The Effect of Hepatic Surgical Margins of Colorectal Liver Metastases on Prognosis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol and Guidelines
2.2. Eligibility Criteria
- Population: adult patients with colorectal liver metastases, either at the initial diagnosis or following liver resection.
- Intervention: surgical resection of liver metastases arising from colorectal carcinomas with clearly defined hepatic surgical margins.
- Comparison: evaluation of surgical margins categorized into two groups: margins ranging from 1 to 10 mm and margins greater than 10 mm.
- Outcome: provision of hazard ratios for overall survival comparing the two margin groups.
2.3. Information Sources, Search Strategy, and Selection Process
2.4. Data Collection Process and Data Items
2.5. Study Risk of Bias Assessment
2.6. Statistical Analysis
3. Results
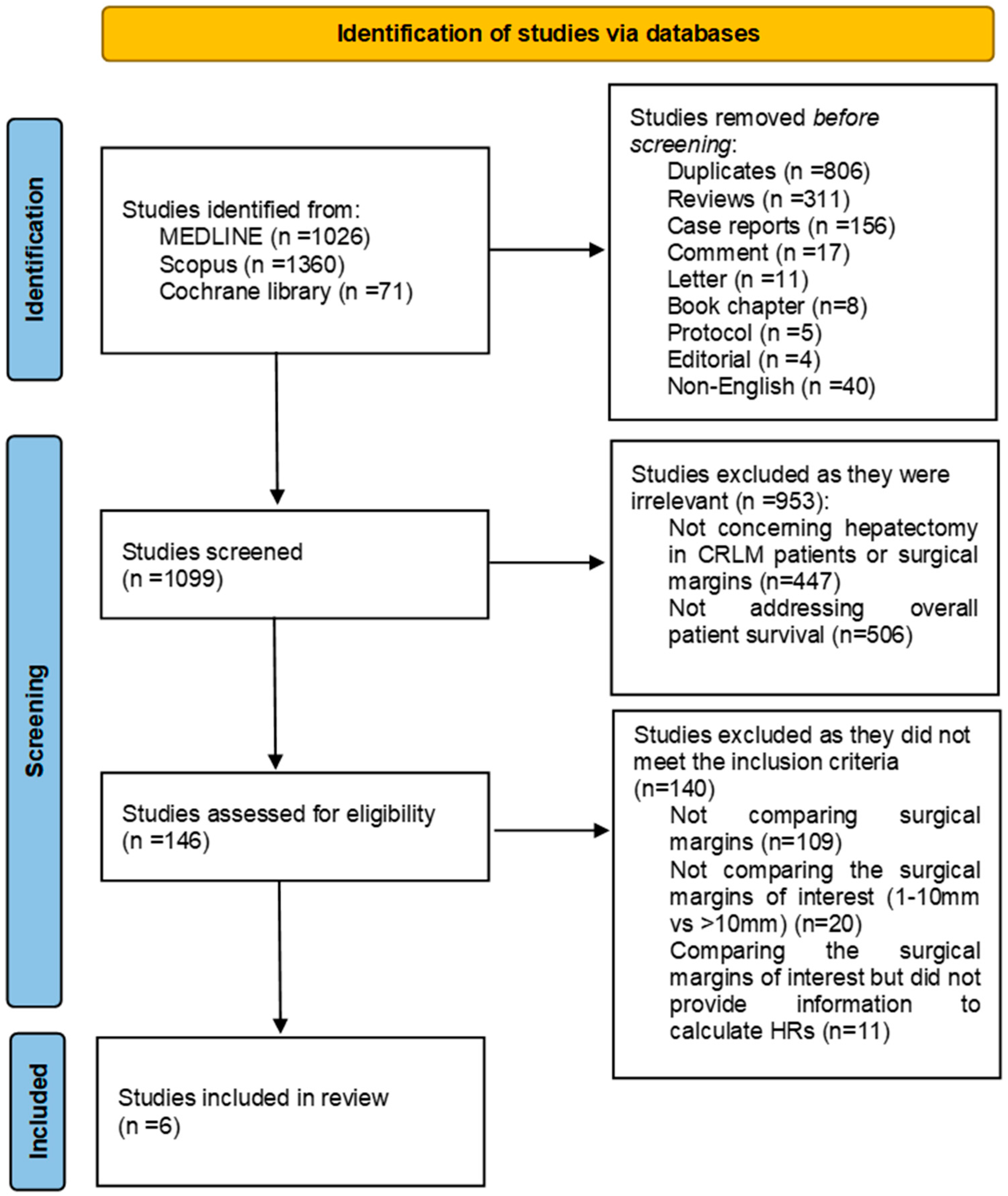
3.1. Study Selection
3.2. Study Characteristics
3.3. Findings of the Studies
3.3.1. Tumor Characteristics
3.3.2. Hepatectomy Characteristics
3.3.3. Neoadjuvant Chemotherapy
3.3.4. Overall Survival
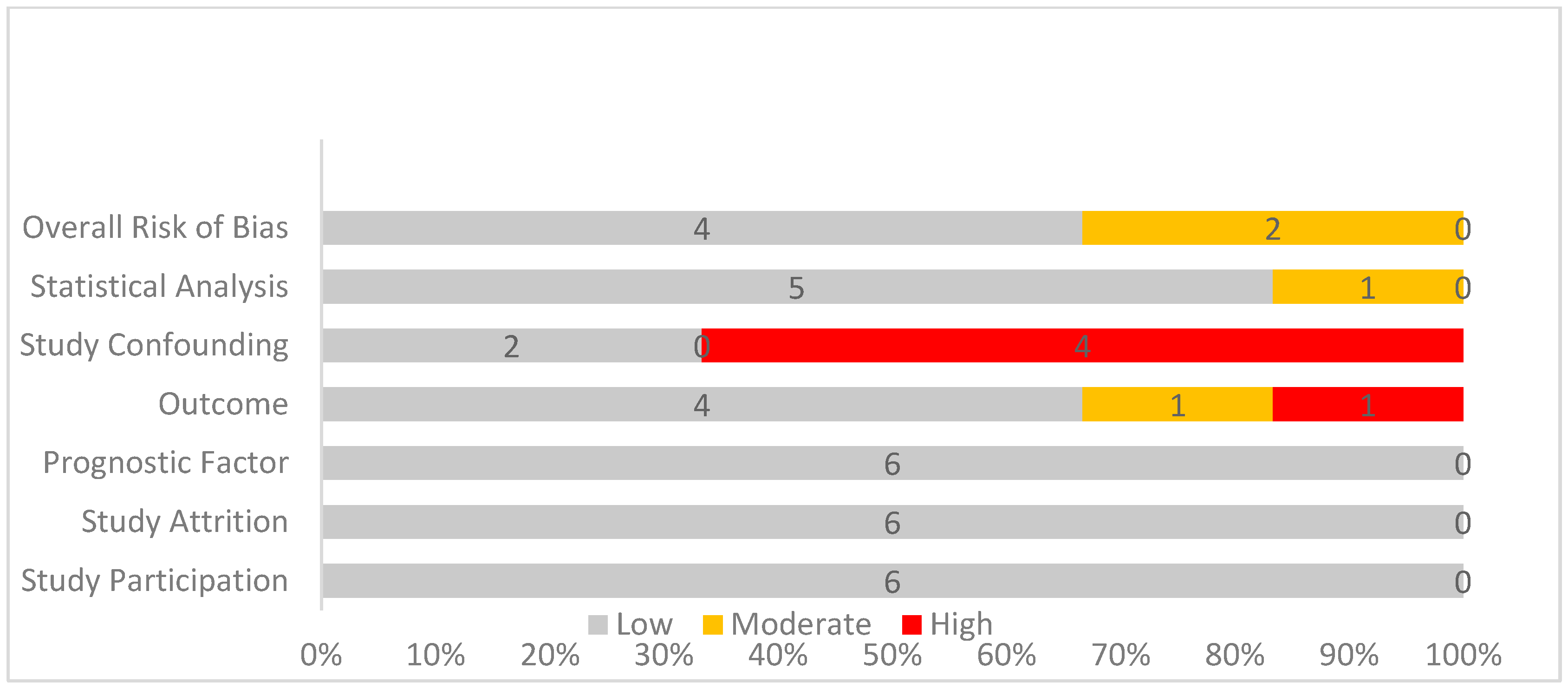
3.4. Quality Assessment
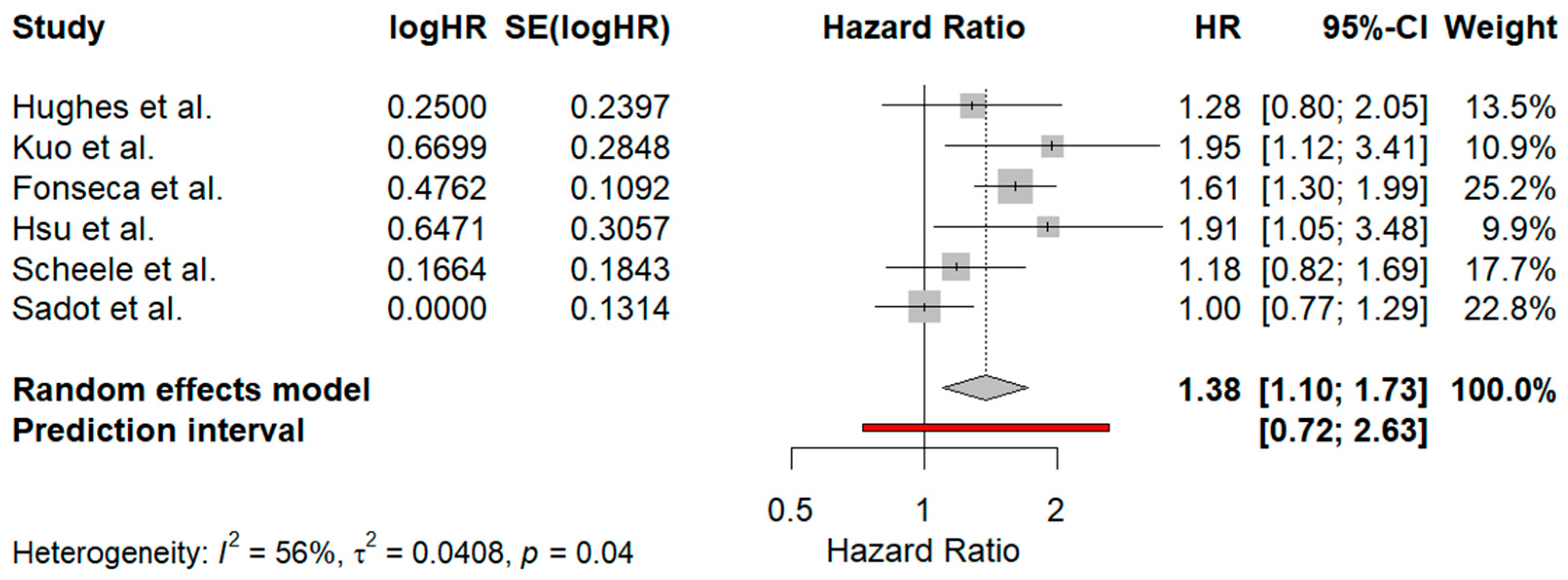
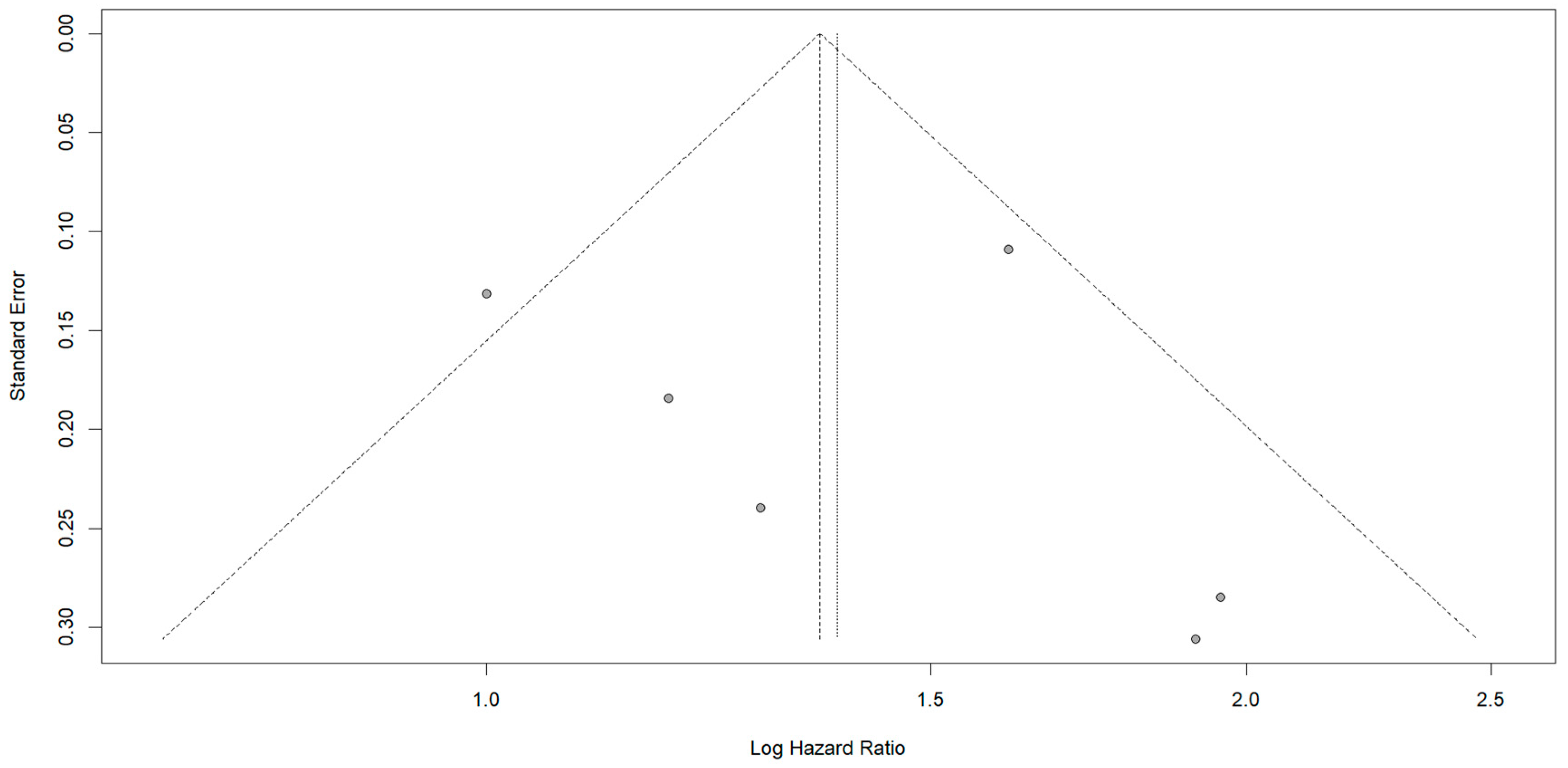
3.5. Meta-Analysis Results
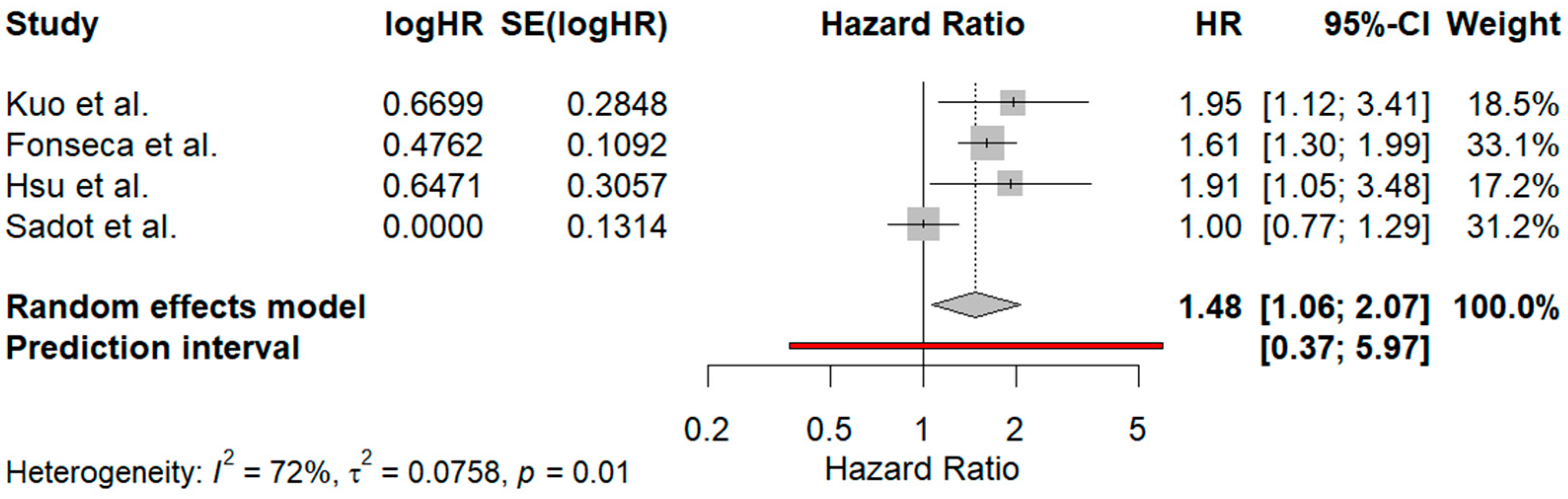
3.5.1. Comparison Between Surgical Margins of 1–10 mm and Those Exceeding 10 mm
3.5.2. Subgroup Analysis Based on Preoperative Chemotherapy
4. Discussion
- Restrospective studies only.
- Data from other reports, with inadequate information reporting, could not be included in the present synthesis.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; Schoen, R.E.; Sung, J.J.Y.; Young, G.P.; Kuipers, E.J. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal cancer epidemiology: Recent trends and impact on outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Haraldsdóttir, S.; Einarsdóttir, H.M.; Smáradóttir, A.; Gunnlaugsson, A.; Halfdanarson, T.R. Colorectal cancer—Review. Laeknabladid 2014, 100, 75–82. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Chakravarti, D.; Shalapour, S.; DePinho, R.A. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 2021, 35, 787–820. [Google Scholar] [CrossRef]
- Weitz, J.; Koch, M.; Debus, J.; Höhler, T.; Galle, P.R.; Büchler, M.W. Colorectal cancer. Lancet 2005, 365, 153–165. [Google Scholar] [CrossRef]
- Sninsky, J.A.; Shore, B.M.; Lupu, G.V.; Crockett, S.D. Risk factors for colorectal polyps and cancer. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 195–213. [Google Scholar] [CrossRef]
- Zhao, W.; Dai, S.; Yue, L.; Xu, F.; Gu, J.; Dai, X.; Qian, X. Emerging mechanisms progress of colorectal cancer liver metastasis. Front. Endocrinol. 2022, 13, 1081585. [Google Scholar] [CrossRef]
- Jin, K.; Gao, W.; Lu, Y.; Lan, H.; Teng, L.; Cao, F. Mechanisms regulating colorectal cancer cell metastasis into liver (Review). Oncol. Lett. 2012, 3, 11–15. [Google Scholar] [CrossRef][Green Version]
- Oki, E.; Ando, K.; Nakanishi, R.; Sugiyama, M.; Nakashima, Y.; Kubo, N.; Kudou, K.; Saeki, H.; Nozoe, T.; Emi, Y.; et al. Recent advances in treatment for colorectal liver metastasis. Ann. Gastroenterol. Surg. 2018, 2, 167–175. [Google Scholar] [CrossRef]
- Kemeny, N. The management of resectable and unresectable liver metastases from colorectal cancer. Curr. Opin. Oncol. 2010, 22, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; Van De Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Russolillo, N.; Ferrero, A.; Langella, S.; Sperti, E.; Capussotti, L. Evolution of long-term outcome of liver resection for colorectal metastases: Analysis of actual 5-year survival rates over two decades. Ann. Surg. Oncol. 2012, 19, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; De Gramont, A.; Figueras, J.; Guthrie, A.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; et al. The oncosurgery approach to managing liver metastases from colorectal cancer: A multidisciplinary international consensus. Oncologist 2012, 17, 1225–1239. [Google Scholar] [CrossRef] [PubMed]
- Spolverato, G.; Ejaz, A.; Azad, N.; Pawlik, T.M. Surgery for colorectal liver metastases: The evolution of determining prognosis. World J. Gastrointest. Oncol. 2013, 5, 207–221. [Google Scholar] [CrossRef]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann. Surg. 1999, 230, 309–318; discussion 318–321. [Google Scholar] [CrossRef]
- John, S.; Robinson, S.; Rehman, S.; Harrison, B.; Vallance, A.; French, J.; Jaques, B.; Charnley, R.; Manas, D.; White, S. Prognostic factors and survival after resection of colorectal liver metastasis in the era of preoperative chemotherapy: An 11-year single-centre study. Dig. Surg. 2013, 30, 293–301. [Google Scholar] [CrossRef]
- Makowiec, F.; Bronsert, P.; Klock, A.; Hopt, U.T.; Neeff, H.P. Prognostic influence of hepatic margin after resection of colorectal liver metastasis: Role of modern preoperative chemotherapy. Int. J. Color. Dis. 2018, 33, 71–78. [Google Scholar] [CrossRef]
- Ai, X.-N.; Zhang, Q.M.; Jin, C.-G.; Hu, H.; Zhang, W.-X.M.; Wu, Z.-Y.M.; Xiu, D.-R. Relationship between hepatic surgical margins of colorectal cancer liver metastases and prognosis: A review. Medicine 2024, 103, e37038. [Google Scholar] [CrossRef]
- Cucchetti, A.; Ercolani, G.; Cescon, M.; Bigonzi, E.; Peri, E.; Ravaioli, M.; Pinna, A.D. Impact of subcentimeter margin on outcome after hepatic resection for colorectal metastases: A meta-regression approach. Surgery 2012, 151, 691–699. [Google Scholar] [CrossRef]
- Liu, W.; Sun, Y.; Zhang, L.; Xing, B.-C. Negative surgical margin improved long-term survival of colorectal cancer liver metastases after hepatic resection: A systematic review and meta-analysis. Int. J. Color. Dis. 2015, 30, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Shapera, E.; Ross, S.; Crespo, K.; Syblis, C.; Przetocki, V.; Rosemurgy, A.; Sucandy, I. Analysis of surgical approach and tumor distance to margin after liver resection for colorectal liver metastasis. J. Robot. Surg. 2022, 16, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Konopke, R.; Kersting, S.; Makowiec, F.; Gaßmann, P.; Kuhlisch, E.; Senninger, N.; Hopt, U.; Saeger, H.D. Resection of Colorectal Liver Metastases: Is a Resection Margin of 3 mm Enough?: A multicenter analysis of the GAST Study Group. World J. Surg. 2008, 32, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Wray, C.; Lowy, A.; Mathews, J.; Park, S.; Choe, K.; Hanto, D.; James, L.; Soldano, D.; Ahmad, S. The significance and clinical factors associated with a subcentimeter resection of colorectal liver metastases. Ann. Surg. Oncol. 2005, 12, 374–380. [Google Scholar] [CrossRef]
- Wakai, T.; Shirai, Y.; Sakata, J.; Valera, V.A.; Korita, P.V.; Akazawa, K.; Ajioka, Y.; Hatakeyama, K. Appraisal of 1 cm hepatectomy margins for intrahepatic micrometastases in patients with colorectal carcinoma liver metastasis. Ann. Surg. Oncol. 2008, 15, 2472–2481. [Google Scholar] [CrossRef]
- Shirabe, K.; Takenaka, K.; Gion, T.; Fujiwara, Y.; Shimada, M.; Yanaga, K.; Kajiyama, M.K.; Sugimachi, K. Analysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br. J. Surg. 1997, 84, 1077–1080. [Google Scholar]
- Are, C.; Gonen, M.; Zazzali, K.; DeMatteo, R.P.; Jarnagin, W.R.; Fong, Y.; Blumgart, L.H.; D’Angelica, M. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann. Surg. 2007, 246, 295–300. [Google Scholar] [CrossRef]
- Lordan, J.; Karanjia, N. ‘Close shave’ in liver resection for colorectal liver metastases. Eur. J. Surg. Oncol. (EJSO) 2010, 36, 47–51. [Google Scholar] [CrossRef][Green Version]
- Jayme, V.R.; Fonseca, G.M.; Amaral, I.M.A.; Coelho, F.F.; Kruger, J.A.P.; Jeismann, V.B.; Pinheiro, R.S.N.; de Mello, E.S.; Herman, P. Infiltrative tumor borders in colorectal liver metastasis: Should we enlarge margin size? Ann. Surg. Oncol. 2021, 28, 7636–7646. [Google Scholar] [CrossRef]
- Hamady, Z.Z.R.; Lodge, J.P.A.; Welsh, F.K.; Toogood, G.J.; White, A.; John, T.; Rees, M. One-millimeter cancer-free margin is curative for colorectal liver metastases: A propensity score case-match approach. Ann. Surg. 2014, 259, 543–548. [Google Scholar] [CrossRef]
- Figueras, J.; Burdio, F.; Ramos, E.; Torras, J.; Llado, L.; Lopez-Ben, S.; Codina-Barreras, A.; Mojal, S. Effect of subcentimeter nonpositive resection margin on hepatic recurrence in patients undergoing hepatectomy for colorectal liver metastases. Evidences from 663 liver resections. Ann. Oncol. 2007, 18, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Postriganova, N.; Kazaryan, A.M.; Røsok, B.I.; Fretland, A.; Barkhatov, L.; Edwin, B. Margin status after laparoscopic resection of colorectal liver metastases: Does a narrow resection margin have an influence on survival and local recurrence? HPB 2014, 16, 822–829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Grooten, W.J.A.; Tseli, E.; Äng, B.O.; Boersma, K.; Stålnacke, B.-M.; Gerdle, B.; Enthoven, P. Elaborating on the assessment of the risk of bias in prognostic studies in pain rehabilitation using QUIPS—Aspects of interrater agreement. Diagn. Progn. Res. 2019, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.S.; Rosenstein, R.B.; Songhorabodi, S.; Adson, M.A.; Ilstrup, D.M.; Fortner, J.G.; Maclean, B.J.; Foster, J.H.; Daly, J.M.; Fitzherbert, D.; et al. Resection of the liver for colorectal carcinoma metastases. A multi-institutional study of long-term survivors. Dis. Colon Rectum 1988, 31, 1–4. [Google Scholar] [CrossRef]
- Scheele, J.; Stang, R.; Altendorf-Hofmann, A.; Paul, M. Resection of colorectal liver metastases. World J. Surg. 1995, 19, 59–71. [Google Scholar] [CrossRef]
- Kuo, I.-M.; Huang, S.-F.; Chiang, J.-M.; Yeh, C.-Y.; Chan, K.-M.; Chen, J.-S.; Yu, M.-C. Clinical features and prognosis in hepatectomy for colorectal cancer with centrally located liver metastasis. World J. Surg. Oncol. 2015, 13, 92. [Google Scholar] [CrossRef]
- Sadot, E.; Koerkamp, B.G.; Leal, J.N.; Shia, J.; Gonen, M.; Allen, P.J.; DeMatteo, R.P.; Kingham, T.P.; Kemeny, N.; Blumgart, L.H.; et al. Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: Surgical technique or biologic surrogate? Ann. Surg. 2015, 262, 476–485; discussion 483–485. [Google Scholar] [CrossRef]
- Fonseca, G.M.; de Mello, E.S.; Faraj, S.F.; Kruger, J.A.; Jeismann, V.B.; Coelho, F.F.; Alves, V.A.; Herman, P. Histopathological factors versus margin size in single colorectal liver metastases: Does a 1-cm margin size matter? Scand. J. Surg. 2022, 111, 14574969211069329. [Google Scholar] [CrossRef]
- Hsu, Y.-J.; Chern, Y.-J.; Wu, Z.-E.; Yu, Y.-L.; Liao, C.-K.; Tsai, W.-S.; You, J.-F.; Lee, C.-W. The oncologic outcome and prognostic factors for solitary colorectal liver metastasis after liver resection. J. Gastrointest. Surg. 2024, 28, 267–275. [Google Scholar] [CrossRef]
- Elias, D.; Bonnet, S.; Honoré, C.; Kohneh-Shahri, N.; Tomasic, G.; Lassau, N.; Dromain, C.; Goere, D. Comparison between the minimum margin defined on preoperative imaging and the final surgical margin after hepatectomy for cancer: How to manage it? Ann. Surg. Oncol. 2008, 15, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Sugihara, K.; Kosuge, T.; Takayama, T.; Shimada, K.; Yamasaki, S.; Sakamoto, M.; Hirohashi, S. Pathologic support for limited hepatectomy in the treatment of liver metastases from colorectal cancer. Ann. Surg. 1995, 221, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Kokudo, N.; Miki, Y.; Sugai, S.; Yanagisawa, A.; Kato, Y.; Sakamoto, Y.; Yamamoto, J.; Yamaguchi, T.; Muto, T.; Makuuchi, M. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: Minimum surgical margins for successful resection. Arch. Surg. 2002, 137, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Nordlinger, B.; Van Cutsem, E.; Gruenberger, T.; Glimelius, B.; Poston, G.; Rougier, P.; Sobrero, A.; Ychou, M. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: Recommendations from an expert panel. Ann. Oncol. 2009, 20, 985–992. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; van Krieken, J.H.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Watanabe, T.; Japanese Society for Cancer of the Colon and Rectum; Muro, K.; Ajioka, Y.; Hashiguchi, Y.; Ito, Y.; Saito, Y.; Hamaguchi, T.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2018, 23, 1–34. [Google Scholar] [CrossRef]
- Adam, R.; Kitano, Y. Multidisciplinary approach of liver metastases from colorectal cancer. Ann. Gastroenterol. Surg. 2019, 3, 50–56. [Google Scholar] [CrossRef]
- Kitano, Y.; Hayashi, H.; Matsumoto, T.; Kinoshita, S.; Sato, H.; Shiraishi, Y.; Nakao, Y.; Kaida, T.; Imai, K.; Yamashita, Y.-I.; et al. Borderline resectable for colorectal liver metastases: Present status and future perspective. World J. Gastrointest. Surg. 2021, 13, 756–763. [Google Scholar] [CrossRef]
- Sena, G.; Picciariello, A.; Marino, F.; Goglia, M.; Rocca, A.; Meniconi, R.L.; Gallo, G. One-stage total laparoscopic treatment for colorectal cancer with synchronous metastasis. is it safe and feasible? Front. Surg. 2021, 8, 752135. [Google Scholar] [CrossRef]
- Rocca, A.; Cipriani, F.; Belli, G.; Berti, S.; Boggi, U.; Bottino, V.; Cillo, U.; Cescon, M.; Cimino, M.; Corcione, F.; et al. The Italian Consensus on minimally invasive simultaneous resections for synchronous liver metastasis and primary colorectal cancer: A Delphi methodology. Updates Surg. 2021, 73, 1247–1265. [Google Scholar] [CrossRef]
- Gumiero, J.L.; de Oliveira, B.M.S.; Neto, P.A.d.O.; Pandini, R.V.; Gerbasi, L.S.; Figueiredo, M.N.; Kruger, J.A.P.; Seid, V.E.; Araujo, S.E.A.; Tustumi, F. Timing of resection of synchronous colorectal liver metastasis: A systematic review and meta-analysis. J. Surg. Oncol. 2022, 126, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Bonney, G.K.; Chew, C.A.; Lodge, P.; Hubbard, J.; Halazun, K.J.; Trunecka, P.; Muiesan, P.; Mirza, D.F.; Isaac, J.; Laing, R.W.; et al. Liver transplantation for non-resectable colorectal liver metastases: The international hepato-pancreato-biliary association consensus guidelines. Lancet Gastroenterol. Hepatol. 2021, 6, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Germani, M.M.; Borelli, B.; Boraschi, P.; Antoniotti, C.; Ugolini, C.; Urbani, L.; Morelli, L.; Fontanini, G.; Masi, G.; Cremolini, C.; et al. The management of colorectal liver metastases amenable of surgical resection: How to shape treatment strategies according to clinical, radiological, pathological and molecular features. Cancer Treat. Rev. 2022, 106, 102382. [Google Scholar] [CrossRef] [PubMed]
- She, W.H.; Cheung, T.T.; Tsang, S.H.Y.; Dai, W.C.; Lam, K.O.; Chan, A.C.Y.; Lo, C.M. Long-term survival in colorectal liver metastasis. Langenbeck’s Arch. Surg. 2022, 407, 3533–3541. [Google Scholar] [CrossRef]
- Bartolini, I.; Ringressi, M.N.; Melli, F.; Risaliti, M.; Brugia, M.; Mini, E.; Batignani, G.; Bechi, P.; Boni, L.; Taddei, A. Analysis of prognostic factors for resected synchronous and metachronous liver metastases from colorectal cancer. Gastroenterol. Res. Pract. 2018, 2018, 5353727. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.; Scheele, J.; Sugarbaker, P.H. Surgery for colorectal cancer metastatic to the liver: Optimizing the results of treatment. Surg. Clin. North Am. 1989, 69, 339–359. [Google Scholar] [CrossRef]
- Lemke, J.; Cammerer, G.; Ganser, J.; Scheele, J.; Xu, P.; Sander, S.; Henne-Bruns, D.; Kornmann, M. Survival and prognostic factors of colorectal liver metastases after surgical and nonsurgical treatment. Clin. Color. Cancer 2016, 15, e183–e192. [Google Scholar] [CrossRef]
- Chun, Y.S.M.; Passot, G.; Nishioka, Y.; Katkhuda, R.; Arvide, E.M.P.; Benzerdjeb, N.; Lopez, J.; Kopetz, S.E.; Maru, D.M.; Vauthey, J.-N.M. Colorectal liver micrometastases: Association with RAS/TP53 Co-mutation and prognosis after surgery. J. Am. Coll. Surg. 2022, 235, 8–16. [Google Scholar] [CrossRef]
- Plúa-Muñiz, K.; Bailón-Cuadrado, M.; Pérez-Saborido, B.; Pacheco-Sánchez, D.; Pinto, P.; Asensio-Díaz, E. Survival analysis and identification of prognostic factors in colorectal liver metastasis after liver resection. Cir. Esp. 2023, 101, 160–169. [Google Scholar] [CrossRef]
- Fukami, Y.; Maeda, A.; Takayama, Y.; Takahashi, T.; Uji, M.; Kaneoka, Y. Adverse oncological outcome of surgical site infection after liver resection for colorectal liver metastases. Surg. Today 2019, 49, 170–175. [Google Scholar] [CrossRef]
- Makowiec, F.; Menzel, M.; Bronsert, P.; Holzner, P.A.; Klock, A.; Lang, S.A.; Fichtner-Feigl, S.; Neeff, H.P. Does the site of primary colorectal cancer influence the outcome after resection of isolated liver metastases? Dig. Liver Dis. 2018, 50, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- McVey, J.C.; Sasaki, K.; Margonis, G.A.; Nowacki, A.S.; Firl, D.J.; He, J.; Berber, E.; Wolfgang, C.; Miller, C.C.; Weiss, M.; et al. The impact of resection margin on overall survival for patients with colon cancer liver metastasis varied according to the primary cancer location. HPB 2019, 21, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Boyd-Carson, H.; Irving, G.; Navarro, A.; Cameron, I.; Gomez, D.; Nottingham HPB Surgery Group. Anti-platelet therapy does not influence the outcome of patients undergoing hepatic resection for colorectal liver metastases, an observational study. Int. J. Surg. 2018, 53, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, M.; Czerwonko, M.; Ardiles, V.; Claria, R.S.; Mazza, O.; de Santibañes, E.; Pekolj, J.; de Santibañes, M. Laparoscopic vs open liver resection for metastatic colorectal cancer: Analysis of surgical margin status and survival. Langenbeck’s Arch. Surg. 2022, 407, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Paro, A.; Hyer, J.M.; Avery, B.S.; Tsilimigras, D.I.; Bagante, F.; Guglielmi, A.; Ruzzenente, A.; Alexandrescu, S.; Poultsides, G.; Sasaki, K.; et al. Using the win ratio to compare laparoscopic versus open liver resection for colorectal cancer liver metastases. Hepatobiliary Surg. Nutr. 2023, 12, 692–703. [Google Scholar] [CrossRef]
- Rahimli, M.; Perrakis, A.; Gumbs, A.A.; Andric, M.; Al-Madhi, S.; Arend, J.; Croner, R.S. The LiMAx test as selection criteria in minimally invasive liver surgery. J. Clin. Med. 2022, 11, 3018. [Google Scholar] [CrossRef]
- Alvikas, J.; Lo, W.; Tohme, S.; Geller, D.A. Outcomes and patient selection in laparoscopic vs. open liver resection for HCC and colorectal cancer liver metastasis. Cancers 2023, 15, 1179. [Google Scholar] [CrossRef]
- Naito, S.; Kajiwara, M.; Nakashima, R.; Sasaki, T.; Hasegawa, S. Application of extended reality (virtual reality and mixed reality) technology in laparoscopic liver resections. Cureus 2023, 15, e44520. [Google Scholar] [CrossRef]
- Chang, W.; Ye, Q.; Xu, D.; Liu, Y.; Zhou, S.; Ren, L.; He, G.; Zhou, G.; Liang, F.; Fan, J.; et al. Robotic versus open surgery for simultaneous resection of rectal cancer and liver metastases: A randomized controlled trial. Int. J. Surg. 2023, 109, 3346–3353. [Google Scholar] [CrossRef]
- Masetti, M.; Fallani, G.; Ratti, F.; Ferrero, A.; Giuliante, F.; Cillo, U.; Guglielmi, A.; Ettorre, G.M.; Torzilli, G.; Vincenti, L.; et al. Minimally invasive treatment of colorectal liver metastases: Does robotic surgery provide any technical advantages over laparoscopy? A multicenter analysis from the IGoMILS (Italian Group of Minimally Invasive Liver Surgery) registry. Updates Surg. 2022, 74, 535–545. [Google Scholar] [CrossRef]
- Sarpel, U.; Bonavia, A.S.; Grucela, A.; Roayaie, S.; Schwartz, M.E.; Labow, D.M. Does anatomic versus nonanatomic resection affect recurrence and survival in patients undergoing surgery for colorectal liver metastasis? Ann. Surg. Oncol. 2009, 16, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Sui, C.-J.; Cao, L.; Li, B.; Yang, J.-M.; Wang, S.-J.; Su, X.; Zhou, Y.-M. Anatomical versus nonanatomical resection of colorectal liver metastases: A meta-analysis. Int. J. Color. Dis. 2012, 27, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Mayo, S.C.; Sutton, T.; Kearney, M.R.; Kardosh, A.; Vaccaro, G.M.; Billingsley, K.G.; Lopez, C.D. Effect of time to surgery of colorectal liver metastases on survival. J. Gastrointest. Cancer 2021, 52, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, Y.; Wen, N.; Wang, S.; Li, B.; Liu, G. Prognostic factors associated with early recurrence following liver resection for colorectal liver metastases: A systematic review and meta-analysis. BMC Cancer 2024, 24, 426. [Google Scholar] [CrossRef]
- Butte, J.M.; Gönen, M.; Allen, P.J.; Kingham, T.P.; Sofocleous, C.T.; DeMatteo, R.P.; Fong, Y.; Kemeny, N.E.; Jarnagin, W.R.; D’angelica, M.I. Recurrence after partial hepatectomy for metastatic colorectal cancer: Potentially curative role of salvage repeat resection. Ann. Surg. Oncol. 2015, 22, 2761–2771. [Google Scholar] [CrossRef]
- Takahashi, M.; Hasegawa, K.; Oba, M.; Aoki, T.; Sakamoto, Y.; Sugawara, Y.; Kokudo, N. Repeat resection leads to long-term survival: Analysis of 10-year follow-up of patients with colorectal liver metastases. Am. J. Surg. 2015, 210, 904–910. [Google Scholar] [CrossRef]
- Nanji, S.; Tsang, M.E.; Wei, X.; Booth, C.M. Outcomes after repeat hepatic resection for recurrent metastatic colorectal cancer: A population-based study. Am. J. Surg. 2017, 213, 1053–1059. [Google Scholar] [CrossRef]
- Andreou, A.; Brouquet, A.; Abdalla, E.K.; Aloia, T.A.; Curley, S.A.; Vauthey, J.-N. Repeat hepatectomy for recurrent colorectal liver metastases is associated with a high survival rate. HPB 2011, 13, 774–782. [Google Scholar] [CrossRef]
- Wang, S.-J.; Si, X.-Y.; Cai, Z.-B.; Zhou, Y.-M. Survival after repeat hepatectomy for recurrent colorectal liver metastasis: A review and meta-analysis of prognostic factors. Hepatobiliary Pancreat. Dis. Int. 2019, 18, 313–320. [Google Scholar] [CrossRef]
- Reijonen, P.; Nordin, A.; Savikko, J.; Poussa, T.; Arola, J.; Isoniemi, H. Histopathological Helsinki score of colorectal liver metastases predicts survival after liver resection. APMIS 2023, 131, 249–261. [Google Scholar] [CrossRef]
- Dupré, A.; Berhane, S.; Chan, A.; Rivoire, M.; Chong, C.; Lai, P.; Cucchetti, A.; Poston, G.J.; Malik, H.; Johnson, P. Multicentre validation of a clinical prognostic score integrating the systemic inflammatory response to the host for patients treated with curative-intent for colorectal liver metastases: The liverpool score. Eur. J. Surg. Oncol. (EJSO) 2019, 45, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Jin, K.; Bao, Q.; Li, J.; Wang, K.; Xing, B. The prognostic impact of resection margin status varies according to the genetic and morphological evaluation (GAME) score for colorectal liver metastasis. J. Surg. Oncol. 2021, 124, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-C.; Yip, A.S.-M. Prognostic factors of survival and a new scoring system for liver resection of colorectal liver metastasis. World J. Hepatol. 2022, 14, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-S.; Kim, M.J.; Yun, S.H.; Chun, H.-K.; Lee, W.Y.; Kim, S.-J.; Choi, S.-H.; Heo, J.-S.; Joh, J.W.; Kim, Y.I. Risk factor stratification after simultaneous liver and colorectal resection for synchronous colorectal metastasis. Langenbeck’s Arch. Surg. 2008, 393, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Cacciaguerra, A.B.; Görgec, B.; Cipriani, F.; Aghayan, D.; Borelli, G.; Aljaiuossi, A.; Dagher, I.; Gayet, B.; Fuks, D.; Rotellar, F.; et al. Risk factors of positive resection margin in laparoscopic and open liver surgery for colorectal liver metastases: A new perspective in the perioperative assessment. Ann. Surg. 2022, 275, e213–e221. [Google Scholar] [CrossRef]
- Nierop, P.M.; Höppener, D.J.; van der Stok, E.P.; Galjart, B.; Buisman, F.E.; Balachandran, V.P.; Jarnagin, W.R.; Kingham, T.P.; Allen, P.J.; Shia, J.; et al. Histopathological growth patterns and positive margins after resection of colorectal liver metastases. HPB 2020, 22, 911–919. [Google Scholar] [CrossRef]
- de Haas, R.J.; Wicherts, D.A.; Flores, E.; Azoulay, D.; Castaing, D.; Adam, R. R1 resection by necessity for colorectal liver metastases: Is it still a contraindication to surgery? Ann. Surg. 2008, 248, 626–637. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, J.; Ye, M.; Weng, W.; Tan, C.; Ni, S.; Huang, D.; Sheng, W.; Wang, L. KRAS mutation predicted more mirometastases and closer resection margins in patients with colorectal cancer liver metastases. Ann. Surg. Oncol. 2020, 27, 1164–1173. [Google Scholar] [CrossRef]
- Brudvik, K.W.; Mise, Y.; Chung, M.H.; Chun, Y.S.; Kopetz, S.E.; Passot, G.; Conrad, C.; Maru, D.M.; Aloia, T.A.; Vauthey, J.-N. RAS mutation predicts positive resection margins and narrower resection margins in patients undergoing resection of colorectal liver metastases. Ann. Surg. Oncol. 2016, 23, 2635–2643. [Google Scholar] [CrossRef]
- Procopio, F.; Viganò, L.; Cimino, M.; Donadon, M.; Del Fabbro, D.; Torzilli, G. Does KRAS mutation status impact the risk of local recurrence after R1 vascular resection for colorectal liver metastasis? An observational cohort study. Eur. J. Surg. Oncol. (EJSO) 2020, 46, 818–824. [Google Scholar] [CrossRef]
- Hatta, A.A.Z.; Pathanki, A.M.; Hodson, J.; Sutcliffe, R.P.; Marudanayagam, R.; Roberts, K.J.; Chatzizacharias, N.; Isaac, J.; Muiesan, P.; Taniere, P.; et al. The effects of resection margin and KRAS status on outcomes after resection of colorectal liver metastases. HPB 2021, 23, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, K.; Kaihara, S.; Koyama, T.; Nakao, K.; Matsuda, S.; Toriguchi, K.; Kitamura, K.; Oshima, N.; Kondo, M.; Hashida, H.; et al. The impact of KRAS status on the required surgical margin width for colorectal liver metastasis resection. J. Clin. Med. 2023, 12, 2313. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hong, H.; Pan, W.; Chen, J.; Jiang, L.; Du, Q.; Li, G.; Lin, S.; Chen, Y. Does using indocyanine green fluorescence imaging for tumors help in determining the safe surgical margin in real-time navigation of laparoscopic hepatectomy? A retrospective study. Ann. Surg. Oncol. 2023, 30, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.; Rehman, S.; McKay, S.; Bartlett, D.; Mirza, D. Use of near-infrared fluorescence techniques in minimally invasive surgery for colorectal liver metastases. J. Clin. Med. 2023, 12, 5536. [Google Scholar] [CrossRef] [PubMed]
- Karmarkar, R.; Benjafield, A.; Aroori, S. The role of colour segmented fluorescence (CSF) mode and same-day administration of low-dose indocyanine green in liver surgery: Our initial experience: Indocyanine green fluorescence guided resection of liver tumours. J. Fluoresc. 2023, 34, 2133–2138. [Google Scholar] [CrossRef]
- Achterberg, F.B.; Bijlstra, O.D.; Slooter, M.D.; Mulder, B.G.S.; Boonstra, M.C.; Bouwense, S.A.; Bosscha, K.; Coolsen, M.M.E.; Derksen, W.J.M.; Gerhards, M.F.; et al. ICG-fluorescence imaging for margin assessment during minimally invasive colorectal liver metastasis resection. JAMA Netw. Open 2024, 7, e246548. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Dai, H.-Y.; Li, C.-Y.; Jin, Y.; Zhu, L.-L.; Zhang, T.-F.; Zhang, Y.-X.; Mai, W.-H. Improved sensitivity and positive predictive value of contrast-enhanced intraoperative ultrasound in colorectal cancer liver metastasis: A systematic review and meta-analysis. J. Gastrointest. Oncol. 2022, 13, 221–230. [Google Scholar] [CrossRef]
- Galli, R.; Siciliano, T.; Aust, D.; Korn, S.; Kirsche, K.; Baretton, G.B.; Weitz, J.; Koch, E.; Riediger, C. Label-free multiphoton microscopy enables histopathological assessment of colorectal liver metastases and supports automated classification of neoplastic tissue. Sci. Rep. 2023, 13, 4274. [Google Scholar] [CrossRef]
- Dhir, M.; Lyden, E.R.; Wang, A.; Smith, L.M.; Ullrich, F.; Are, C. Influence of margins on overall survival after hepatic resection for colorectal metastasis. Ann. Surg. 2011, 254, 234–242. [Google Scholar] [CrossRef]
- Margonis, G.A.; Sergentanis, T.N.; Ntanasis-Stathopoulos, I.; Andreatos, N.; Tzanninis, I.-G.; Sasaki, K.; Psaltopoulou, T.; Wang, J.; Buettner, S.; Papalois, A.E.; et al. Impact of surgical margin width on recurrence and overall survival following R0 hepatic resection of colorectal metastases. Ann. Surg. 2018, 267, 1047–1055. [Google Scholar] [CrossRef]

| Study ID | Location | Type of Research | Total Subjects (%Male/Age) | Recruitment Period | Substratification of Margins (n) | Substratification of Negative Margins (n) | Median Follow-Up (Months) |
|---|---|---|---|---|---|---|---|
| Hughes et al., 1988 [35] | California, US | ORCS | 100 - | 1948–1985 | R0 (26) R1 (1) | 1–10 mm: 9 >10 mm: 17 | ≈60 |
| Scheele et al., 1995 [36] | Erlanger, Germany | ORCS | 434 (52.8/59) | 1960–1992 | R0 (350) R1 (48) | 1–10 mm: 204 >10 mm: 146 | >60 |
| Kuo et al., 2015 [37] | Taoyuan, Taiwan | ORCS | 159 (55.9/-) | 1991–2006 | - | 1–10 mm: 128 >10 mm: 31 | 38.5 |
| Sadot et al., 2015 [38] | New York, US | ORCS | 2368 (57/61) | 1992–2012 | - | 1–10 mm: 1191 >10 mm: 765 | 55 |
| Fonseca et al., 2022 [39] | Sao Paulo, Brazil | ORCS | 101 (54.5/63) | 2007–2015 | - | 1–10 mm: 66 >10 mm: 35 | 34 |
| Hsu et al., 2024 [40] | Taoyuan, Taiwan | ORCS | 230 (63.5/62.4) | 2010–2019 | R0 (224) R1 (4) | 1–10 mm: 166 >10 mm: 62 | 39 |
| Study ID | Tumor Size (mm) | No. of Metastases | Location of the Primary Site | Metastases | Distribution for Multiple Metastases | Status of Primary Tumor Lymph Nodes | Preoperative CEA Level | Differentiation | Disease-Free Interval | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <5 cm | >5 cm | Solitary | Multiple | Colon | Rectum | Synchronous | Metachronous | Unilobar | Bilobar | Negative | Positive | < | > | W | M | P | <1 Year | >1 Year | |
| Hughes et al., 1988 [35] | - | - | 81 | 16 | - | - | - | - | 13 | 3 | 41 | 30 | - | - | - | - | - | 64 | 34 |
| Scheele et al., 1995 [36] | 233 | 117 | 203 | 147 | 189 | 161 | 142 | 208 | 90 | 57 | 116 | 216 | 82 <5 ng/mL | 197 >5 ng/mL | - | - | - | 77 | 111 |
| Kuo et al., 2015 [37] | 129 | 30 | 101 | 58 | 63 | 69 | 104 | 55 | 114 | 45 | 32 | 127 | - | - | 15 | 138 | 6 | - | - |
| Sadot et al., 2015 [38] | 1654 | 714 | 1010 | 1358 | - | - | 1181 | 1187 | - | - | 907 | 1440 | 1950 <200 μg/L | 178 ≥200 μg/L | - | - | - | 1504 | 864 |
| Fonseca et al., 2022 [39] | 80 | 21 | - | - | 57 | 44 | 62 | 39 | - | - | 47 | 45 | 65 <200 μg/L | 32 ≥200 μg/L | - | - | - | - | - |
| Hsu et al., 2024 [40] | - | - | 230 | 0 | 230 | 0 | 121 | 109 | - | - | 146 | 82 | 97 <5 ng/mL | 131 >5 ng/mL | 12 | 203 | 14 | - | - |
| Total | 2096 | 882 | 1625 | 1579 | 539 | 274 | 1610 | 1598 | 217 | 105 | 1289 | 1940 | - | - | 27 | 241 | 20 | 1645 | 1009 |
| Study ID | Major Hepatectomy | Minor Hepatectomy | Anatomical | Nonanatomical |
|---|---|---|---|---|
| Hughes et al., 1988 [35] | 47 | 44 | - | - |
| Scheele et al., 1995 [36] | - | - | 291 | 59 |
| Kuo et al., 2015 [37] | - | - | 32 | 127 |
| Sadot et al., 2015 [38] | 1215 | 871 | - | - |
| Fonseca et al., 2022 [39] | 36 | 65 | - | - |
| Hsu et al., 2024 [40] | - | - | - | - |
| Total | 1298 | 980 | 323 | 186 |
| Study ID | Number of Patients | Regimen | 5-Year Overall Survival (%) | |
|---|---|---|---|---|
| Yes | No | |||
| Hughes et al., 1988 [35] | - | - | - | - |
| Scheele et al., 1995 [36] | - | - | - | - |
| Kuo et al., 2015 [37] | 15 (9.4%) | 5-fluorouracil-based | 35.7 | 20 |
| Sadot et al., 2015 [38] | 2193 (93%) | Oxaliplatin or irinotecan | 43 | 36 |
| Fonseca et al., 2022 [39] | 101 (100%) | Oxaliplatin | - | - |
| Hsu et al., 2024 [40] | - | - | - | - |
| Study ID | 5-Year Overall Survival (%) | 5-Year Disease-Free (%) | ||
|---|---|---|---|---|
| 1–10 mm | >10 mm | 1–10 mm | >10 mm | |
| Hughes et al., 1988 [35] | 20 * | 30 * | - | - |
| Scheele et al., 1995 [36] | 37 | 43 | 31 | 37 |
| Kuo et al., 2015 [37] | 18.2 | 40.2 | 28.4 | 58.1 |
| Sadot et al., 2015 [38] | 46 | 48 | - | - |
| Fonseca et al., 2022 [39] | - | - | - | - |
| Hsu et al., 2024 [40] | - | - | - | - |
| Study ID | Study Participation | Study Attrition |
Prognostic Factor Measurement |
Outcome Measurement | Study Confounding |
Statistical Analysis and Reporting | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|
| Hughes et al., 1988 [35] |  |  |  |  |  |  |  |
| Scheele et al., 1995 [36] |  |  |  |  |  |  |  |
| Kuo et al., 2015 [37] |  |  |  |  |  |  |  |
| Sadot et al., 2015 [38] |  |  |  |  |  |  |  |
| Fonseca et al., 2022 [39] |  |  |  |  |  |  |  |
| Hsu et al., 2024 [40] |  |  |  |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paramythiotis, D.; Karlafti, E.; Tsavdaris, D.; Apostolidou Kiouti, F.; Haidich, A.-B.; Ioannidis, A.; Panidis, S.; Michalopoulos, A. The Effect of Hepatic Surgical Margins of Colorectal Liver Metastases on Prognosis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 7776. https://doi.org/10.3390/jcm13247776
Paramythiotis D, Karlafti E, Tsavdaris D, Apostolidou Kiouti F, Haidich A-B, Ioannidis A, Panidis S, Michalopoulos A. The Effect of Hepatic Surgical Margins of Colorectal Liver Metastases on Prognosis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(24):7776. https://doi.org/10.3390/jcm13247776
Chicago/Turabian StyleParamythiotis, Daniel, Eleni Karlafti, Dimitrios Tsavdaris, Fani Apostolidou Kiouti, Anna-Bettina Haidich, Aristeidis Ioannidis, Stavros Panidis, and Antonios Michalopoulos. 2024. "The Effect of Hepatic Surgical Margins of Colorectal Liver Metastases on Prognosis: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 24: 7776. https://doi.org/10.3390/jcm13247776
APA StyleParamythiotis, D., Karlafti, E., Tsavdaris, D., Apostolidou Kiouti, F., Haidich, A.-B., Ioannidis, A., Panidis, S., & Michalopoulos, A. (2024). The Effect of Hepatic Surgical Margins of Colorectal Liver Metastases on Prognosis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(24), 7776. https://doi.org/10.3390/jcm13247776








