.V.; O’Mahony, S.M.; Bayer, A.J.; Hubbard, R.E. The impact of frailty and delirium on mortality in older inpatients. Age Ageing 2012, 41, 412–416. [Google Scholar] [CrossRef]
- Bellelli, P.G.; Biotto, M.; Morandi, A.; Meagher, D.; Cesari, M.; Mazzola, P.; Annoni, G.; Zambon, A. The relationship among frailty, delirium and attentional tests to detect delirium: A cohort study. Eur. J. Intern. Med. 2019, 70, 33–38. [Google Scholar] [CrossRef]
- Wightman, H.; Quinn, T.J.; Mair, F.S.; Lewsey, J.; McAllister, D.A.; Hanlon, P. Frailty in randomised controlled trials for dementia or mild cognitive impairment measured via the frailty index: Prevalence and prediction of serious adverse events and attrition. Alzheimers Res. Ther. 2023, 15, 110. [Google Scholar] [CrossRef]
- Pearson, E.; Siskind, D.; Hubbard, R.E.; Gordon, E.H.; Coulson, E.J.; Warren, N. Frailty and severe mental illness: A systematic review and narrative synthesis. J. Psychiatr. Res. 2022, 147, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Arnautovska, U.; Siskind, D.; Pearson, E.; Baker, A.; Reid, N.; Kwan, W.W.L.; Wang, N.; Gordon, E.; Hubbard, R.; Warren, N. Comprehensive Geriatric Assessment for younger outpatients with severe mental illness: Protocol for a feasibility study. BMJ Open 2023, 13, e069518. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.A.; Nguyen, T.T. Physical frailty as an important indicator of accelerated biological ageing in serious mental illnesses. Int. Psychogeriatr. 2022, 34, 955–957. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.T.; Lee, S.M.; Chen, H.K.; Wu, B.J. Association between frailty and its individual components with the risk of falls in patients with schizophrenia spectrum disorders. Schizophr. Res. 2018, 197, 138–143. [Google Scholar] [CrossRef]
- Bersani, F.S.; Mellon, S.H.; Reus, V.I.; Wolkowitz, O.M. Accelerated ageing in serious mental disorders. Curr. Opin. Psychiatry 2019, 32, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Bersani, F.S.; Canevelli, M.; Cesari, M.; Maggioni, E.; Pasquini, M.; Wolkowitz, O.M.; Ferracuti, S.; Biondi, M.; Bruno, G. Frailty Index as a clinical measure of biological age in psychiatry. J. Affect. Disord. 2020, 268, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Cao, X.; Li, X.; Zhang, J.; Ma, C.; Zhang, N.; Lu, Q.; Crimmins, E.M.; Gill, T.M.; Chen, X.; et al. Association of Unhealthy Lifestyle and Childhood Adversity With Acceleration of Ageing Among UK Biobank Participants. JAMA Netw. Open 2022, 5, e2230690. [Google Scholar] [CrossRef]
- Shahab, S.; Mulsant, B.H.; Levesque, M.L.; Calarco, N.; Nazeri, A.; Wheeler, A.L.; Foussias, G.; Rajji, T.K.; Voineskos, A.N. Brain structure, cognition, and brain age in schizophrenia, bipolar disorder, and healthy controls. Neuropsychopharmacology. 2019, 44, 898–906. [Google Scholar] [CrossRef]
- Yang, C.; Hou, X.; Ma, X.; Wu, D. Frailty among inpatients with Schizophrenia: Status, influencing factors, and their correlation with quality of life. Front. Psychiatry 2023, 13, 1067260. [Google Scholar] [CrossRef]
- Pizzol, D.; Trott, M.; Butler, L.; Barnett, Y.; Ford, T.; Neufeld, S.A.; Ragnhildstveit, A.; Parris, C.N.; Underwood, B.R.; López Sánchez, G.F.; et al. Relationship between severe mental illness and physical multimorbidity: A meta-analysis and call for action. BMJ Ment. Health 2023, 26, e300870. [Google Scholar] [CrossRef]
- Leng, S.X.; Hung, W.; Cappola, A.R.; Yu, Q.; Xue, Q.L.; Fried, L.P. White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 499–502. [Google Scholar] [CrossRef]
- Leng, S.X.; Cappola, A.R.; Andersen, R.E.; Blackman, M.R.; Koenig, K.; Blair, M.; Walston, J.D. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Ageing Clin. Exp. Res. 2004, 16, 153–157. [Google Scholar] [CrossRef]
- Landin-Wilhelmsen, K.; Wilhelmsen, L.; Lappas, G.; Rosen, T.; Lindstedt, G.; Lundberg, P.A.; Bengtsson, B.A. Serum insulin-like growth factor I in a random population sample of men and women: Relation to age, sex, smoking habits, coffee consumption and physical activity, blood pressure and concentrations of plasma lipids, fibrinogen, parathyroid hormone and osteocalcin. Clin. Endocrinol. 1994, 41, 351–357. [Google Scholar]
- Papadakis, M.A.; Grady, D.; Tierney, M.J.; Black, D.; Wells, L.; Grunfeld, C. Insulin-like growth factor 1 and functional status in healthy older men. J. Am. Geriatr. Soc. 1995, 43, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Goodman-Gruen, D.; Barrett-Connor, E. Epidemiology of insulin-like growth factor-I in elderly men and women. The Rancho Bernardo Study. Am. J. Epidemiol. 1997, 145, 970–976. [Google Scholar] [CrossRef]
- Harris, T.B.; Kiel, D.; Roubenoff, R.; Langlois, J.; Hannan, M.; Havlik, R.; Wilson, P. Association of insulin-like growth factor-I with body composition, weight history, and past health behaviors in the very old: The Framingham Heart Study. J. Am. Geriatr. Soc. 1997, 45, 133–139. [Google Scholar] [CrossRef]
- O’Connor, K.G.; Tobin, J.D.; Harman, S.M.; Plato, C.C.; Roy, T.A.; Sherman, S.S.; Blackman, M.R. Serum levels of insulin-like growth factor-I are related to age and not to body composition in healthy women and men. J. Gerontol. A Biol. Sci. Med. Sci. 1998, 53, M176–M182. [Google Scholar] [CrossRef]
- Rosen, C.J. Growth hormone and ageing. Endocrine 2000, 12, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Cappola, A.R.; Bandeen-Roche, K.; Wand, G.S.; Volpato, S.; Fried, L.P. Association of IGF-I levels with muscle strength and mobility in older women. J. Clin. Endocrinol. Metab. 2001, 86, 4139–4146. [Google Scholar] [CrossRef]
- Cappola, A.R.; Xue, Q.L.; Ferrucci, L.; Guralnik, J.M.; Volpato, S.; Fried, L.P. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J. Clin. Endocrinol. Metab. 2003, 88, 2019–2025. [Google Scholar] [CrossRef]
- Nedergaard, A.; Henriksen, K.; Karsdal, M.A.; Christiansen, C. Menopause, estrogens and frailty. Gynecol. Endocrinol. 2013, 29, 418–423. [Google Scholar] [CrossRef]
- Ruan, H.; Hu, J.; Zhao, J.; Tao, H.; Chi, J.; Niu, X.; Zhang, J.; Wang, Y. Menopause and frailty: A scoping review. Menopause 2020, 27, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Chang, M.; Wang, J. Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: A systematic review and meta-analysis. Age Ageing 2021, 50, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Thabane, L.; Papaioannou, A.; Ioannidis, G.; Levine, M.A.; Adachi, J.D. An overview of osteoporosis and frailty in the elderly. BMC Musculoskelet. Disord. 2017, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Cicatiello, A.G.; Di Girolamo, D.; Dentice, M. Metabolic Effects of the Intracellular Regulation of Thyroid Hormone: Old Players, New Concepts. Front. Endocrinol. 2018, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.J. Clinical Review 86: Euthyroid Sick Syndrome: Is it a Misnomer? J. Clin. Endocrinol. Metab. 1997, 82, 329–334. [Google Scholar] [CrossRef]
- Bermudez, F.; Surks, M.I.; Oppenheimer, J.H. High Incidence of Decreased Serum Triiodothyronine Concentration in Patients With Nonthyroidal Disease. J. Clin. Endocrinol. Metab. 1975, 41, 27–40. [Google Scholar] [CrossRef]
- Kaplan, M.M.; Larsen, P.R.; Crantzf, R.; Dzau, V.J.; Rossing, T.H.; Haddow, J.E. Prevalence of Abnormal Thyroid Function Test Results in Patients With Acute Medical Illnesses. Am. J. Med. 1982, 72, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Plikat, K.; Langgartner, J.; Buettner, R.; Bollheimer, L.C.; Woenckhaus, U.; Schölmerich, J.; Wrede, C.E. Frequency and Outcome of Patients With Nonthyroidal Illness Syndrome in a Medical Intensive Care Unit. Metabolism 2007, 56, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Chinga-Alayo, E.; Villena, J.; Evans, A.T.; Zimic, M. Thyroid Hormone Levels Improve the Prediction of Mortality Among Patients Admitted to the Intensive Care Unit. Intensive Care Med. 2005, 31, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Dıéz, J.J. Thyroid Dysfunction and Kidney Disease. Eur. J. Endocrinol. 2009, 160, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Bello, G.; Pennisi, M.A.; Montini, L.; Silva, S.; Maviglia, R.; Cavallaro, F.; Bianchi, A.; De Marinis, L.; Antonelli, M. Nonthyroid Illness Syndrome and Prolonged Mechanical Ventilation in Patients Admitted to the ICU. Chest 2009, 135, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Sharshar, T.; Bastuji-Garin, S.; Polito, A.; De Jonghe, B.; Stevens, R.D.; Maxime, V.; Rodriguez, P.; Cerf, C.; Outin, H.; Touraine, P.; et al. Hormonal Status in Protracted Critical Illness and in-Hospital Mortality. Hormonal Status in Protracted Critical Illness and in-Hospital Mortality. Crit. Care 2011, 15, R47. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Capalbo, C.; Napoli, C.; Anibaldi, P.; Salvati, V.; De Vitis, C.; Mancini, R.; Coluzzi, F.; Rocco, M. Nonthyroidal Illness Syndrome: To Treat or Not to Treat? Have We Answered the Question? A Review of Metanalyses. Front. Endocrinol. 2022, 13, 850328. [Google Scholar] [CrossRef] [PubMed]
- De Vitis, C.; Capalbo, C.; Torsello, A.; Napoli, C.; Salvati, V.; Loffredo, C.; Blandino, G.; Piaggio, G.; Auciello, F.R.; Pelliccia, F.; et al. Opposite Effect of Thyroid Hormones on Oxidative Stress and on Mitochondrial Respiration in COVID-19 Patients. Antioxidants 2022, 11, 1998. [Google Scholar] [CrossRef]
- Sciacchitano, S.; De Vitis, C.; D’Ascanio, M.; Giovagnoli, S.; De Dominicis, C.; Laghi, A.; Anibaldi, P.; Petrucca, A.; Salerno, G.; Santino, I.; et al. Gene signature and immune cell profiling by high-dimensional, single-cell analysis in COVID-19 patients, presenting Low T3 syndrome and coexistent hematological malignancies. J. Transl. Med. 2021, 19, 139. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, W.; Xu, W. Thyroid function analysis in 50 patients with COVID-19: A retrospective study. Thyroid 2020, 31, 8–11. [Google Scholar] [CrossRef]
- Li, Z.; Hou, P.; Mu, S.; Wang, R.; Miao, H.; Feng, M.; Wang, H.; Zhang, W.; Chen, Y.; Feng, T.; et al. Thyroxine changes in COVID-19 pandemic: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1089190. [Google Scholar] [CrossRef]
- Simonides, W.S.; Mulcahey, M.A.; Redout, E.M.; Muller, A.; Zuidwijk, M.J.; Visser, T.J.; Wassen, F.W.; Crescenzi, A.; da-Silva, W.S.; Harney, J.; et al. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J. Clin. Investig. 2008, 118, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Vaccination against the major infectious diseases. C. R. Acad. Sci. III 1999, 322, 943–951. [Google Scholar] [CrossRef]
- Reichert, T.A.; Simonsen, L.; Sharma, A.; Pardo, S.A.; Fedson, D.S.; Miller, M.A. Influenza and the winter increase in mortality in the United States, 1959–1999. Am. J. Epidemiol 2004, 160, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.M.; Elliot, A.J. The impact of influenza on the health and health care utilization of elderly people. Vaccine 2005, 23 (Suppl. 1), S1–S9. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Vaccine Action Plan 2011–2020; World Health Organization: Geneva, Switzerland, 2013; Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/strategies/global-vaccine-action-plan (accessed on 10 October 2023).
- Immunization Agenda 2030 (IA2030). A Global Strategy to Leave No One Behind. Available online: http://www.immunizationagenda2030.org/ (accessed on 10 October 2023).
- Vetrano, D.L.; Triolo, F.; Maggi, S.; Malley, R.; Jackson, T.A.; Poscia, A.; Bernabei, R.; Ferrucci, L.; Fratiglioni, L. Fostering healthy ageing: The interdependency of infections, immunity and frailty. Ageing Res. Rev. 2021, 69, 101351. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.K.; Shinde, V.; Ye, L.; Hatchette, T.; Haguinet, F.; Dos Santos, G.; McElhaney, J.E.; Ambrose, A.; Boivin, G.; Bowie, W.; et al. The Importance of Frailty in the Assessment of Influenza Vaccine Effectiveness Against Influenza-Related Hospitalization in Elderly People. J. Infect. Dis. 2017, 216, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Curran, D.; Him, J.H.; Matthews, S.; Dessart, C.; Levin, M.J.; Oostvogels, L.; Riley, M.E.; Schmader, K.E.; Cunningham, A.L.; McNeil, S.A.; et al. Zoster-064 Study Group. Recombinant Zoster Vaccine Is Efficacious and Safe in Frail Individuals. J. Am. Geriatr. Soc. 2021, 69, 744–752. [Google Scholar] [CrossRef]
- Gruver, A.L.; Hudson, L.L.; Sennpowski, G.D. Immunosenescence of ageing. J. Pathol. 2007, 211, 144–156. [Google Scholar] [CrossRef]
- Collerton, J.; Martin-Ruiz, C.; Davies, K.; Hilkens, C.M.; Isaacs, J.; Kolenda, C.; Parker, C.; Dunn, M.; Catt, M.; Jagger, C.; et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: Cross-sectional findings from the Newcastle 85+ Study. Mech. Ageing Dev. 2012, 133, 456–466. [Google Scholar] [CrossRef]
- McElhaney, J.E.; Zhou, X.; Talbot, H.K.; Soethout, E.; Bleackley, R.C.; Granville, D.J.; Pawelec, G. The unmet need in the elderly: How immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 2012, 30, 2060–2067. [Google Scholar] [CrossRef]
- Torres, I.; Albert, E.; Giménez, E.; Alcaraz, M.J.; Botija, P.; Amat, P.; Remigia, M.J.; Beltrán, M.J.; Rodado, C.; Huntley, D.; et al. B and T cell immune responses elicited by the Comirnaty® COVID-19 vaccine in nursing home residents. Clin. Microbiol. Infect. 2021, 27, 1672–1677. [Google Scholar] [CrossRef]
- Lim, J.P.; Low, K.; Lin, N.; Lim, C.; Ong, S.; Tan, W.; Tay, W.C.; Tan, H.N.; Young, B.E.; Lye, D.; et al. Predictors for development of critical illness amongst older adults with COVID-19: Beyond age to age-associated factors. Arch. Gerontol. Geriatr. 2021, 94, 104331. [Google Scholar] [CrossRef]
- Lim, W.S.; Liang, C.K.; Assantachai, P.; Auyeung, T.W.; Kang, L.; Lee, W.J.; Lim, J.Y.; Sugimoto, K.; Akishita, M.; Chia, S.L.; et al. COVID-19 and older people in Asia: Asian Working Group for Sarcopenia calls to actions. Geriatr. Gerontol. Int. 2020, 20, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Standing Recommendations for COVID-19 Issued by the Director-General of the World Health Organization (WHO) in Accordance with the International Health Regulations (2005) (IHR). Available online: https://www.who.int/publications/i/item/9789240074064 (accessed on 6 October 2023).
- Gomez-Cabrero, D.; Walter, S.; Abugessaisa, I.; Miñambres-Herraiz, R.; Palomares, L.B.; Butcher, L.; Erusalimsky, J.D.; Garcia-Garcia, F.J.; Carnicero, J.; Hardman, T.C.; et al. A robust machine learning framework to identify signatures for frailty: A nested case-control study in four ageing European cohorts. GeroScience 2021, 43, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Uliel, N.; Segal, G.; Perri, A.; Turpashvili, N.; Kassif Lerner, R.; Itelman, E. Low ALT, a marker of sarcopenia and frailty, is associated with shortened survival amongst myelodysplastic syndrome patients: A retrospective study. Medicine 2023, 102, e33659. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Frailty as a phenotypic manifestation of underlying oxidative stress. Free Radic. Biol. Med. 2020, 149, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Markle-Reid, M.; Browne, G. Conceptualizations of frailty in relation to older adults. J. Adv. Nurs. 2003, 44, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Faller, J.W.; Pereira, D.d.N.; de Souza, S.; Nampo, F.K.; Orlandi, F.d.S.; Matumoto, S. Instruments for the detection of frailty syndrome in older adults: A systematic review. PLoS ONE 2019, 14, e0216166. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Dykes, L. Communicating an ethical basis for decision-making in relation to frailty and allocation of scarce resources in the COVID-19 pandemic. Can. Geriatr. Soc. CME J. 2020, 10, 1–3. Available online: https://www.geriatricsjournal.ca/s/51-Communicating-an-Ethical-Basis-for-Decision-Making-COVID-19.pdf (accessed on 2 November 2023).
- Montero-Odasso, M.; Hogan, D.B.; Lam, R.; Madden, K.; MacKnight, C.; Molnar, F.; Rockwood, K. Age Alone is not Adequate to Determine Health-care Resource Allocation During the COVID-19 Pandemic. Can. Geriatr. J. 2020, 23, 152–154. [Google Scholar] [CrossRef]
- Rockwood, K.; Theou, O. Using the Clinical Frailty Scale in Allocating Scarce Health Care Resources. Can. Ger. J. 2020, 23, 210–215. [Google Scholar] [CrossRef]
- Aguayo, G.A.; Vaillant, M.T.; Donneau, A.-F.; Schritz, A.; Stranges, S.; Malisoux, L.; Chioti, A.; Guillaume, M.; Muller, M.; Witte, D.R. Comparative analysis of the association between 35 frailty scores and cardiovascular events, cancer, and total mortality in an elderly general population in England: An observational study. PLoS Med. 2018, 15, e1002543. [Google Scholar] [CrossRef]
- Buta, B.J.; Walston, J.D.; Godino, J.G.; Park, M.; Kalyani, R.R.; Xue, Q.-L.; Bandeen-Roche, K.; Varadhan, R. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res. Rev. 2016, 26, 53–61. [Google Scholar] [CrossRef]
- Katz, S. Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J. Am. Geriatr. Soc. 1983, 31, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Fitti, J.E.; Kovar, M.G. The supplement on aging to the 1984 National Health Interview Survey (NHIS). Vital Health Stat. 1987, 21, 1–115. [Google Scholar]
- Fillenbaum, G.C. Multidimensional Functional Assessment of Older Adults: The Duke Older American Resources and Services Procedures; Lawrence Erlbaum Associates: Hillsdale, NJ, USA; Psychology Press: New York, NY, USA, 1988; 192p. [Google Scholar]
- Narain, P.; Rubenstein, L.Z.; Wieland, G.D.; Rosbrook, B.; Strome, L.S.; Pietruszka, F.; Morley, J.E. Predictors of immediate and 6-month outcomes in hospitalized elderly patients: The importance of functional status. J. Am. Geriatr. Soc. 1988, 36, 775–783. [Google Scholar] [CrossRef]
- Katz, S.; Akpom, C.A. A measure of primary sociobiological functions. Int. J. Health Serv. 1976, 6, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Reuben, D.B.; Solomon, D.H. Assessment in geriatrics: Of caveats and names (editorial). J. Am. Geriatr. Soc. 1989, 37, 570–572. [Google Scholar] [CrossRef] [PubMed]
- De Vriendt, P.; Gorus, E.; Cornelis, E.; Bautmans, I.; Petrovic, M.; Mets, T. The advanced activities of daily living: A tool allowing the evaluation of subtle functional decline in mild cognitive impairment. J. Nutr. Health Aging 2013, 17, 64–71. [Google Scholar] [CrossRef]
- Pulok, M.H.; Theou, O.; van der Valk, A.M.; Rockwood, K. The role of illness acuity on the association between frailty and mortality in emergency department patients referred to internal medicine. Age Ageing 2020, 49, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, G.; Gonzalez Lindh, M.; Razmi, R.; Forslin, M.; Parenmark, F.; Bandert, A.; Ehrenborg, C.; Palm, A. Clinical frailty scale as a predictor of disease severity in patients hospitalised with COVID-19—An observational cohort study. Infect. Dis. 2022, 54, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Satake, S.; Shimada, H.; Yamada, M.; Kim, H.; Yoshida, H.; Gondo, Y.; Matsubayashi, K.; Matsushita, E.; Kuzuya, M.; Kozaki, K.; et al. Prevalence of frailty among community-dwellers and outpatients in Japan as defined by the Japanese version of the cardiovascular health study criteria. Geriatr. Gerontol. Int. 2017, 17, 2629–2634. [Google Scholar] [CrossRef] [PubMed]
- Satake, S.; Arai, H. The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria). Geriatr. Gerontol. Int. 2020, 20, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.; Iqbal, J.; Murali-Krishnan, R.; Sultan, A.; Orme, R.; Briffa, N.; Denvir, M.; Gunn, J. Role of frailty assessment in patients undergoing cardiac interventions. Open Heart. 2014, 1, e000033. [Google Scholar] [CrossRef]
- Boxer, R.; Kleppinger, A.; Ahmad, A.; Annis, K.; Hager, D.; Kenny, A. The 6-min walk is associated with frailty and predicts mortality in older adults with heart failure. Congest. Heart Fail. 2010, 16, 208–213. [Google Scholar] [CrossRef]
- Rolfson, D.B.; Majumdar, S.R.; Tsuyuki, R.T.; Tahir, A.; Rockwood, K. Validity and reliability of the edmonton frail scale. Age Ageing 2006, 35, 526–529. [Google Scholar] [CrossRef]
- Aygör, H.E.; Fadıloğlu, Ç.; Şahin, S.; Aykar, F.Ş.; Akçiçek, F. Validation of edmonton frail scale into elderly Turkish population. Arch. Gerontol. Geriatr. 2018, 76, 133–137. [Google Scholar] [CrossRef]
- Aprahamian, I.; Cezar, N.O.; Izbicki, R.; Lin, S.M.; Paulo, D.L.; Fattori, A.; Biella, M.M.; Jacob Filho, W.; Yassuda, M.S. Screening for frailty with the FRAIL scale: A comparison with the phenotype criteria. J. Am. Med. Dir. Assoc. 2017, 18, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.M.; Song, X.; Rockwood, K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J. Am. Geriatr. Soc. 2004, 52, 1929–1933. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Silvius, J.L.; Fox, R.A. Comprehensive geriatric assessment. Helping your elderly patients maintain functional well-being. Postgrad. Med. 1998, 103, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Hilmer, S.N.; Perera, V.; Mitchell, S.; Murnion, B.P.; Dent, J.; Bajorek, B.; Matthews, S.; Rolfson, D.B. The assessment of frailty in older people in acute care. Australas. J. Ageing 2009, 28, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Mañas, L.; García-Sánchez, I.; Hendry, A.; Bernabei, R.; Roller-Wirnsberger, R.; Gabrovec, B.; Liew, A.; Carriazo, A.M.; Redon, J.; Galluzzo, L.; et al. Key Messages for a Frailty Prevention and Management Policy in Europe from the ADVANTAGE JOINT ACTION Consortium. J. Nutr. Health Ageing 2018, 22, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Gabrovec, B.; Antoniadou, E.; Soleymani, D.; Targowski, T.; Kadalska, E.; López- Samaniego, L.; Carriazo, A.M.; Csizmadia, P.; Hendry, A.; Mair, A.; et al. European Guide for Management of Frailty at Individual Level Including Recommendations and Roadmap. Advanced REcords System (ARES) documents of the European commision 2019, 6690697. Available online: https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5c8c87747&appId=PPGMS (accessed on 2 November 2023).
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Woodhouse, L.; Rodríguez-Mañas, L.; Fried, L.P.; Woo, J.; Aprahamian, I.; Sanford, A.; Lundy, J.; et al. Physical frailty: ICFSR International Clinical Practice Guidelines for identification and management. J. Nutr. Health Ageing 2019, 23, 771–787. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Stewart, R.; White, H. Importance of frailty in patients with cardiovascular disease. Eur. Heart J. 2014, 35, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Chalcraft, J.R.; Cardinal, L.M.; Wechsler, P.J.; Hollis, B.W.; Gerow, K.G.; Alexander, B.M.; Keith, J.F.; Larson-Meyer, D.E. Vitamin D Synthesis Following a Single Bout of Sun Exposure in Older and Younger Men and Women. Nutrients 2020, 12, 2237. [Google Scholar] [CrossRef]
- Murad, M.H.; Elamin, K.B.; Abu Elnour, N.O.; Elamin, M.B.; Alkatib, A.A.; Fatourechi, M.M.; Almandoz, J.P.; Mullan, R.J.; Lane, M.A.; Liu, H.; et al. Clinical review: The effect of vitamin D on falls: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2011, 96, 2997–3006. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Willett, W.C.; Orav, E.J.; Lips, P.; Meunier, P.J.; Lyons, R.A.; Flicker, L.; Wark, J.; Jackson, R.D.; Cauley, J.A.; et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N. Engl. J. Med. 2012, 567, 40–49. [Google Scholar] [CrossRef]
- Rejnmark, L.; Avenell, A.; Masud, T.; Anderson, F.; Meyer, H.E.; Sanders, K.M.; Salovaara, K.; Cooper, C.; Smith, H.E.; Jacobs, E.T.; et al. Vitamin D with calcium reduces mortality: Patient level pooled analysis of 70,528 patients from eight major vitamin D trials. J. Clin. Endocrinol. Metab. 2012, 97, 2670–2681. [Google Scholar] [CrossRef]
- Ricci, A.; Pagliuca, A.; D’Ascanio, M.; Innammorato, M.; De Vitis, C.; Mancini, R.; Giovagnoli, S.; Facchiano, F.; Sposato, B.; Anibaldi, P.; et al. Circulating Vitamin D levels status and clinical prognostic indices in COVID-19 patients. Respir. Res. 2021, 22, 76. [Google Scholar] [CrossRef]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubée, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrients 2020, 12, 3377. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Laviano, A.; Cruz-Jentoft, A.J. The anorexia of ageing: Is it a geriatric syndrome? J. Am. Med. Dir. Assoc. 2010, 11, 153–156. [Google Scholar] [CrossRef]
- Morley, J.E. Undernutrition: A major problem in nursing homes. J. Am. Med. Dir. Assoc. 2011, 12, 243–246. [Google Scholar] [CrossRef]
- Neelemat, F.; Bosmans, J.E.; Thijs, A.; Seidell, J.C.; van Bokhorst-de van der Schueren, M.A. Post-discharge nutritional support in malnourished elderly individuals improves functional limitations. J. Am. Med. Dir. Assoc. 2011, 12, 295–301. [Google Scholar] [CrossRef]
- Morley, J.E. Weight loss in older persons: New therapeutic approaches. Curr. Pharm. Des. 2007, 13, 3637–3647. [Google Scholar] [CrossRef]
- Morley, M.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.; Doehner, W.; Fearon, K.C.; Ferrucci, L.; Hellerstein, M.K.; et al. Nutritional recommendations for the management of sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef]
- Blanco-Reina, E.; Aguilar-Cano, L.; García-Merino, M.R.; Ocaña-Riola, R.; Valdellós, J.; Bellido-Estévez, I.; Ariza-Zafra, G. Assessing Prevalence and Factors Related to Frailty in Community-Dwelling Older Adults: A Multinomial Logistic Analysis. J. Clin. Med. 2021, 10, 3576. [Google Scholar] [CrossRef]
- van Dam, C.S.; Labuschagne, H.A.; van Keulen, K.; Kramers, C.; Kleipool, E.E.; Hoogendijk, E.O.; Knol, W.; Nanayakkara, P.W.B.; Muller, M.; Trappenburg, M.C.; et al. Polypharmacy, comorbidity and frailty: A complex interplay in older patients at the emergency department. Eur. Geriatr. Med. 2022, 13, 849–857. [Google Scholar] [CrossRef]
- Ory, M.G.; Schechtman, K.B.; Miller, J.P.; Hadley, E.C.; Fiatarone, M.A.; Province, M.A.; Arfken, C.L.; Morgan, D.; Weiss, S.; Kaplan, M. Frailty and injuries in later life: The FICSIT trials. J. Am. Geriatr. Soc. 1993, 41, 283–296. [Google Scholar] [CrossRef]
- Fiatarone, M.A.; O’Neill, E.F.; Ryan, N.D.; Clements, K.M.; Solares, G.R.; Nelson, M.E.; Roberts, S.B.; Kehayias, J.J.; Lipsitz, L.A.; Evans, W.J. Exercise training and nutritional supplementation for physical frailty in very elderly people. N. Engl. J. Med. 1994, 330, 1769–1775. [Google Scholar] [CrossRef]
- Villareal, D.T.; Chode, S.; Parimi, N.; Sinacore, D.R.; Hilton, T.; Armamento-Villareal, R.; Napoli, N.; Qualls, C.; Shah, K. Weight loss, exercise, or both and physical function in obese older adults. N. Engl. J. Med. 2011, 364, 1218–1229. [Google Scholar] [CrossRef]
- Singh, N.A.; Quine, S.; Clemson, L.M.; Williams, E.J.; Williamson, D.A.; Stavrinos, T.M.; Grady, J.N.; Perry, T.J.; Lloyd, B.D.; Smith, E.U.; et al. Effects of high-intensity progressive resistance training and targeted multidisciplinary treatment of frailty on mortality and nursing home admissions after hip fracture: A randomized controlled trial. J. Am. Med. Dir. Assoc. 2012, 13, 24–30. [Google Scholar] [CrossRef]
- Goel, K.; Lennon, R.J.; Tilbury, R.T.; Squires, R.W.; Thomas, R.J. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation 2011, 123, 2344–2352. [Google Scholar] [CrossRef]
- Suaya, J.A.; Stason, W.B.; Ades, P.A.; Normand, S.L.; Shepard, D.S. Cardiac rehabilitation and survival in older coronary patients. J. Am. Coll. Cardiol. 2009, 54, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Pack, Q.R.; Goel, K.; Lahr, B.D.; Greason, K.L.; Squires, R.W.; Lopez-Jimenez, F.; Zhang, Z.; Thomas, R.J. Participation in cardiac rehabilitation and survival after coronary artery bypass graft surgery: A community-based study. Circulation 2013, 128, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Theou, O.; Rockwood, K. Should frailty status always be considered when treating the elderly patient? Ageing Health 2012, 8, 261–271. [Google Scholar] [CrossRef]
- Roller-Wirnsberger, R.; Lindner, S.; Liew, A.; O’Caoimh, R.; Koula, M.L.; Moody, D.; Espinosa, J.M.; van Durme, T.; Dimitrov, P.; Benjak, T.; et al. European Collaborative and Interprofessional Capability Framework for Prevention and Management of Frailty—A consensus process supported by the Joint Action for Frailty Prevention (ADVANTAGE) and the European Geriatric Medicine Society (EuGMS). Aging Clin. Exp. Res. 2020, 32, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Prince, M.; Thiyagarajan, J.A.; De Carvalho, I.A.; Bernabei, R.; Chan, P.; Gutierrez-Robledo, L.M.; Michel, J.P.; Morley, J.E.; Ong, P.; et al. Frailty: An emerging public health priority. J. Am. Med. Dir. Assoc. 2016, 17, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Ageing Europe—Statistics on Population Developments. EU-27 Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/SEPDF/cache/80393.pdf (accessed on 2 November 2023).
- Mitnitski, A.B.; Graham, J.E.; Mogilner, A.J.; Rockwood, K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, C.; Veronese, N.; Maggi, S.; Baggio, G.; Toffanello, E.D.; Zambon, S.; Sartori, L.; Musacchio, E.; Perissinotto, E.; Crepaldi, G.; et al. Factors influencing transitions between frailty states in elderly adults: The Progetto Veneto Anziani Longitudinal Study. J. Am. Geriatr. Soc. 2017, 65, 179–184. [Google Scholar] [CrossRef]
- Rodriguez-Mañas, L.; Laosa, O.; Vellas, B.; Paolisso, G.; Topinkova, E.; Oliva-Moreno, J.; Bourdel-Marchasson, I.; Izquierdo, M.; Hood, K.; Zeyfang, A.; et al. Effectiveness of a multimodal intervention in functionally impaired older people with type 2 diabetes mellitus. J. Cachexia Sarcopenia Muscle 2019, 10, 721–733. [Google Scholar] [CrossRef]
- Trombetti, A.; Hars, M.; Hsu, F.-C.; Reid, K.F.; Church, T.S.; Gill, T.M.; King, A.C.; Liu, C.K.; Manini, T.M.; McDermott, M.M.; et al. Effect of physical activity on frailty. Ann. Intern. Med. 2018, 168, 309–316. [Google Scholar] [CrossRef]
- Espinoza, S.E.; Jung, I.; Hazuda, H. Frailty transitions in the San Antonio longitudinal study of ageing. J. Am. Geriatr. Soc. 2012, 60, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Lanziotti Azevedo da Silva, S.; Campos Cavalcanti Maciel, Á.; de Sousa Máximo Pereira, L.; Domingues Dias, J.M.; Guimarães de Assis, M.; Corrêa Dias, R. Transition patterns of frailty syndrome in community-dwelling elderly individuals: A longitudinal study. J. Frailty Ageing 2015, 4, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Auyeung, T.W.; Leung, J.; Kwok, T.; Woo, J. Transitions in frailty states among community-living older adults and their associated factors. J. Am. Med. Dir. Assoc. 2014, 15, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Wei, Y.Z.; Wei, L.Q.; Jiang, X.Y.; Wang, X.F.; Shi, Y.; Hai, H. Frailty transitions and types of death in Chinese older adults: A population-based cohort study. Clin. Interv. Ageing 2018, 13, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Pollack, L.R.; Litwack-Harrison, S.; Cawthon, P.M.; Ensrud, K.; Lane, N.E.; Barrett-Connor, E.; Dam, T.T. Patterns and predictors of frailty transitions in older men: The osteoporotic fractures in men study. J. Am. Geriatr. Soc. 2017, 65, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Liljas, A.E.M.; Iliffe, S. Frailty syndrome: Implications and challenges for health care policy. Risk Manag. Healthc. Policy 2019, 12, 23–30. [Google Scholar] [CrossRef]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Bytyçi, I.; Henein, M.Y. Stride Length Predicts Adverse Clinical Events in Older Adults: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2670. [Google Scholar] [CrossRef]
- Greene, B.R.; Doheny, E.P.; Kenny, R.A.; Caulfield, B. Classification of frailty and falls history using a combination of sensor-based mobility assessments. Physiol. Meas. 2014, 35, 2053–2066. [Google Scholar] [CrossRef]
- Lee, H.; Lim, J.A.; Nam, H.K. Effect of a digital literacy program on older adults’ digital social behavior: A quasi-experimental study. Int. J. Environ. Res. Public Health 2022, 19, 12404. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.S.; Asch, D.A.; Volpp, K.G. Wearable devices as facilitators, not drivers, of health behavior change. JAMA 2015, 313, 459–460. [Google Scholar] [CrossRef]
- Kato, Y.; Sakamoto, R.; Hori, A.; Momosaki, R. Innovation in Digital Health Interventions for Frailty and Sarcopenia. J. Clin. Med. 2023, 12, 2341. [Google Scholar] [CrossRef]
- De Luca, V.; Femminella, G.D.; Leonardini, L.; Patumi, L.; Palummeri, E.; Roba, I.; Aronni, W.; Toccoli, S.; Sforzin, S.; Denisi, F.; et al. Digital Health Service for Identification of Frailty Risk Factors in Community-Dwelling Older Adults: The SUNFRAIL+ Study Protocol. Int. J. Environ. Res. Public Health 2023, 20, 3861. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; Barbolini, M.; Longobucco, Y.; Barbieri, L.; Benedetti, C.; Bono, F.; Cacciapuoti, I.; Donatini, A.; Iezzi, E.; Papini, D.; et al. A Novel Tool for the Early Identification of Frailty in Elderly People: The Application in Primary Care Settings. J. Frailty Ageing 2020, 9, 101–106. [Google Scholar] [CrossRef]
- Gobbens, R.J.; van Assen, M.A.; Luijkx, K.G.; Wijnen-Sponselee, M.T.; Schols, J.M. Determinants of frailty. J. Am. Med. Dir. Assoc. 2010, 11, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Zampino, M. A mitochondrial root to accelerated ageing and frailty. Nat. Rev. Endocrinol. 2020, 16, 133–134. [Google Scholar] [CrossRef]
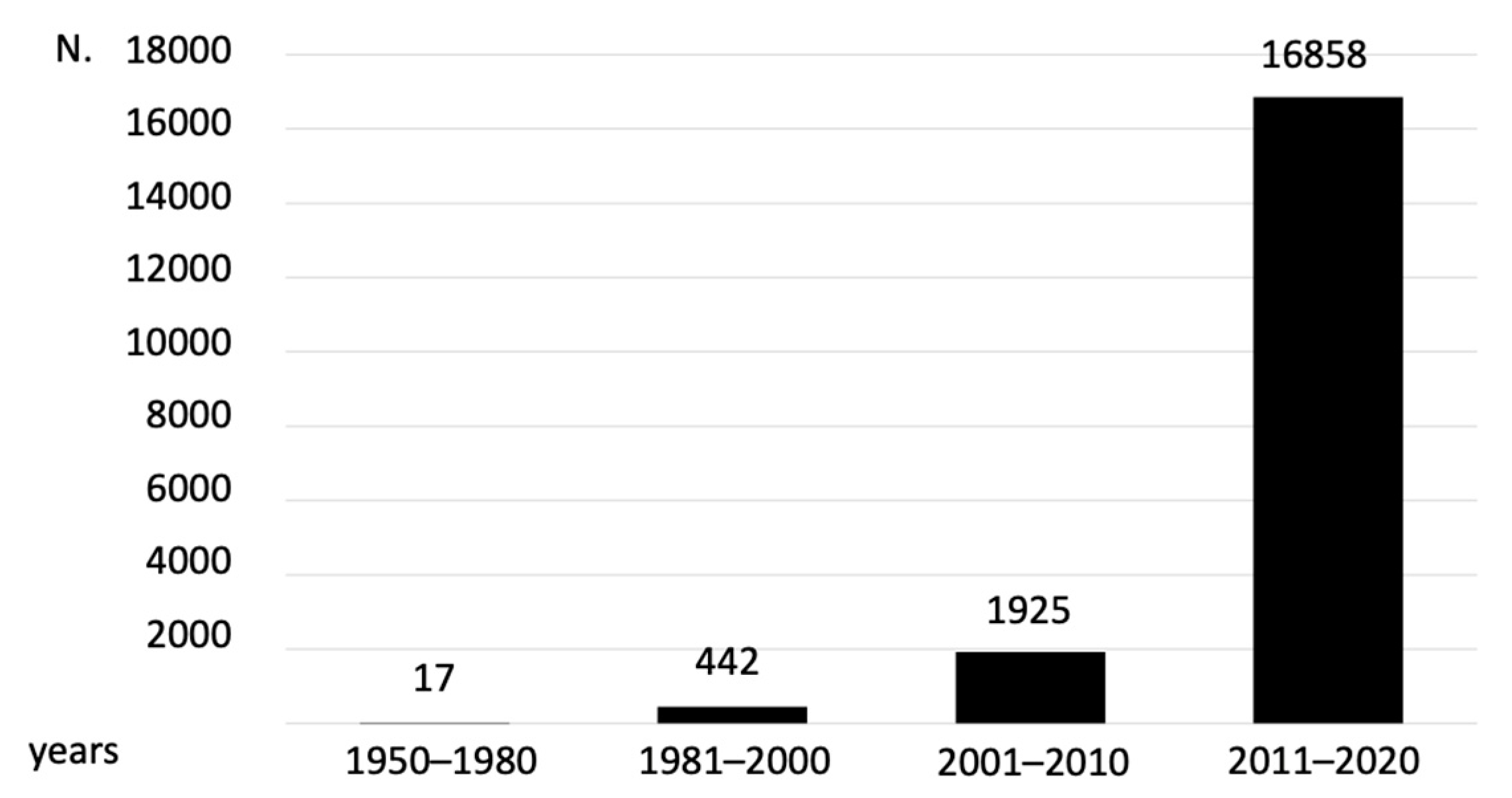
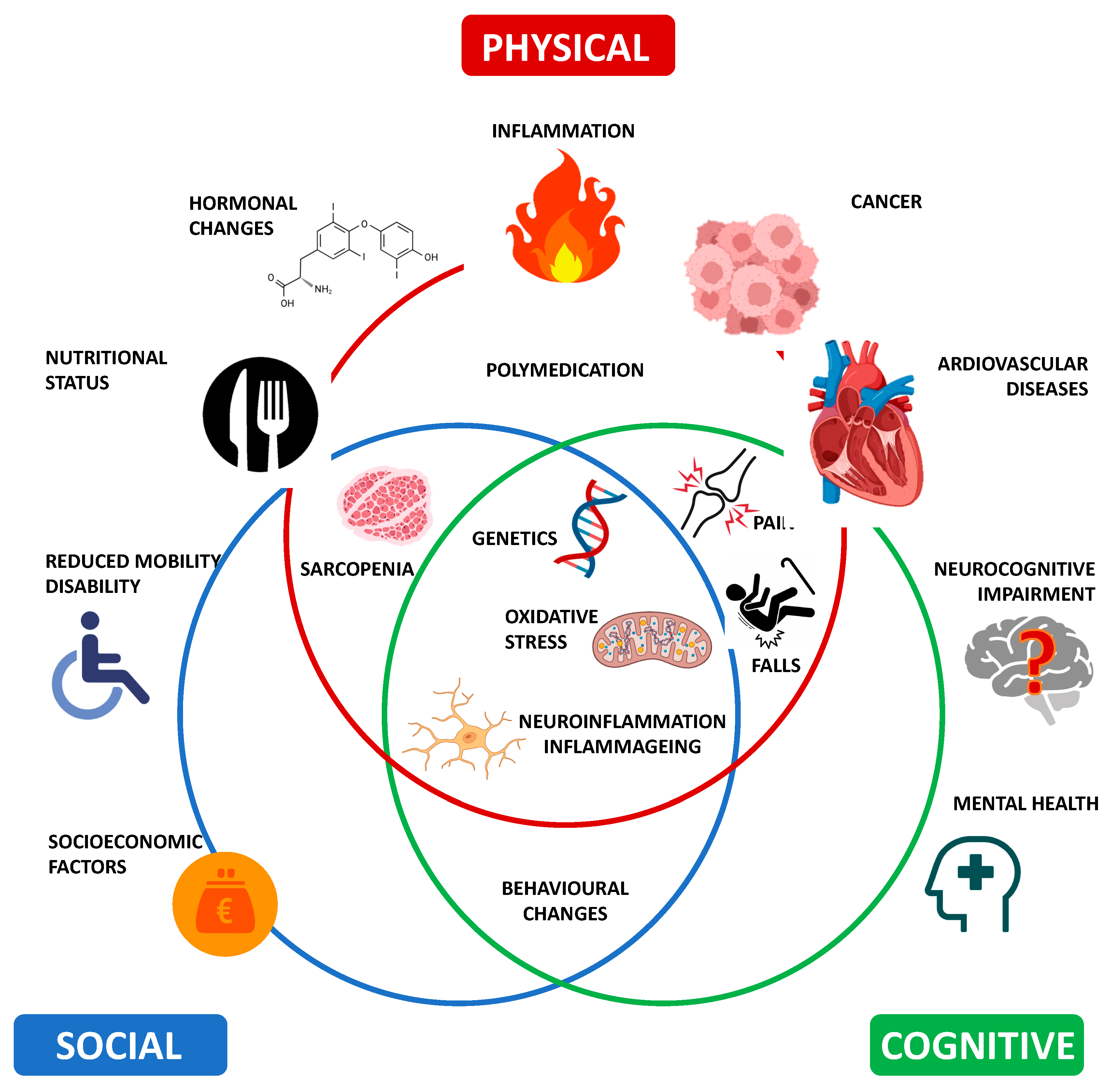

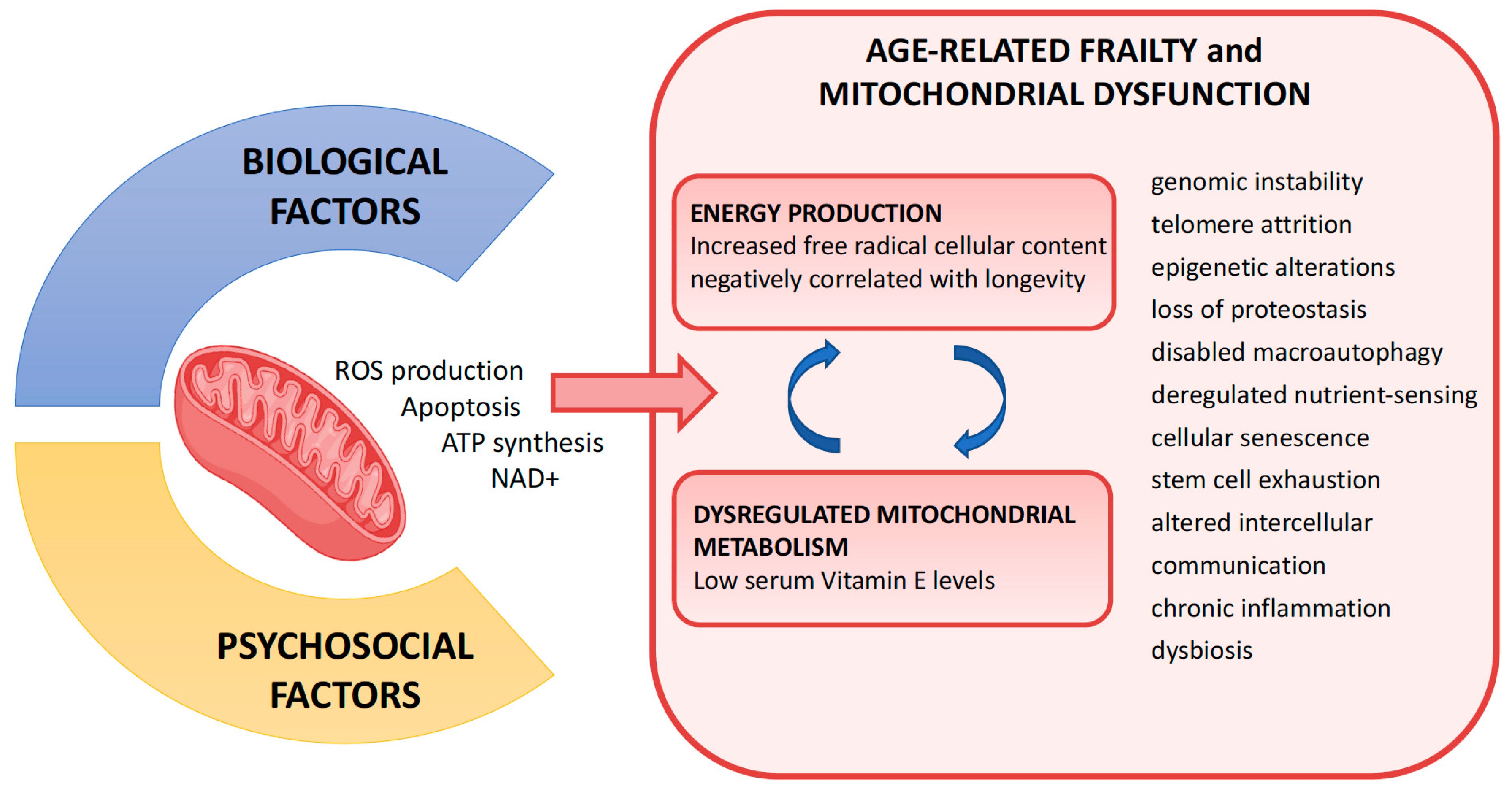

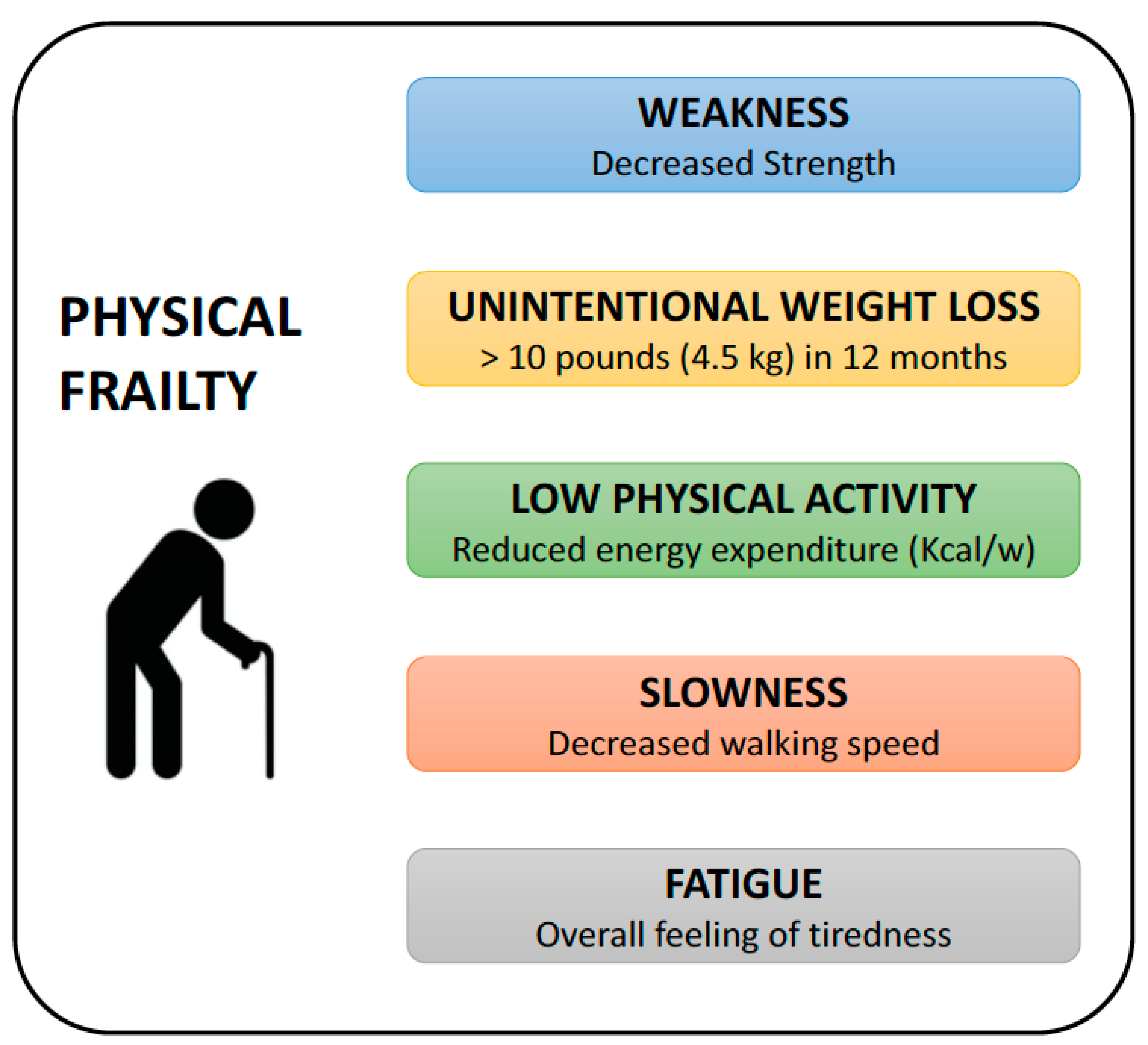
| Physical/Lifestyle | Psychological | Sociodemographic | Economic | Educational | Jurisprudential |
|---|---|---|---|---|---|
| Clinical | Psychological | Social | Economic | Educational | Fragilitas sexus |
| Physical | Cognitive | Migration | Global | Health Literacy | |
| Skeletal | Perceived | ||||
| Neurological | |||||
| Endocrine | |||||
| Oral | |||||
| Tendon | |||||
| Joint | |||||
| Renal | |||||
| Skin | |||||
| Handgrip Strength | |||||
| Sensorial | |||||
| Sedentary Behavior |
| (A) | |||
| Pooled Estimates of Incidence | Pre-Frail People | Frail People | Reference |
| Community-dwelling older adults | 150.64 cases per 1000 person-years (95% CI = 123.26–184.08) (21 studies, n = 1163) | 43.36 cases per 1000 person-years (95% CI = 37.29–50.41) (46 studies, n = 1163) | [100] |
| (B) | |||
| Pooled estimates of prevalence | Pre-frail People | Frail People | Reference |
| Nursing homes | 52.3% (95% CI = 37.9%–66.5%) (9 studies, n = 1373) | 40.2% (CI = 28.9%–52.1%) (7 studies, n = 1163) | [101] |
| Pooled Estimates of Prevalence in Europe (22 Countries) | Frail People | Reference |
|---|---|---|
| Overall | 18% (95% CI, 15–21) (62 studies, representing 68 unique datasets, n = 1163) | [106] |
| Community | 12% (95% CI = 10–15) (53 datasets, n = 1163) | [106] |
| Non-community | 45% (95% CI = 27–63) (15 datasets, n = 1163) | [106] |
| Chronic Disease | Overall Prevalence in Frail patients (%) | Prevalence in Frail Women (%) |
|---|---|---|
| Hypertension | 50.8–53.1 | 60.8 |
| Chronic kidney disease | - | 54.3 |
| Osteoarthritis | 25.9–70.8 | 78.2 |
| Depressive symptoms | - | 46.3 |
| Coronary heart disease | - | 17.2–41.5 |
| Diabetes mellitus | 13.6–25.0 | 9.9–21.3 |
| Chronic lower respiratory tract diseases | 12.3–14.1 | 9.8–15.5 |
| Myocardial infarction alone | 8.6–13.3 | - |
| Rheumatoid arthritis | - | 6.4 |
| Stroke | 12.3 | 4.4 |
| Peripheral arterial disease | 3.8–14.8 | - |
| Congestive heart failure | 12.3–13.6 | 3.5 |
| Frailty Criterion | Definition |
|---|---|
| Unintentional Weight loss | Loss of more than 5% body weight unintentionally in the last year or BMI is less than 18.5 kg/m2 |
| Exhaustion | Feeling unusually tired/weak (by rating the usual energy level on a self-reported scale from 0 to 10) |
| Slowness | Walking speed measured in a 4-m length in m/s |
| Low Activity Level | Physical expenditure measured in Kcal/week |
| Weakness | Grip strength measured using a hand dynamometer and by squeezing the dynamometer maximally 3 times with the dominant hand |
| # | Frailty Categories | Definition |
|---|---|---|
| 1 | Very fit | People who are robust, active, energetic, and motivated. These people exercise regularly and are among the fittest for their age. |
| 2 | Well | People who have no active disease symptoms but are less fit than the “very fit” category. They exercise or are very active occasionally, e.g., seasonally. |
| 3 | Well | People whose medical problems are well-controlled but are not regularly active beyond routine walking. |
| 4 | Apparently vulnerable | While not dependent on others for daily help, their symptoms often limit activities. A common complaint is being “slowed up” and/or being tired during the day. |
| 5 | Mildly frail | These people often have more evident symptoms of slowing and need help in high order IADLs (finances, transportation, heavy housework, and medications). Typically, mild frailty progressively impairs shopping, outdoor walks on their own, meal preparation, and housework. |
| 6 | Moderately frail | People need help with all outdoor activities and housekeeping. Indoors, they often have problems with stairs, need help with bathing, and might need minimal assistance (cuing and standby) with dressing. |
| 7 | Severely frail | They are completely dependent for personal care, regardless of the cause (physical or cognitive). Even so, they seem stable and not at high risk of dying (within ~ 6 months). |
| 8 | Very severely frail | They are completely dependent, approaching the end of life. Typically, they may not even recover from a minor illness. |
| 9 | Terminally ill | They are approaching the end of life. This category applies to people with a short life expectancy. |
| # | Frailty Categories | Definition | 0 Points | 1 Point | 2 Points |
|---|---|---|---|---|---|
| 1 | Cognition | Based on the Clock Drawing Test (Placing numbers in the correct positions on a pre-drawn circle and placing hands to indicate the time of “ten after eleven” | No Errors | Minor spacing errors | Other errors |
| 2 | General Health Status | Previous admittances to a hospital Self-description of health | 0 time Excellent, Very good, or Good | 1–2 times Fair | >2 times Poor |
| 3 | Functional Independence | Help needed for daily activities such as: meal preparation, shopping, transportation, telephone, housekeeping, laundry, managing money, and taking medications | 0–1 | 2–4 | 5–8 |
| 4 | Social Support | Reliance on someone who is willing and able to meet their needs | Always | Sometimes | Never |
| 5 | Medication Use | Assumption of five or more different medications on a regular basis They forget to take the medicine at the right time | No No | Yes Yes | |
| 6 | Nutrition | Weight is lost such that the clothing has become looser | No | Yes | |
| 7 | Mood | Feeling sad or depressed | No | Yes | |
| 8 | Continence | Losing urine control | No | Yes | |
| 9 | Function Performance (balance and mobility) | Based on the Timed Up and Go test (They will sit in a chair with their back and arms resting and then stand up on the command “GO” and walk at a safe and comfortable pace (approximately 3 m away) before finally returning to the chair and sitting down) | 0–10 s | 11–20 s | >20 s unwilling needs Assistance |
| Totals | /17 | ||||
| # | Frailty Categories | Definition |
|---|---|---|
| 1 | Fatigue | Too tired to exercise |
| 2 | Resistance | Able to climb a flight of stairs without assistance |
| 3 | Ambulation | Able to walk one block without assistance |
| 4 | Illnesses | Presence of five or more illnesses (Heart attack, angina, heart failure, stroke, dementia, COPD, diabetes, malignancy, osteoarthritis, hypertension, asthma, and kidney disease) |
| 5 | Loss of weight | Loss of more than 5% of body weight in one year |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciacchitano, S.; Carola, V.; Nicolais, G.; Sciacchitano, S.; Napoli, C.; Mancini, R.; Rocco, M.; Coluzzi, F. To Be Frail or Not to Be Frail: This Is the Question—A Critical Narrative Review of Frailty. J. Clin. Med. 2024, 13, 721. https://doi.org/10.3390/jcm13030721
Sciacchitano S, Carola V, Nicolais G, Sciacchitano S, Napoli C, Mancini R, Rocco M, Coluzzi F. To Be Frail or Not to Be Frail: This Is the Question—A Critical Narrative Review of Frailty. Journal of Clinical Medicine. 2024; 13(3):721. https://doi.org/10.3390/jcm13030721
Chicago/Turabian StyleSciacchitano, Salvatore, Valeria Carola, Giampaolo Nicolais, Simona Sciacchitano, Christian Napoli, Rita Mancini, Monica Rocco, and Flaminia Coluzzi. 2024. "To Be Frail or Not to Be Frail: This Is the Question—A Critical Narrative Review of Frailty" Journal of Clinical Medicine 13, no. 3: 721. https://doi.org/10.3390/jcm13030721
APA StyleSciacchitano, S., Carola, V., Nicolais, G., Sciacchitano, S., Napoli, C., Mancini, R., Rocco, M., & Coluzzi, F. (2024). To Be Frail or Not to Be Frail: This Is the Question—A Critical Narrative Review of Frailty. Journal of Clinical Medicine, 13(3), 721. https://doi.org/10.3390/jcm13030721











