Can Quality of Life Tests Be Useful in Patients Affected by Alpha-1 Antitrypsin Deficiency?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
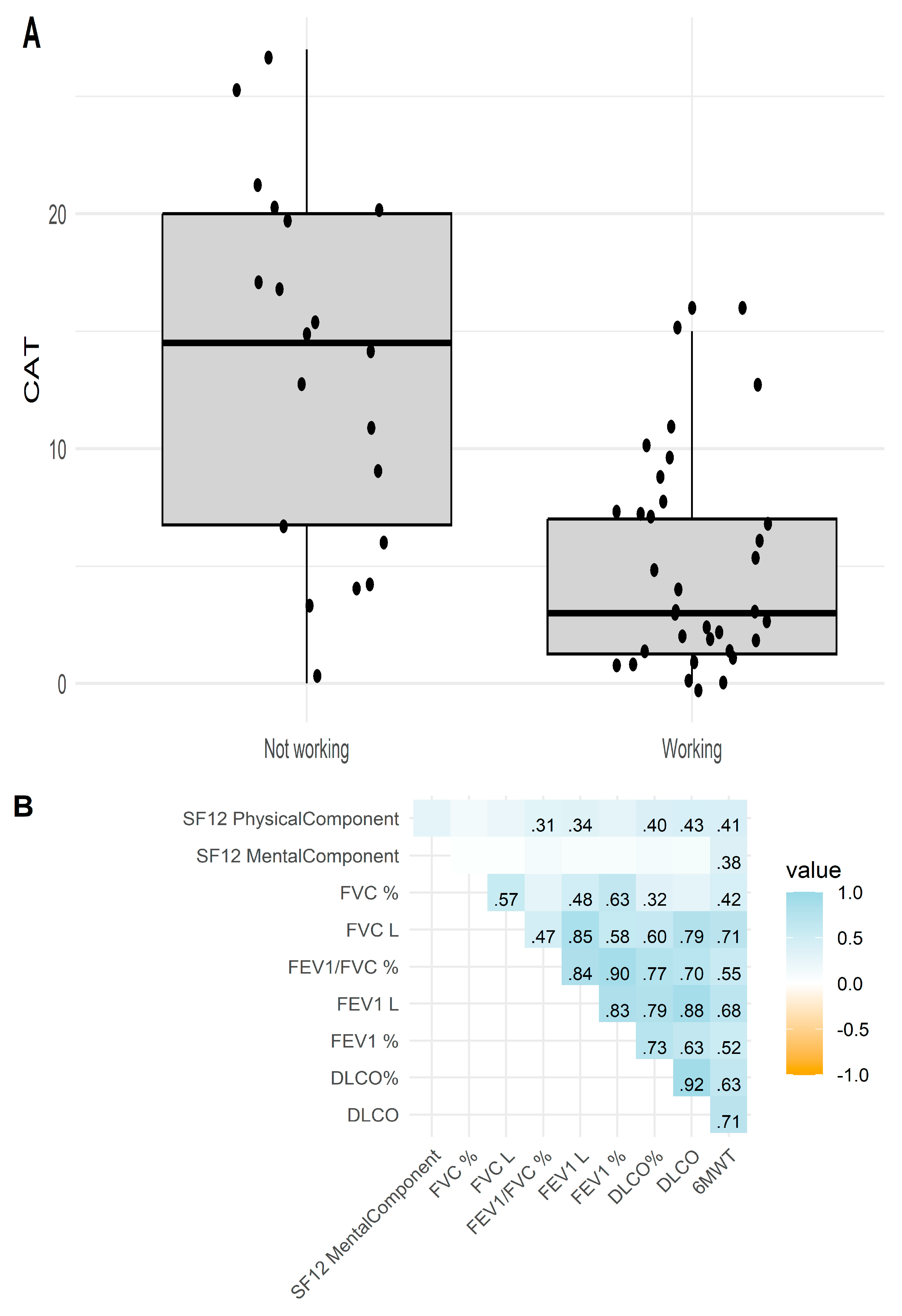
3.1. Baseline Characteristics
3.2. Relationship Between Quality of Life Tests and Characteristics Clinical and Functional
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernández-Pérez, J.M.; Ramos-Díaz, R.; Vaquerizo-Pollino, C.; Pérez, J.A. Frequency of alleles and genotypes associated with alpha-1 antitrypsin deficiency in clinical and general populations: Revelations about underdiagnosis. Pulmonology 2023, 29, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Turner, A.M.; Torres-Duran, M.; Tanash, H.; Rodríguez-García, C.; López-Campos, J.L.; Chlumsky, J.; Guimaraes, C.; Rodriguez-Hermosa, J.L.; Corsico, A.; et al. Clinical and functional characteristics of individuals with alpha-1 antitrypsin deficiency: EARCO international registry. Respir. Res. 2022, 23, 352, Erratum in Respir. Res. 2023, 24, 57. [Google Scholar] [CrossRef] [PubMed]
- Strange, C.; McElvaney, N.G.; Vogelmeier, C.F.; Marin-Galiano, M.; Buch-Haensel, M.; Zhang, X.; Chen, Y.; Vit, O.; Wencker, M.; Chapman, K.R. The effect of exacerbations on lung density in α1-antitrypsin deficiency. ERJ Open Res. 2023, 9, 00457–2022. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Villasante, C.; Alonso, J.; Sobradillo, V.; Gabriel, R.; Vilagut, G.; Masa, J.F.; Viejo, J.L.; Jiménez-Ruiz, C.A.; Miravitlles, M. Interpretation ofi quality of life scores from the St George’s Respiratory Questionnaire. Eur. Respir. J. 2002, 19, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Sanjuas, C.; Alonso, J.; Sanchis, J.; Casan, P.; Broquetas, J.M.; Ferrie, P.J.; Juniper, E.F.; Anto, J.M. The quality of life questionnaire with asthma patients: The Spanish version of the Asthma Quality of Life Questionnaire. Arch. Bronconeumol. 1995, 31, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Kline Leidy, N. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Miravilles, M.; Iriberri, M.; Barrueco, M.; Lleonart, M.; Villarrubia, E.; Galera, J. Usefulness of the LCOPD, CAFS and CASIS scales in understanding the impact of COPD on patients. Respiration 2013, 86, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Vilagut, G.; Garin, O.; Cunillera, O.; Tresserras, R.; Brugulat, P.; Mompart, A.; Medina, A.; Ferrer, M.; Alonso, J. Normas de referencia para el cuestionario de salud SF-12, versión 2 basadas en población general de Cataluña. Med. Clin. 2012, 139, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.; Garin, O.; Pardo, Y.; Vilagut, G.; Pont, À.; Suárez, M.; Neira, M.; Rajmil, L.; Gorostiza, I.; Ramallo-Fariña, Y.; et al. Validity of the EQ-5D-5L and Reference Norms for the Spanish Population. Qual. Life Res. 2018, 27, 2337–2348. Available online: http://www.jstor.org/stable/44856478 (accessed on 11 December 2023). [CrossRef] [PubMed]
- Sampol, J.; Miravitlles, M.; Sáez, M.; Pallero, M.; Sampol, G.; Ferrer, J. Poor sleep quality, COPD severity and survival according to CASIS and Pittsburgh questionnaires. Sci. Rep. 2023, 13, 18656. [Google Scholar] [CrossRef] [PubMed]
- Choate, R.; Holm, K.E.; Sandhaus, R.A.; Mannino, D.M.; Strange, C. Health-related Quality of Life in Alpha-1 Antitrypsin Deficiency-associated Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2023, 208, 1132–1134. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.R.; Holm, K.E.; Choate, R.; Mannino, D.M.; Stockley, R.A.; Sandhaus, R.A.; Turner, A.M. Quality of Life and Mortality Outcomes for Augmentation Naïve and Augmented Patients with Severe Alpha-1 Antitrypsin Deficiency. Chronic Obstr. Pulm. Dis. 2023, 10, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Torres-Durán, M.; López-Campos, J.L.; Rodríguez-Hermosa, J.L.; Esquinas, C.; Martínez-González, C.; Hernández-Pérez, J.M.; Rodríguez, C.; Bustamante, A.; Casas-Maldonado, F.; Barrecheguren, M.; et al. Demographic and clinical characteristics of patients with α1-antitrypsin deficiency genotypes PI*ZZ and PI*SZ in the Spanish registry of EARCO. ERJ Open Res. 2022, 8, 00213–2022. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Decramer, M.; Wedzicha, J.A.; Wilson, K.C.; Agustí, A.; Criner, G.J.; MacNee, W.; Make, B.J.; Rennard, S.I. An official American thoracic society/European respiratory society statement: Research questions in chronic obstructive pulmonary disease. Am. J. Respir. Crit Care Med. 2015, 191, e4–e27. [Google Scholar] [CrossRef] [PubMed]
- Shorofsky, M.; Bourbeau, J.; Kimoff, J.; Jen, R.; Malhotra, A.; Ayas, N.; Tan, W.C.; Aaron, S.D.; Sin, D.D.; Road, J.; et al. Impaired Sleep Quality in COPD Is Associated with Exacerbations: The CanCOLD Cohort Study. Chest 2019, 156, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Gauvain, C.; Mornex, J.F.; Pison, C.; Cuvelier, A.; Balduyck, M.; Pujazon, M.C.; Fournier, M.; AitIlalne, B.; Thabut, G. Health-related quality of life in patients with alpha-1 antitrypsin deficiency: The French experience. COPD J. Chronic Obstr. Pulm. Dis. 2015, 12, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Luisetti, M.; Ferrarotti, I.; Corda, L.; Ottaviani, S.; Gatta, N.; Tinelli, C.; Bruletti, G.; Bertella, E.; Balestroni, G.; Confalonieri, M.; et al. Italian registry of patients with alpha-1 antitrypsin deficiency: General data and quality of life evaluation. COPD J. Chronic Obstr. Pulm. Dis. 2015, 12 (Suppl. 1), 52–57. [Google Scholar] [CrossRef] [PubMed]

| Variable | Mean ± SD n (%) | Median ± 25th–75th Percentile |
|---|---|---|
| Sex, male | 23 (42.6%) | |
| Age | 51.5 ± 13.7 | 53.5 (45.2–62) |
| Age at onset of symptoms | 35 ± 12.9 | 32.5 (28–37.8) |
| Age at diagnosis | 43.3 ± 13.9 | 44 (35–52.8) |
| Genotypes | ||
| Pi*SZ | 23 (43.4%) | |
| Pi*ZZ | 18 (34.0%) | |
| Pi*SS | 5 (9.4%) | |
| Other genotypes | 7 (12.3%) | |
| Levels of AAT | 49.5 ± 25.3 | 48.2 (29.5–59.6) |
| Smoking status | ||
| Never smoked | 4 (7.4%) | |
| Ex smoker | 26 (48.1%) | |
| Currently smoking | 24 (44.4%) | |
| Number of packs/year | 29 ± 19.4 | 27 (13–37) |
| Charlson index | 2.5 ± 1.8 | 3 (1–4) |
| BMI | 26.4 ± 4.6 | 26.1 (23–30.5) |
| Cardiac frequency | 72.4 ± 4.1 | 72 (72–73.5) |
| Respiratory frequency | 15.8 ± 1.9 | 16 (14–16) |
| SpO2 | 95.7 ± 2 | 96 (94–97) |
| FVC (L) | 3.7 ± 1.2 | 3.6 (2.6–4.6) |
| FVC (%) | 96.9 ± 17.2 | 98 (90.3–108.4) |
| FEV1 (L) | 2.4 ± 1.3 | 2.3 (1.2–3.4) |
| FEV1 (%) | 76.2 ± 30.7 | 83 (41–103) |
| FEV/FVC % | 0.6 ± 0.2 | 0.7 (0.4–0.8) |
| DLCO | 6.6 ± 3.4 | 6.7 (4.1–8.8) |
| DLCO (%) | 72.9 ± 26.7 | 75.2 (53.5–93) |
| KCO | 1.8 ± 2.7 | 1.3 (0.9–1.6) |
| KCO (%) | 76.4 ± 25.9 | 76.8 (56–96.5) |
| RV (L) | 2.6 ± 1 | 2.6 (1.9–3.1) |
| RV (%) | 136.1 ± 48.3 | 134 (102–161.9) |
| TLC (L) | 6.4 ± 1.3 | 5.9 (5.3–7.4) |
| TLC (%) | 110.6 ± 24.2 | 113.2 (102.8–120.6) |
| Distance traveled | 493.8 ± 170.8 | 524 (415.5–602.5) |
| Variables | Not Working (n = 15) | Working (n = 32) | p |
|---|---|---|---|
| Age | 58 (48.5–62) | 48 (40.5–57) | 0.069 |
| Sex female n (%) | 10 (37.0) | 17 (63.0%) | 0.576 |
| AAT | 48.5 (35–61.2) | 52.2 (2.3–59.0) | 0.584 |
| FEV1/FVC % | 0.56 ± 0.25 | 0.69 ± 0.18 | 0.069 |
| FEV1% | 50 (35.17–104.25) | 89 (64.18–103) | 0.154 |
| FEV1 (L) | 2.13 ± 1.42 | 2.81 ± 1.19 | 0.136 |
| FVC% | 92.81 ± 18.69 | 98.23 ± 17.35 | 0.369 |
| FVC (L) | 3.48 ± 1.03 | 3.94 ± 1.18 | 0.202 |
| DLCO% | 62.2 ± 25.9 | 79.2 ± 26.9 | 0.113 |
| Distance traveled (m) | 415.5 (392.3–468.8) | 573 (511.3–629.3) | <0.001 |
| CAT | 13.6 ± 7.75 | 4.59 ± 4.32 | <0.001 |
| Low impact | 5 (33.3%) | 29 (90.6%) | <0.001 |
| Medium impact | 8 (53.3%) | 3 (3.4%) | 0.002 |
| High impact | 2 (13.3%) | 0 (0%) | 0.097 |
| SF-12 Physical component | 35.3 (24.3–40.8) | 49.9 (36.5–54.1) | 0.001 |
| SF-12 Mental component | 48.5 (36.6–55.3) | 55.3 (40.4–58.7) | 0.197 |
| CASIS | 46.4 ± 26.6 | 32.2 ± 21.4 | 0.082 |
| EQ5D5L VAS | 60 (50–70) | 70 (50–90) | 0.129 |
| EQ5D5L index value | 0.53 (0.13–0.66) | 0.89 (0.74–1) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Pérez, J.M.; Khadour-Khadour, H.; Romero-Romero, G.; García-Bello, M.Á. Can Quality of Life Tests Be Useful in Patients Affected by Alpha-1 Antitrypsin Deficiency? J. Clin. Med. 2024, 13, 7711. https://doi.org/10.3390/jcm13247711
Hernández-Pérez JM, Khadour-Khadour H, Romero-Romero G, García-Bello MÁ. Can Quality of Life Tests Be Useful in Patients Affected by Alpha-1 Antitrypsin Deficiency? Journal of Clinical Medicine. 2024; 13(24):7711. https://doi.org/10.3390/jcm13247711
Chicago/Turabian StyleHernández-Pérez, José María, Hassan Khadour-Khadour, Gema Romero-Romero, and Miguel Ángel García-Bello. 2024. "Can Quality of Life Tests Be Useful in Patients Affected by Alpha-1 Antitrypsin Deficiency?" Journal of Clinical Medicine 13, no. 24: 7711. https://doi.org/10.3390/jcm13247711
APA StyleHernández-Pérez, J. M., Khadour-Khadour, H., Romero-Romero, G., & García-Bello, M. Á. (2024). Can Quality of Life Tests Be Useful in Patients Affected by Alpha-1 Antitrypsin Deficiency? Journal of Clinical Medicine, 13(24), 7711. https://doi.org/10.3390/jcm13247711







