Anticoagulation Management in V-V ECMO Patients: A Multidisciplinary Pragmatic Protocol
Abstract
1. Introduction
2. Materials and Methods
3. Relevant Sections
3.1. Choosing Anticoagulation
3.1.1. Unfractionated Heparin (UFH)
3.1.2. Bivalirudin (Direct Thrombin Inhibitor—DTI)
3.2. Monitoring and Available Laboratory Testing
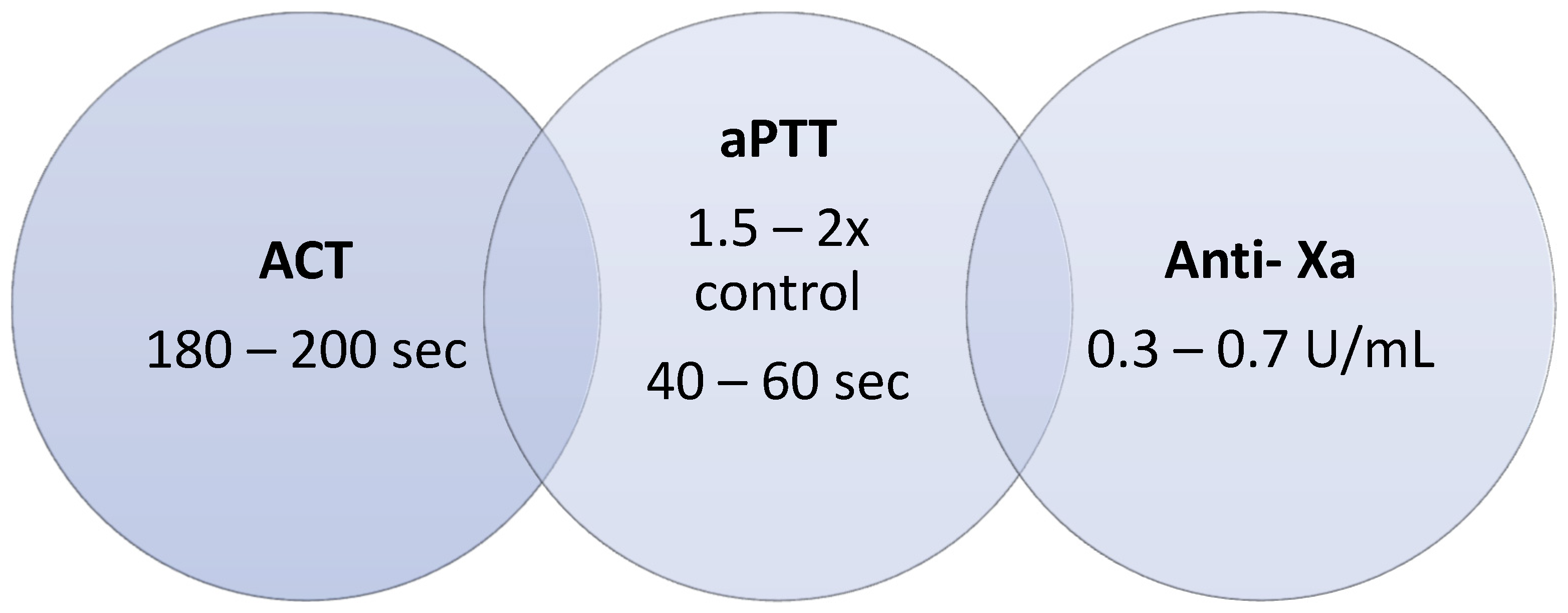
3.2.1. Activated Partial Thromboplastin Time (aPTT)
3.2.2. Anti-Xa Activity (Anti-Xa)
3.2.3. Activated Coagulation Time (ACT)
3.2.4. Viscoelastic Testing (VET)
3.2.5. Dilute Thrombin Time (dTT)
3.2.6. Antithrombin (AT)
3.3. Anticoagulation Complications
3.3.1. Heparin Resistance (HR)
- Pseudo-heparin resistance: occurs when high FVIII and/or fibrinogen levels interfere with aPTT measurement, giving falsely low results and leading to misinterpretation of low heparin efficiency.
- Antithrombin (AT) deficiency: is a common cause of HR [14]. Heparin works by binding to AT so that low levels will cause an effective low heparin effect. Acquired AT deficiency can be secondary to liver disease, sepsis, nephrotic syndrome, malnutrition, acute thrombosis, increased consumption during bleeding or disseminated intravascular coagulation, associated with extracorporeal circuits such as ECMO, and the use of heparin [10,14]. ECMO-related AT deficiency is frequently seen upon ECMO initiation and may be attributed to reduced synthesis plus accelerated consumption [14].
- Low heparin concentration: especially due to the binding of acute phase proteins that change the pharmacokinetics and volume of distribution of the drug. Systemic inflammation increases the production of proteins that bind to heparin—e.g., PF4, causing lower heparin concentration in blood with lower anticoagulant effect and frequent dosage adjustments.
- Multifactorial: Combination of the causes mentioned above.
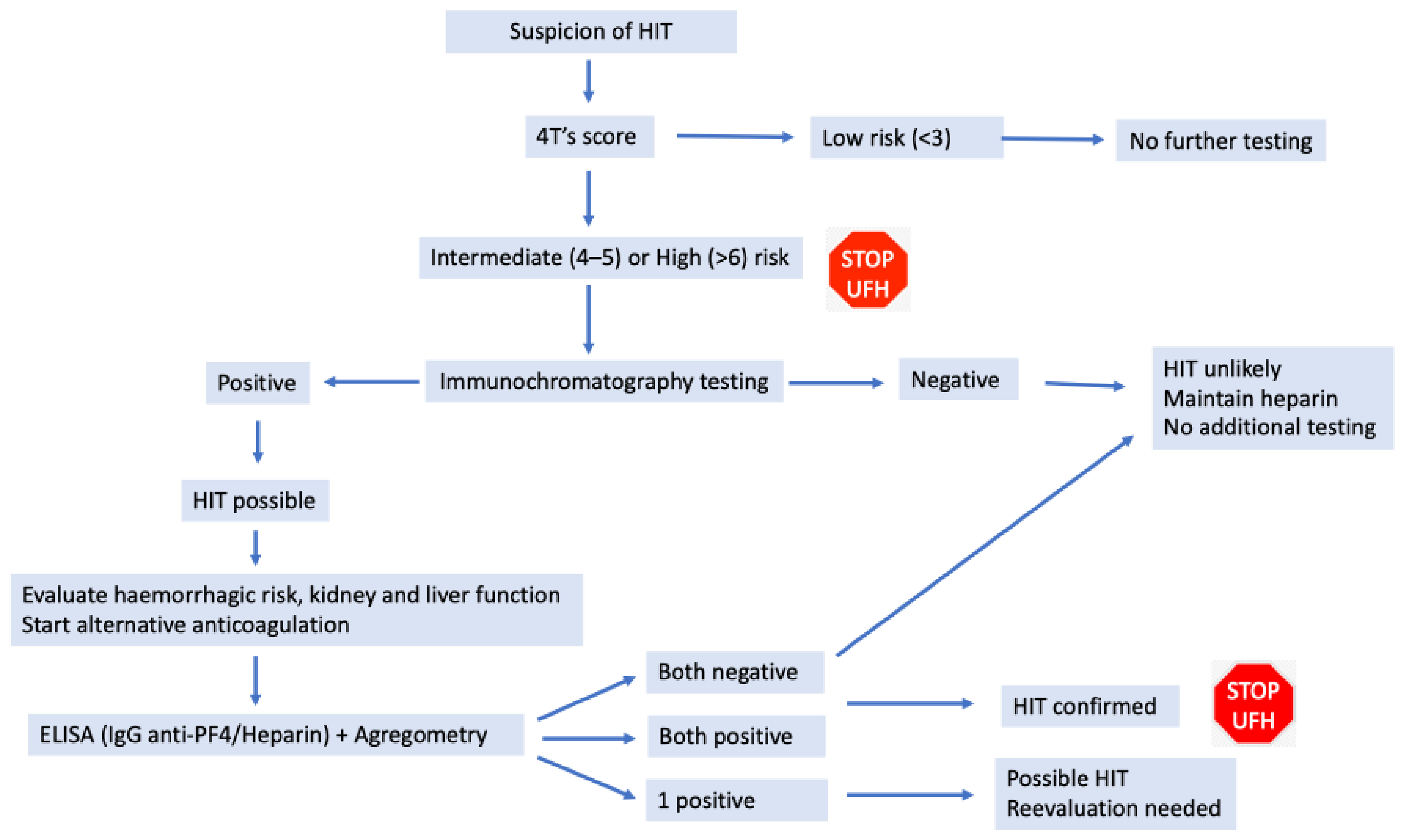
3.3.2. Heparin Induced Thrombocytopenia (HIT)
3.4. Ecmo Complications
3.4.1. Thrombotic Events
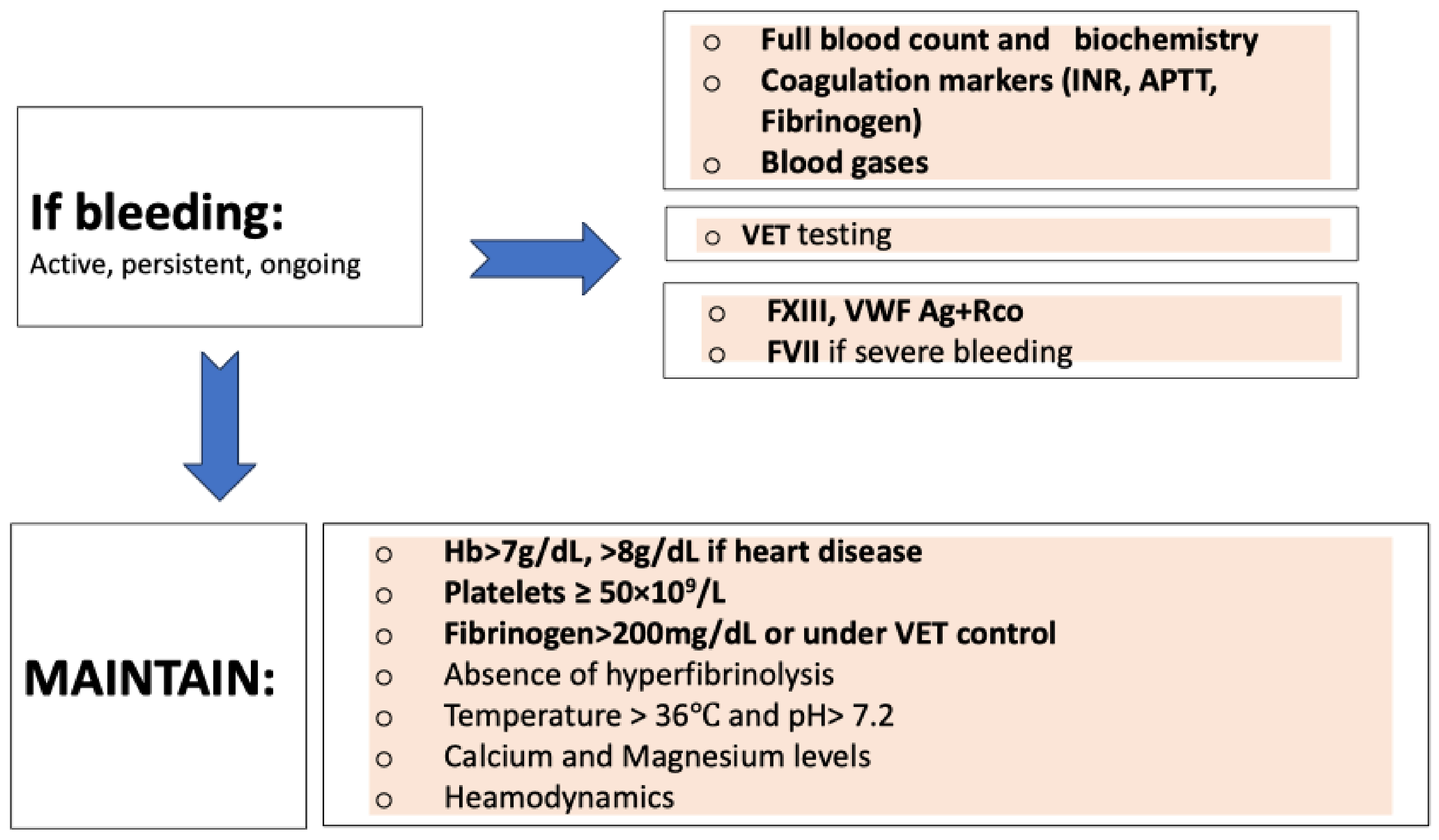
3.4.2. Bleeding Events Management
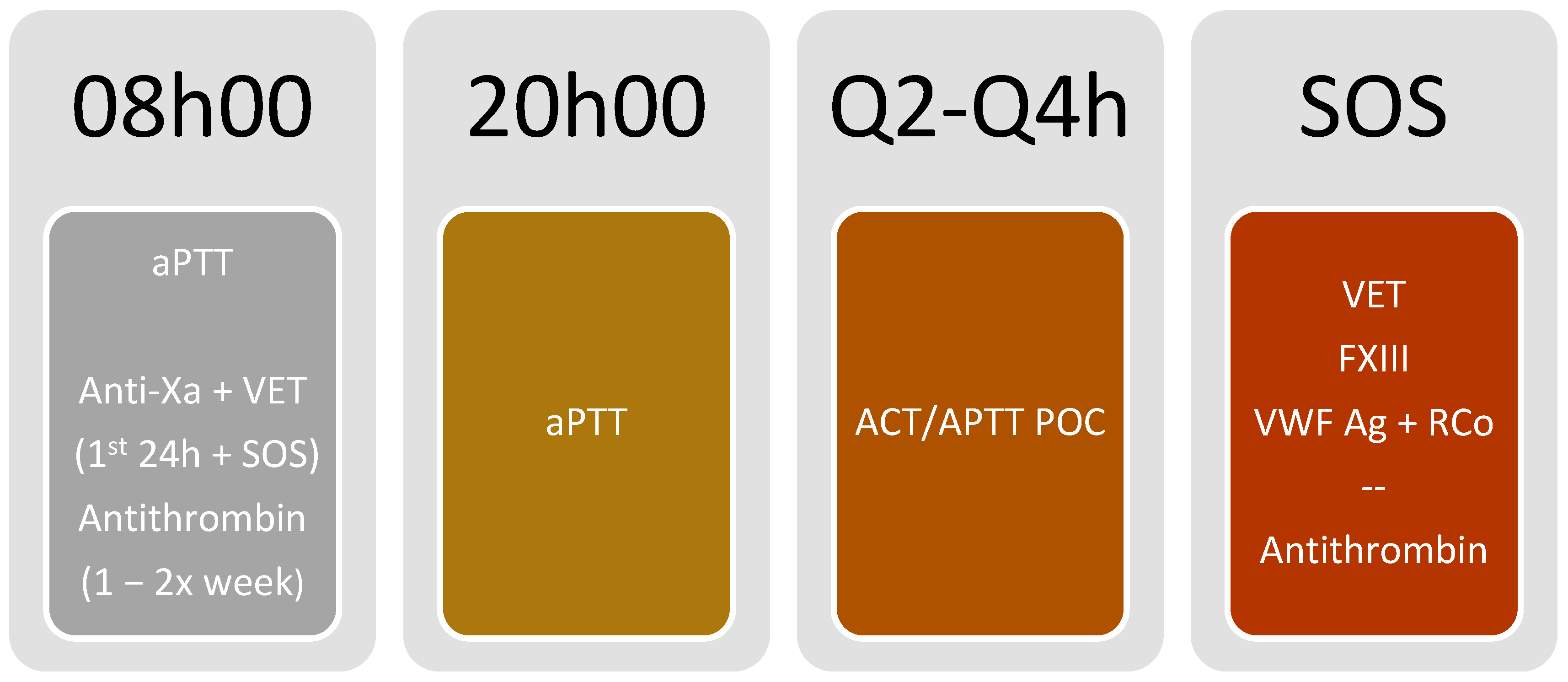
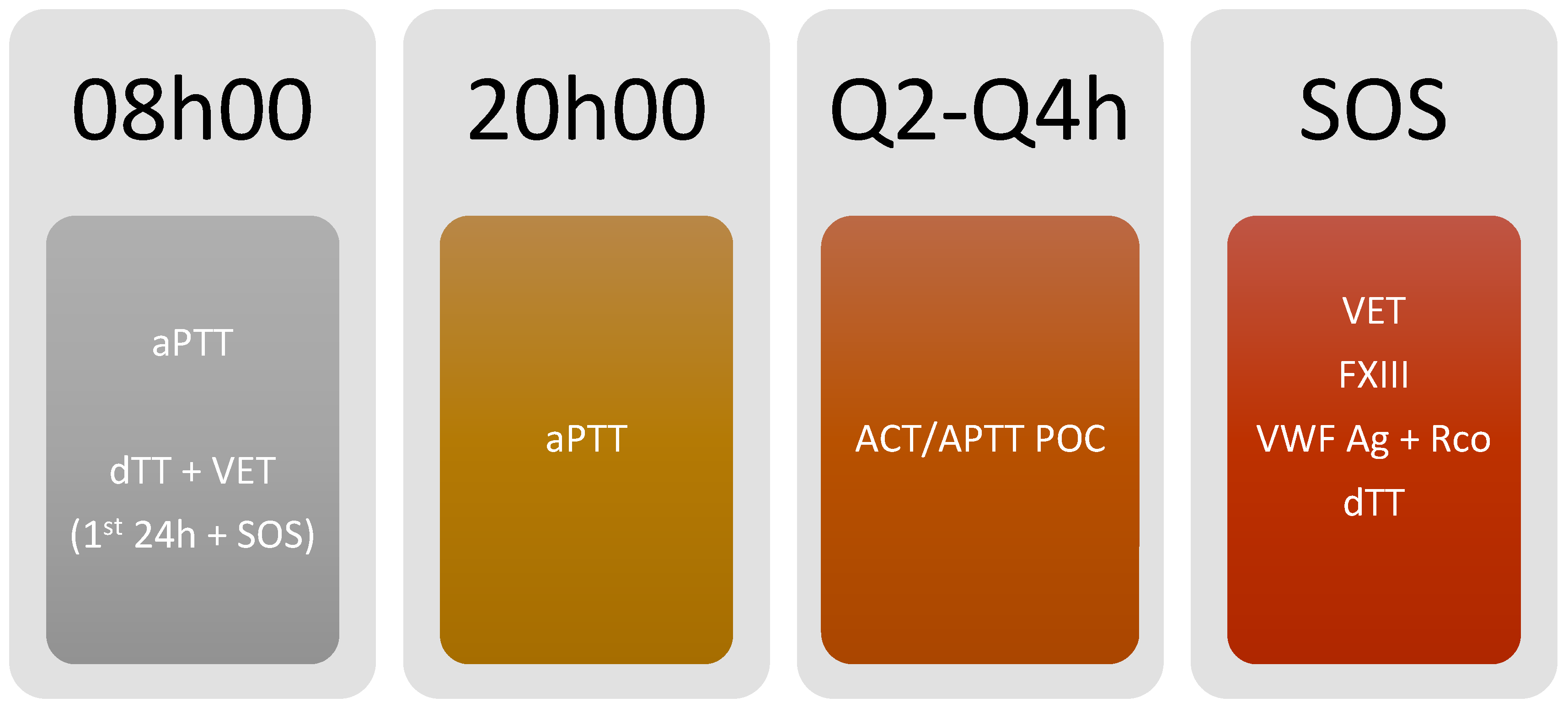
3.5. Monitoring Algorithm (Figure 3 and Figure 4)
- ○
- Due to the possibility of using a bedside point of care (POC) test, with the advantage of fast test results and timely monitoring, we propose using either point of care aPTT or ACT for coagulation monitoring every 2 h or 4 h, according to patient status and difficulty to achieve the target (Figure 5).
- (A)
- Verify if these assays were performed 20 min after blood collection
- (B)
- Verify if the blood was collected under the recommended conditions
- (C)
- Verify if the ACT equipment is under adequate quality control and correct work conditions (reagents, instrument).
- (D)
- These ranges can be reagent-dependent and must be checked by each laboratory
- (E)
- Even if all previous items were ok, preferably follow the anti-Xa results and repeat all tests shortly after.
- ○
- If POC methods are not available, aPTT should be monitored every 2 h or 4 h, according to patient status and difficulty in achieving the target.
- ○
- If POC aPTT is not available, aPTT monitoring should be performed every 12 h, using a multimodal evaluation focusing on the anticoagulant effect.
- ○
- Anti-Xa activity monitoring (using heparin) and dTT (for bivalirudin) should be performed in the first 24 h of monitoring and once daily.
- ○
- In patients on heparin anticoagulation, AT levels should be measured regularly (once or twice a week according to clinical status—i.e., if suspecting heparin resistance or not able to achieve heparin effect), and supplementation with AT concentrate should be granted when deemed necessary.
- ○
- When thrombotic complications occur, consider AT measurement. Maintain AT level over 80% (ideally between 80–120%).
- ○
- Viscoelastic testing (ROTEM®, Quantra®) should be considered in the first 24 h of anticoagulation to have qualitative monitoring. Testing should be repeated when thrombotic or bleeding complications occur.
- ○
- If there is a hemorrhagic event, FXIII, VWF (both antigen and functional), and FVII monitoring should be considered according to the volume of blood loss and the clinical scenario.
- ○
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Paulis, S.; Cavaliere, F. Anticoagulation Management in High Bleeding-Risk ECMO in Adults. Front. Cardiovasc. Med. 2022, 9, 884063. [Google Scholar] [CrossRef]
- Doymaz, S. Anticoagulation during ECMO: The Past, Present and Future. J. Intensive Crit. Care 2018, 4, 1–6. [Google Scholar] [CrossRef][Green Version]
- Staessens, S.; Moussa, M.D.; Pierache, A.; Rauch, A.; Rousse, N.; Boulleaux, E.; Ung, A.; Desender, L.; Pradines, B.; Vincentelli, A.; et al. Thrombus formation during ECMO: Insights from a detailed histological analysis of thrombus composition. J. Thromb. Haemost. 2022, 20, 2058–2069. [Google Scholar] [CrossRef]
- Olson, S.R.; Murphree, C.R.; Zonies, D.; Meyer, A.D.; Mccarty, O.J.T.; Deloughery, T.G.; Shatzel, J.J. Thrombosis and Bleeding in Extracorporeal Membrane Oxygenation (ECMO) Without Anticoagulation: A Systematic Review. ASAIO J. 2021, 67, 290–296. [Google Scholar] [CrossRef]
- Kalbhenn, J.; Zieger, B. Bleeding During Veno-Venous ECMO: Prevention and Treatment. Front. Med. 2022, 9, 879579. [Google Scholar] [CrossRef]
- Mulder, M.M.G.; Fawzy, I.; Lancé, M.D. ECMO and anticoagulation: A comprehensive review. Neth. J. Crit. Care 2018, 26, 6–13. [Google Scholar]
- Zakhary, B.; Vercaemst, L.; Mason, P.; Antonini, M.V.; Lorusso, R.; Brodie, D. How I approach membrane lung dysfunction in patients receiving ECMO. Crit. Care 2020, 24, 671. [Google Scholar] [CrossRef]
- Chlebowski, M.M.; Baltagi, S.; Carlson, M.; Levy, J.H.; Spinella, P.C. Clinical controversies in anticoagulation monitoring and antithrombin supplementation for ECMO. Crit. Care 2020, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.H.; Staudinger, T.; Steiner, M.E. How to manage anticoagulation during extracorporeal membrane oxygenation. Intensive Care Med. 2022, 48, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Maskey, A. Anticoagulation in ECMO patients: An overview. Indian J. Thorac. Cardiovasc. Surg. 2021, 37, 241–247. [Google Scholar] [CrossRef] [PubMed]
- McMichael, A.B.; Ryerson, L.M.; Ratano, D.; Fan, E.; Faraoni, D.; Annich, G.M. 2021 ELSO Adult and Pediatric Anticoagulation Guidelines. ASAIO J. 2022, 68, 303–310. [Google Scholar] [CrossRef]
- Helms, J.; Frere, C.; Thiele, T.; Tanaka, K.A.; Neal, M.D.; Steiner, M.E.; Connors, J.M.; Levy, J.H. Anticoagulation in adult patients supported with extracorporeal membrane oxygenation: Guidance from the Scientific and Standardization Committees on Perioperative and Critical Care Haemostasis and Thrombosis of the International Society on Thrombosis and Haemostasis. J. Thromb. Haemost. 2023, 21, 373–396. [Google Scholar] [CrossRef] [PubMed]
- Shirejini, S.Z.; Carberry, J.; McQuilten, Z.K.; Burrell, A.J.C.; Gregory, S.D.; Hagemeyer, C.E. Current and future strategies to monitor and manage coagulation in ECMO patients. Thromb. J. 2023, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Rajsic, S.; Breitkopf, R.; Jadzic, D.; Krneta, M.P.; Tauber, H.; Treml, B. Clinical Medicine Anticoagulation Strategies during Extracorporeal Membrane Oxygenation: A Narrative Review. J. Clin. Med. 2022, 2022, 5147. [Google Scholar] [CrossRef]
- Nath, S.S.; Pandey, C.K.; Kumar, S. Clinical application of viscoelastic point-of-care tests of coagulation-shifting paradigms. Ann. Card. Anaesth. 2022, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; Asmussen, S.; Maybauer, D.M.; Santonocito, C.; Fraser, J.F.; Erdoes, G.; Maybauer, M.O. Bivalirudin for Alternative Anticoagulation in Extracorporeal Membrane Oxygenation: A Systematic Review. J. Intensive Care Med. 2017, 32, 312–319. [Google Scholar] [CrossRef]
- Koster, A.; Ljajikj, E.; Faraoni, D.; Ortuno, S.; Delmas, C.; Diehl, J.-L.; Bailleul, C.; Lancelot, A.; Naili, M.; Cholley, B.; et al. Traditional and non-traditional anticoagulation management during extracorporeal membrane oxygenation. Ann. Cardiothorac. Surg. 2019, 8, 129–136. [Google Scholar] [CrossRef]
- Love, J.E.; Ferrell, C.; Chandler, W.L. Monitoring direct thrombin inhibitors with a plasma diluted thrombin time. Thromb. Haemost. 2007, 98, 234–243. [Google Scholar] [CrossRef]
- Levy, J.H.; Frere, C.; Koster, A. Resistance to unfractionated heparin in the ICU: Evaluation and management options. Intensive Care Med. 2023, 49, 1005–1007. [Google Scholar] [CrossRef]
- Arepally, G.M. Heparin-induced thrombocytopenia. Blood 2017, 129, 2864–2872. [Google Scholar] [CrossRef]
- Farner, B.; Kroll, H.; Kohlmann, T.; Warkentin, T.E.; Eichler, P.; Greinacher, A. Clinical features of heparin-induced thrombocytopenia including risk factors for thrombosis. A retrospective analysis of 408 patients. Thromb. Haemost. 2005, 94, 132–135. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Greinacher, A. Management of heparin-induced thrombocytopenia. Curr. Opin. Hematol. 2016, 23, 462–470. [Google Scholar] [CrossRef]
- Lewis, B.; Wallis, D.; Leya, F.; Hursting, M.J.; Kelton, J.G. Argatroban Anticoagulation in Patients with Heparin-Induced Thrombocytopenia. Available online: https://jamanetwork.com/journals/jamainternalmedicine/article-abstract/755826 (accessed on 28 February 2023).
- Hong, A.P.; Cook, D.J.; Sigouin, C.S.; Warkentin, T.E. Central venous catheters and upper-extremity deep-vein thrombosis complicating immune heparin-induced thrombocytopenia. Blood 2003, 101, 3049–3051. [Google Scholar] [CrossRef]
- Lo, G.K.; Juhl, D.; Warkentin, T.E.; Sigouin, C.S.; Eichler, P.; Greinacher, A. Evaluation of pretest clinical score (4 T’s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J. Thromb. Haemost. 2006, 4, 759–765. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Cook, R.J.; Marder, V.J.; Sheppard, J.-A.I.; Moore, J.C.; Eriksson, B.I.; Greinacher, A.; Kelton, J.G. Anti-platelet factor 4/heparin antibodies in orthopedic surgery patients receiving antithrombotic prophylaxis with fondaparinux or enoxaparin. Blood 2005, 106, 3791–3796. [Google Scholar] [CrossRef]
- Favaloro, E.J.; McCaughan, G.; Mohammed, S.; Lau, K.K.E.; Gemmell, R.; Cavanaugh, L.; Donikian, D.; Kondo, M.; Brighton, T.; Pasalic, L. HIT or miss? A comprehensive contemporary investigation of laboratory tests for heparin induced thrombocytopenia. Pathology 2018, 50, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.; Davidson, S.; Keeling, D. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: Second edition. Br. J. Haematol. 2012, 159, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Rodrigues, A.; Gomes, M.; Carrilho, A.; Nunes, A.R.; Orfão, R.; Alves, Â.; Aguiar, J.; Campos, M. Interventional Algorithms for the Control of Coagulopathic Bleeding in Surgical, Trauma, and Postpartum Settings: Recommendations From the Share Network Group. Clin. Appl. Thromb. Hemost. 2016, 22, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.; Kaserer, A.; Sprengel, K.; Wanner, G.A.; Seifert, B.; Theusinger, O.M.; Spahn, D.R. Change of transfusion and treatment paradigm in major trauma patients. Anaesthesia 2017, 72, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.; Oakes, M.; Kim, M.; Desai, A.; Olson, S.R.; Raghunathan, V.; Shatzel, J.J. Anticoagulation strategies in extracorporeal circulatory devices in adult populations. Eur. J. Haematol. 2021, 106, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Panigada, M.; Spinelli, E.; Cucino, A.; Cipriani, E.; De Falco, S.; Panarello, G.; Occhipinti, G.; Arcadipane, A.; Sales, G.; Fanelli, V.; et al. Antithrombin supplementation during extracorporeal membrane oxygenation: Study protocol for a pilot randomized clinical trial. Trials 2019, 20, 349. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; Wheeler, K.E. Weight-based heparin protocol using antifactor Xa monitoring. Am. J. Health Syst. Pharm. 2010, 67, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Ha, M.; Liu, M.H.; Reardon, D.P. Direct Thrombin Inhibitor Resistance and Possible Mechanisms. Hosp. Pharm. 2016, 51, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Domingues, T.D.; Jesus, G.N.; Garção, A.; Rodrigues, A.R.; Correia, C.J.; Pereira, C.L.; Correia, D.; Beleza, Á.; Ribeiro, J.M. COVID-19-associated Coagulopathy Characterization using Rotational Thromboelastometry in a Prospective, Observational Cohort Study: The HemoCoV Study. Acta Med. Port. 2023, 36, 496–505. [Google Scholar] [CrossRef]
| Heparin Resistance Causes | Anti-Xa Activity | aPTT Level | AT Level | Management |
|---|---|---|---|---|
| Pseudo-resistance | Therapeutic | Low | Normal or Moderate/low | Adjust heparin dosage according to anti-Xa activity |
| Low heparin concentration (high clearance or increased binding to proteins) | Low | Low | Normal or Moderate/low | Increase UFH dosage or change to DTI |
| AT deficiency | Low | Low | Very low (<40–50%) | AT supplementation or change to DTI |
| Criteria | Points | |
|---|---|---|
| Thrombocytopenia | Count drop > 50% Nadir ≥ 20 × 109/L | 2 |
| Count drop 30–50% Nadir 10–19 × 109/L | 1 | |
| Count drop < 30% Nadir < 10 × 109/L | 0 | |
| Timing of the decrease in platelet count | 5 to 10 days * | 2 |
| >10 days or possible start in 5–10 days | 1 | |
| <4 days | 0 | |
| Thrombosis manifestations | Acute thrombosis Cutaneous necrosis Systemic reaction | 2 |
| Recurring thrombosis Other skin lesions Suspicion of thrombosis, no confirmation | 1 | |
| Asymptomatic | 0 | |
| Other causes of thrombocytopenia | Apparently none (absence) Possible | 2 1 |
| Confirmed | 0 |
| ACT (s) | <140 | 141–160 | 161–179 | 180–200 | 201–240 | 241–270 | >270 |
|---|---|---|---|---|---|---|---|
| UFH bolus (U/kg) | 20 | 10 | - | - | - | - | - |
| STOP UFH (min) | - | - | - | - | - | 30 | 60 |
| UFH adjustment | +30% | +20% | +10% | - | −10% | −20% | −30% |
| aPTT ratio | <1.2 | 1.21–1.30 | 1.31–1.49 | 1.5–2 | 2.01–2.25 | 2.26–2.49 | >2.5 |
| UFH bolus (U/kg) | 20 | 10 | - | - | - | - | - |
| STOP UFH (min) | - | - | - | - | - | 30 | 60 |
| UFH adjustment | +20% | +15% | +10% | - | −10% | −20% | −30% |
| Anti-Xa (units/mL) | <0.20 | 0.20–0.29 | 0.30–0.7 | 0.71–0.80 | 0.81–0.99 | >1.00 | |
| UFH bolus (U/kg) | 26 | - | - | - | - | - | |
| STOP UFH (min) | - | - | - | - | - | 60 | |
| UFH adjustment | +4 U/kg/h | +2 U/kg/h | - | −1 U/kg/h | −2 U/kg/h | −3 U/kg/h |
| ACT (s) | <180 | 180–200 | 200–250 | >250 | |||
|---|---|---|---|---|---|---|---|
| Adjustment | ↑ 0.25–0.5 mg/Kg/h (if <140 s consider bolus 0.1–0.5 mg/Kg/h) → ↑ 10 to 20% each time | - | ↓ dose 10 to 20% | ↓ 30–50% | |||
| STOP (min) | - | - | - | - | - | - | 30 |
| APTT ratio | <1.2 | 1.21–1.30 | 1.31–1.49 | 1.5–2.0 | 2.01–2.25 | 2.26–2.49 | >2.5 |
| Adjustment | +20% (×1.2) | +10% (×1.1) | - | −10% (×0.9) | −50% (×0.5) | ||
| STOP (min) | - | - | - | - | - | 30 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.B.; Rodrigues, A.; Correia, C.J.; Jesus, G.N.; Ribeiro, J.M. Anticoagulation Management in V-V ECMO Patients: A Multidisciplinary Pragmatic Protocol. J. Clin. Med. 2024, 13, 719. https://doi.org/10.3390/jcm13030719
Rodrigues AB, Rodrigues A, Correia CJ, Jesus GN, Ribeiro JM. Anticoagulation Management in V-V ECMO Patients: A Multidisciplinary Pragmatic Protocol. Journal of Clinical Medicine. 2024; 13(3):719. https://doi.org/10.3390/jcm13030719
Chicago/Turabian StyleRodrigues, Ana Bento, Anabela Rodrigues, Catarina Jacinto Correia, Gustavo Nobre Jesus, and João Miguel Ribeiro. 2024. "Anticoagulation Management in V-V ECMO Patients: A Multidisciplinary Pragmatic Protocol" Journal of Clinical Medicine 13, no. 3: 719. https://doi.org/10.3390/jcm13030719
APA StyleRodrigues, A. B., Rodrigues, A., Correia, C. J., Jesus, G. N., & Ribeiro, J. M. (2024). Anticoagulation Management in V-V ECMO Patients: A Multidisciplinary Pragmatic Protocol. Journal of Clinical Medicine, 13(3), 719. https://doi.org/10.3390/jcm13030719







