HYSPAIN CLINICAL TRIAL Testing the Efficacy of Paracervical Anesthesia for Pain Control During Office Hysteroscopy: A Randomized, Single-Center, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
1. Introduction
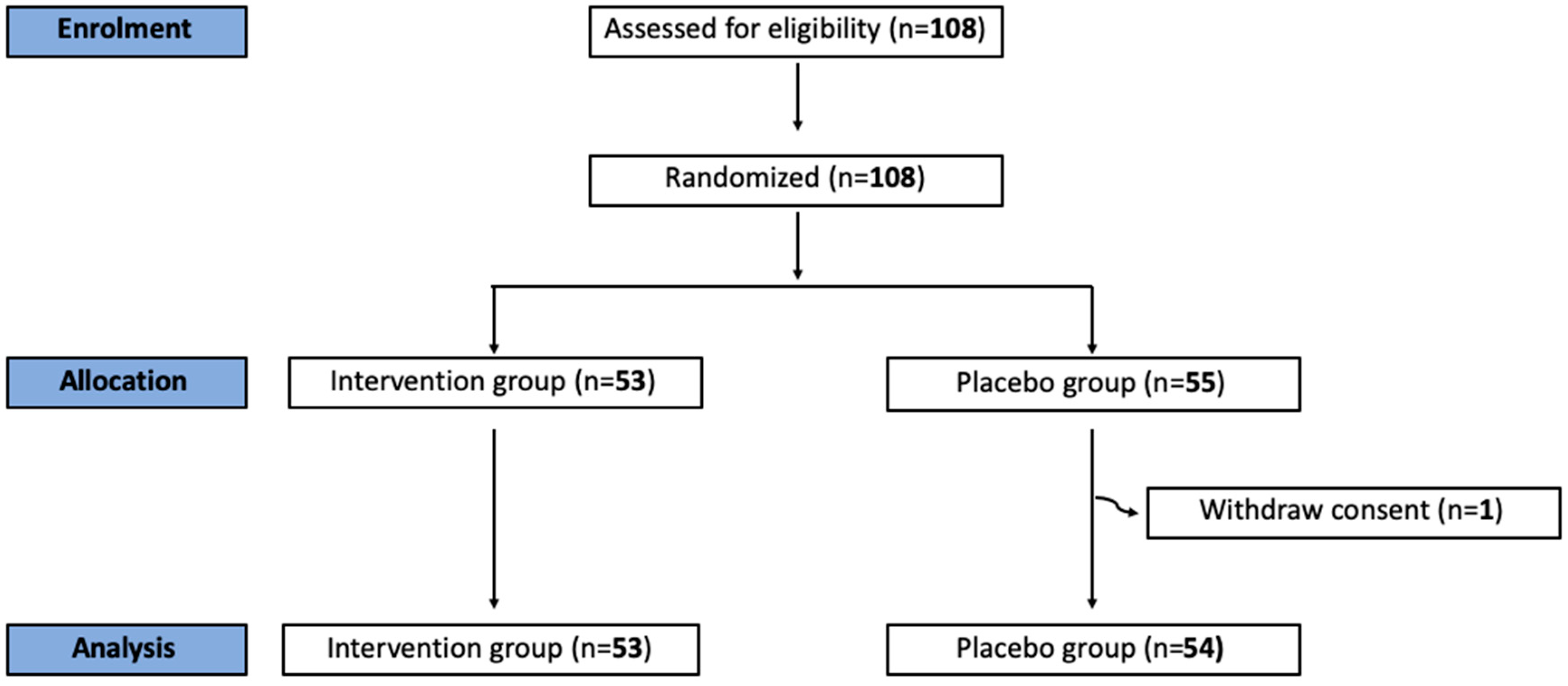
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gkrozou, F.; Dimakopoulos, G.; Vrekoussis, T.; Lavasidis, L.; Koutlas, A.; Navrozoglou, I.; Stefos, T.; Paschopoulos, M. Hysteroscopy in women with abnormal uterine bleeding: A meta-analysis on four major endometrial pathologies. Arch. Gynecol. Obstet. 2015, 291, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Angioni, S.; D’Alterio, M.N.; Ronsini, C.; Saponara, S.; De Franciscis, P.; Riemma, G. Risk of endometrial malignancy in women treated for breast cancer: The BLUSH prediction model—Evidence from a comprehensive multicentric retrospective cohort study. Climacteric 2024, 27, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.N.; Hamilton, K.; Kosturakis, A. An Overview of Office Hysteroscopy. Curr. Obstet. Gynecol. Rep. 2024, 13, 88–96. [Google Scholar] [CrossRef]
- De Iaco, P.; Marabini, A.; Stefanetti, M.; Del Vecchio, C.; Bovicelli, L. Acceptability and pain of outpatient hysteroscopy. J. Am. Assoc. Gynecol. Laparosc. 2000, 7, 71–75. [Google Scholar] [PubMed]
- Bettocchi, S.; Selvaggi, L. A vaginoscopic approach to reduce the pain of office hysteroscopy. J. Am. Assoc. Gynecol. Laparosc. 1997, 4, 255–258. [Google Scholar] [CrossRef]
- Cicinelli, E.; Didonna, T.; Ambrosi, G.; Schönauer, L.M.; Fiore, G.; Matteo, M.G. Topical anaesthesia for diagnostic hysteroscopy and endometrial biopsy in postmenopausal women: A randomised placebo-controlled double-blind study. BJOG 1997, 104, 316–319. [Google Scholar] [CrossRef]
- Costello, M.F.; Horrowitz, S.D.; Williamson, M. A prospective randomized double-blind placebo-controlled study of local anaesthetic injected through the hysteroscope for outpatient hysteroscopy and endometrial biopsy. Gynaecol. Endosc. 1998, 7, 121–126. [Google Scholar] [CrossRef]
- Lau, W.C.; Tam, W.H.; Lo, W.K.; Yuen, P.M. A randomised double-blind placebo-controlled trial of transcervical intrauterine local anaesthesia in outpatient hysteroscopy. BJOG 2000, 107, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Esteve, M.; Schindler, S.; Machado, S.B.; Borges, S.A.; Santos, C.R.; Coutinho, E. The efficacy of intracervical lidocaine in outpatient hysteroscopy. Gynaecol. Endosc. 2002, 11, 33–36. [Google Scholar] [CrossRef]
- Kokanali, M.K.; Güzel, A.I.; Özer, I.; Topçu, H.O.; Cavkaytar, S.; Doʇanay, M. Pain experienced during and after office hysteroscopy with and without intracervical anesthesia. J. Exp. Ther. Oncol. 2014, 10, 243–246. [Google Scholar] [PubMed]
- Cicinelli, E.; Didonna, T.; Schonauer, L.M.; Stragapede, S.; Falco, N.; Pansini, N. Paracervical anesthesia for hysteroscopy and endometrial biopsy in postmenopausal women. A randomized, double-blind, placebo-controlled study. J. Reprod. Med. 1998, 43, 1014. [Google Scholar] [PubMed]
- Lau, W.C. Paracervical anaesthesia in outpatient hysteroscopy: A randomised double-blind placebo controlled trial. BJOG 2000, 107, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.Y.K.; Wong, K.S.; Tang, L.C.H. Stepwise pain score analysis of the effect of local lignocaine on outpatient hysteroscopy: A randomized, double-blind, placebo-controlled trial. Fertil. Steril. 2000, 73, 1234–1237. [Google Scholar] [CrossRef]
- Soriano, D.; Ajaj, D.; Ajaj, S.; Chuong, T.; Deval, B.; Fauconnier, A.; Daraï, E. Lidocaine spray and outpatient hysteroscopy: Randomized placebo-controlled trial. Obstet. Gynecol. 2000, 96, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Arnau, B.; Jovell, E.; Redón, S.; Canals, M.; Mir, V.; Jiménez, E. Lidocaine-prilocaine (EMLA®) cream as analgesia in hysteroscopy practice: A prospective, randomized, non-blinded, controlled study. Acta Obstet. Gynecol. Scand. 2013, 92, 978–981. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; for the CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Huskisson, E.C. Measurement of pain. Lancet 1974, 304, 1127–1131. [Google Scholar] [CrossRef]
- International Conference on Harmonisation. ICH Topic E9: Statistical Principles for Clinical Trials. CPMP/ICH/363/96. 1998. Available online: https://database.ich.org/sites/default/files/E9_Guideline.pdf (accessed on 21 July 2024).
| Characteristic | Anaesthesia (n = 53) | Placebo (n = 54) |
|---|---|---|
| Age (y) | 44y (31–74) | 44.5y (31–72) |
| Race | ||
| Caucasian | 42 (79.25%) | 43 (79.44%) |
| Hispanoamerican | 10 (18.87%) | 11 (20.37%) |
| Interracial | 1 (1.89%) | 0 |
| Parity | ||
| Nulliparous | 21 (39.62%) | 32 (38.89%) |
| Primiparous | 14 (26.42%) | 9 (16.67%) |
| Multiparous | 18 (33.96%) | 24 (44.44%) |
| Previous Cesarean section | 10 (18.87%) | 16 (29.63%) |
| Menopause | 15 (28.30%) | 15 (27.78%) |
| Previous D&C | 9 (16.98%) | 11 (20.37%) |
| Previous Conization | 4 (7.55%) | 3 (5.56%) |
| Previous TMX treatment | 5 (9.43%) | 3 (5.56%) |
| Previous QRT | 4 (7.55%) | 1 (1.85%) |
| Indication | ||
| Infertility | 9 (16.98%) | 9 (16.67%) |
| Us finding | 19 (35.85%) | 18 (33.33%) |
| Abnormal uterine bleading | 16 (30.19%) | 16 (29.63%) |
| Postmenopause haemorrage | 8 (35.85%) | 8 (14.81%) |
| Other | 1 (15.09%) | 3 (5.56%) |
| Hysteroscopyc Findings and Procedures | Anaesthesia (n = 53) | Placebo (n = 54) |
|---|---|---|
| Entrance | ||
| Normal–easy | 41 (77.36%) | 39 (72.22%) |
| Stenotic | 5 (9.43%) | 5 (9.26%) |
| Tortuous | 6 (11.32%) | 10 (18.52%) |
| Failed | 1 (1.89%) | 0 |
| Endocervical canal | ||
| Normal | 45 (84.91%) | 49 (90.74%) |
| Pathology | 8 (15.09%) | 5 (9.26%) |
| Uterine cavity findings | 38 (73.08%) | 38 (70.37%) |
| Polyp | 26 (50%) | 28 (51.85%) |
| Myoma | 8 (15.38%) | 7 (12.96%) |
| RPOC (retained products of conception) | 5 (9.62%) | 3 (5.56%) |
| Procedure | ||
| Diagnostic | 11 (21.15%) | 14 (26.42%) |
| Therapeutic | 41 (78.85%) | 39 (73.58%) |
| Polypectomy | 26 (49.06%) | 30 (55.56%) |
| Myomectomy | 7 (13.21%) | 7 (12.96%) |
| Septoplasty/metroplasty | 1 (1.89%) | 0 |
| Isthmoplasty | 2 (3.77%) | 0 |
| RPOC resection | 5 (9.43%) | 3 (5.56%) |
| Instruments used | ||
| Bipolar | 37 (74%) | 38 (76%) |
| Mechanical | 13 (26%) | 12 (24%) |
| Complications | ||
| Failed hysteroscopy | 1 (1.89%) | 1 (1.85%) |
| Insuficient cavity distension | 1 (1.89%) | 2 (3.70%) |
| Bleeding | 1 (1.89%) | 0 |
| Mean procedure time (min) | 10 (2–60) | 11 (2–60) |
| Mean flow pressure (mmHg) | 75 (65–150) | 75 (65–135) |
| Anaesthesia | Placebo | p | |
|---|---|---|---|
| Global pain | 4 (2–6) | 4 (3–6) | 0.5822 |
| Pain during entrance | 3 (1–6) | 3 (1–6) | 0.3948 |
| Pain related to infiltration of Mepivacaine | 3 (1–4) | 3 (1–4) | 0.4650 |
| EVA: Median (p25–p75) |
| Coefficient (IC 95%) | p | Treatment Coefficient (Placebo) | |
|---|---|---|---|
| Age | −0.00 (−0.05; 0.03) | 0.717 | 0.39 (−0.49; 1.28) |
| Ethnicity | |||
| Caucasian | REF. CAT. | ||
| Hispanoamerican | 0.52 (−0.56; 1.62) | 0.343 | 0.48 (−0.38; 1.35) |
| Other | 5.35 (0.8; 9.89) | 0.021 | |
| Vaginal deliveries | |||
| 0 | REF. CAT. | ||
| >=1 | −1.14 (−2.02; −0.26) | 0.012 | 0.40 (−0.46; 1.26) |
| Cesarean section | 0.39 (−0.64; 1.43) | 0.454 | 0.34 (−0.54; 1.24) |
| D&C (dilatation and curettage) | |||
| Conization | 0.13 (−1.6; 1.93) | 0.882 | 0.39 (−0.49; 1.28) |
| Menopause | 0.49 (−0.49; 1.47) | 0.323 | 0.39 (−0.49; 1.28) |
| Treatment with Tamoxifen | −0.94 (−2.63; 0.74) | 0.268 | 0.35 (−0.53; 1.24) |
| Duration of hysteroscopy | 0.023 (−0.02; 0.06) | 0.290 | 0.32 (−0.56; 1.2) |
| Mean pressure | 0.00 (−0.01; 0.02) | 0.664 | 0.33 (−0.55; 1.22) |
| Instruments used | |||
| Bipolar | REF. CAT. | ||
| Mechanical | −1.11 (−2.10; −0.11) | 0.029 | 0.33 (−0.52; 1.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adrien-Lara, M.; Pereira, A.; Vitale, S.G.; Ríos, M.; Pérez-Medina, T.; Calles-Sastre, L. HYSPAIN CLINICAL TRIAL Testing the Efficacy of Paracervical Anesthesia for Pain Control During Office Hysteroscopy: A Randomized, Single-Center, Double-Blind, Placebo-Controlled Clinical Trial. J. Clin. Med. 2024, 13, 7856. https://doi.org/10.3390/jcm13247856
Adrien-Lara M, Pereira A, Vitale SG, Ríos M, Pérez-Medina T, Calles-Sastre L. HYSPAIN CLINICAL TRIAL Testing the Efficacy of Paracervical Anesthesia for Pain Control During Office Hysteroscopy: A Randomized, Single-Center, Double-Blind, Placebo-Controlled Clinical Trial. Journal of Clinical Medicine. 2024; 13(24):7856. https://doi.org/10.3390/jcm13247856
Chicago/Turabian StyleAdrien-Lara, María, Augusto Pereira, Salvatore Giovanni Vitale, Mar Ríos, Tirso Pérez-Medina, and Laura Calles-Sastre. 2024. "HYSPAIN CLINICAL TRIAL Testing the Efficacy of Paracervical Anesthesia for Pain Control During Office Hysteroscopy: A Randomized, Single-Center, Double-Blind, Placebo-Controlled Clinical Trial" Journal of Clinical Medicine 13, no. 24: 7856. https://doi.org/10.3390/jcm13247856
APA StyleAdrien-Lara, M., Pereira, A., Vitale, S. G., Ríos, M., Pérez-Medina, T., & Calles-Sastre, L. (2024). HYSPAIN CLINICAL TRIAL Testing the Efficacy of Paracervical Anesthesia for Pain Control During Office Hysteroscopy: A Randomized, Single-Center, Double-Blind, Placebo-Controlled Clinical Trial. Journal of Clinical Medicine, 13(24), 7856. https://doi.org/10.3390/jcm13247856







