Advanced Speckle-Tracking Echocardiography Could Play a Crucial Role in the Diagnosis of Post-Implanted Cardiomyopathy Associated with a Leadless Pacemaker System
Abstract
1. Introduction
2. Case Description
3. Discussion
4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, J.Y.; Kim, S.S.; Jeong, H.K.; Choi, I.Y.; Kim, H.K.; Ki, Y.J.; Choi, D.H.; Park, K.H. Pacing-induced cardiomyopathy in patients with preserved ejection fraction undergoing permanent cardiac pacemaker placement. J. Interv. Card. Electrophysiol. 2024, 67, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Wilkoff, B.L.; Cook, J.R.; Epstein, A.E.; Greene, H.L.; Hallstrom, A.P.; Hsia, H.; Kutalek, S.P.; Sharma, A. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA 2002, 288, 3115–3123. [Google Scholar] [PubMed]
- Donal, E.; Tribouilloy, C.; Sadeghpour, A.; Laroche, C.; Tude Rodrigues, A.C.; Pereira Nunes, M.D.C.; Kang, D.H.; Hernadez-Meneses, M.; Kobalava, Z.; De Bonis, M.; et al. Cardiac device-related infective endocarditis need for lead extraction whatever the device according to the ESC EORP EURO-ENDO registry. Eur. Heart J. Open. 2023, 3, oead064. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Kempa, M.; Mitkowski, P.; Kowalski, O.; Sterliński, M.; Przybylski, A.; Kaźmierczak, J. Expert opinion of a Working Group on Leadless Pacing appointed by the National Consultant in Cardiology and the Board of the Heart Rhythm Section of the Polish Cardiac Society. Kardiol. Pol. 2021, 79, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Epstein, A.E.; Verdino, R.J.; Lin, D.; Goldberg, L.R.; Marchlinski, F.E.; Frankel, D.S. Incidence and predictors of right ventricular pacing- induced cardiomyopathy. Heart Rhythm. 2014, 11, 1619–1625. [Google Scholar] [CrossRef]
- Khurshid, S.; Frankel, D.S. Pacing-Induced cardiomyopathy. Card. Electrophysiol. Clin. 2021, 13, 741–753. [Google Scholar] [CrossRef]
- Kiehl, E.L.; Makki, T.; Kumar, R.; Gumber, D.; Kwon, D.H.; Rickard, J.W.; Kanj, M.; Wazni, O.M.; Saliba, W.I.; Varma, N.; et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016, 13, 2272–2278. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Parakh, N.; Gupta, A.; Juneja, R.; Naik, N.; Yadav, R.; Sharma, G.; Roy, A.; Verma, S.K.; Bahl, V.K. Incidence and predictors of pacemaker-induced cardiomyopathy with comparison between apical and non-apical right ventricular pacing sites. J. Interv. Card. Electrophysiol. 2019, 56, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Somma, V.; Ha, F.J.; Palmer, S.; Mohamed, U.; Agarwal, S. Pacing-induced cardiomyopathy: A systematic review and meta-analysis of definition, prevalence, risk factors, and management. Heart Rhythm. 2023, 20, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Madani, R.; Figueroa, J.; Cox, D.; Ferguson, E.; Border, W.; Sachdeva, R.; Fischbach, P.; Whitehill, R. Myocardial deformation as a predictor of right ventricular pacing-induced cardiomyopathy in the pediatric population. J. Cardiovasc. Electrophysiol. 2020, 31, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.Z.; Motwani, M.; Cunnington, C.; Kwok, C.S.; Fullwood, C.; Oceandy, D.; Fitchet, A.; Goode, G.K.; Luckie, M.; Zaidi, A.M.; et al. One-Month global lon- gitudinal strain identifies patients who will develop pacing-induced left ventricular dysfunction over time: The Pacing and Ventricular Dysfunction (PAVD) Study. PLoS ONE 2017, 12, e0162072. [Google Scholar] [CrossRef]
- Chin, J.Y.; Kang, K.W.; Park, S.H.; Choi, Y.J.; Jung, K.T.; Lee, S.; Youn, H.J. Pre-implant global longitudinal strain as an early sign of pacing-induced cardiomyopathy in patients with complete atrioventricular block. Echocardiography 2021, 38, 175–182. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Sanchez, R.; Nadkarni, A.; Buck, B.; Daoud, G.; Koppert, T.; Okabe, T.; Houmsse, M.; Weiss, R.; Augostini, R.; Hummel, J.D.; et al. Incidence of pacing-induced cardiomyopathy in pacemaker-dependent patients is lower with leadless pacemakers compared to transvenous pacemakers. J. Cardiovasc. Electrophysiol. 2021, 32, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, P.P.; Jing, Z.M.; Chen, Y.; Jia, J.J.; Zhao, F.L.; Zhao, Y.N.; Xiao, X.J.; Li, G.C.; Yang, Y.H.; et al. Predictors of pacing-induced cardiomyopathy detection and outcomes demonstration after conduction system pacing upgrade on patients with long-term persistent atrial fibrillation. Pacing Clin. Electrophysiol. 2023, 46, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Duchenne, J.; Garweg, C.; Puvrez, A.; Mao, Y.; Ector, J.; Willems, R.; Voigt, J.U. The effect of leadless pacing on LV and RV systolic function is not inferior to conventional RV pacing. Eur. Heart J. 2022, 43, 708. [Google Scholar] [CrossRef]
- Al-Asad, K.S.; Martinez, A.; Prasad, R.M.; Ukponmwan, E.U.; Baloch, Z.Q.; Ali, A.; Ip, J. Pacing-Induced Cardiomyopathy in Leadless and Traditional Pacemakers: A Single-Center Retrospective Analysis. Cureus 2023, 15, e41393. [Google Scholar] [CrossRef] [PubMed]
- Victor, F.; Mabo, P.; Mansour, H.; Pavin, D.; Kabalu, G.; de Place, C.; Leclercq, C.; Daubert, J.C. A randomized comparison of permanent septal versus apical right ventricular pacing: Short-term results. J. Cardiovasc. Electrophysiol. 2006, 17, 238–242. [Google Scholar] [CrossRef]
- Shantha, G.; Brock, J.; Singleton, M.; Kozak, P.; Bodziock, G.; Bradford, N.; Deshmukh, A.; Liang, J.J.; Pothineni, N.V.K.; Hranitzky, P.; et al. Anatomical location of leadless pacemaker and the risk of pacing-induced cardiomyopathy. J. Cardiovasc. Electrophysiol. 2023, 34, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Pipilas, D.; Frankel, D.S.; Khurshid, S. Pacing-induced cardiomyopathy after leadless pacemaker implant: It’s all about location, location, location. J. Cardiovasc. Electrophysiol. 2023, 34, 1427–1430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smiseth, O.A.; Rider, O.; Cvijic, M.; Valkovič, L.; Remme, E.W.; Voigt, J.U. Myocardial Strain Imaging: Theory, Current Practice, and the Future. JACC Cardiovasc. Imaging 2024. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Aalen, J.M.; Donal, E.; Larsen, C.K.; Duchenne, J.; Lederlin, M.; Cvijic, M.; Hubert, A.; Voros, G.; Leclercq, C.; Bogaert, J.; et al. Imaging predictors of response to cardiac resynchronization therapy: Left ventricular work asymmetry by echocardiography and septal viability by cardiac magnetic resonance. Eur. Heart J. 2020, 41, 3813–3823. [Google Scholar] [CrossRef] [PubMed]
- Cvijic, M.; Duchenne, J.; Ünlü, S.; Michalski, B.; Aarones, M.; Winter, S.; Aakhus, S.; Fehske, W.; Stankovic, I.; Voigt, J.U. Timing of myocardial shortening determines left ventricular regional myocardial work and regional remodelling in hearts with conduction delays. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Lacour, T.; Bisson, A.; Bernard, A.; Fauchier, L.; Babuty, D.; Clementy, N. How to upgrade a leadless pacemaker to cardiac resynchronization therapy. J. Cardiovasc. Electrophysiol. 2019, 30, 2578–2581. [Google Scholar] [CrossRef]
- Palmisano, P.; Parlavecchio, A.; Guido, A.; Accogli, M.; Coluccia, G. Upgrade from leadless to transvenous pacemaker with left bundle branch area pacing: A case report. Pacing Clin. Electrophysiol. 2024. Epub ahead of print. [Google Scholar] [CrossRef]


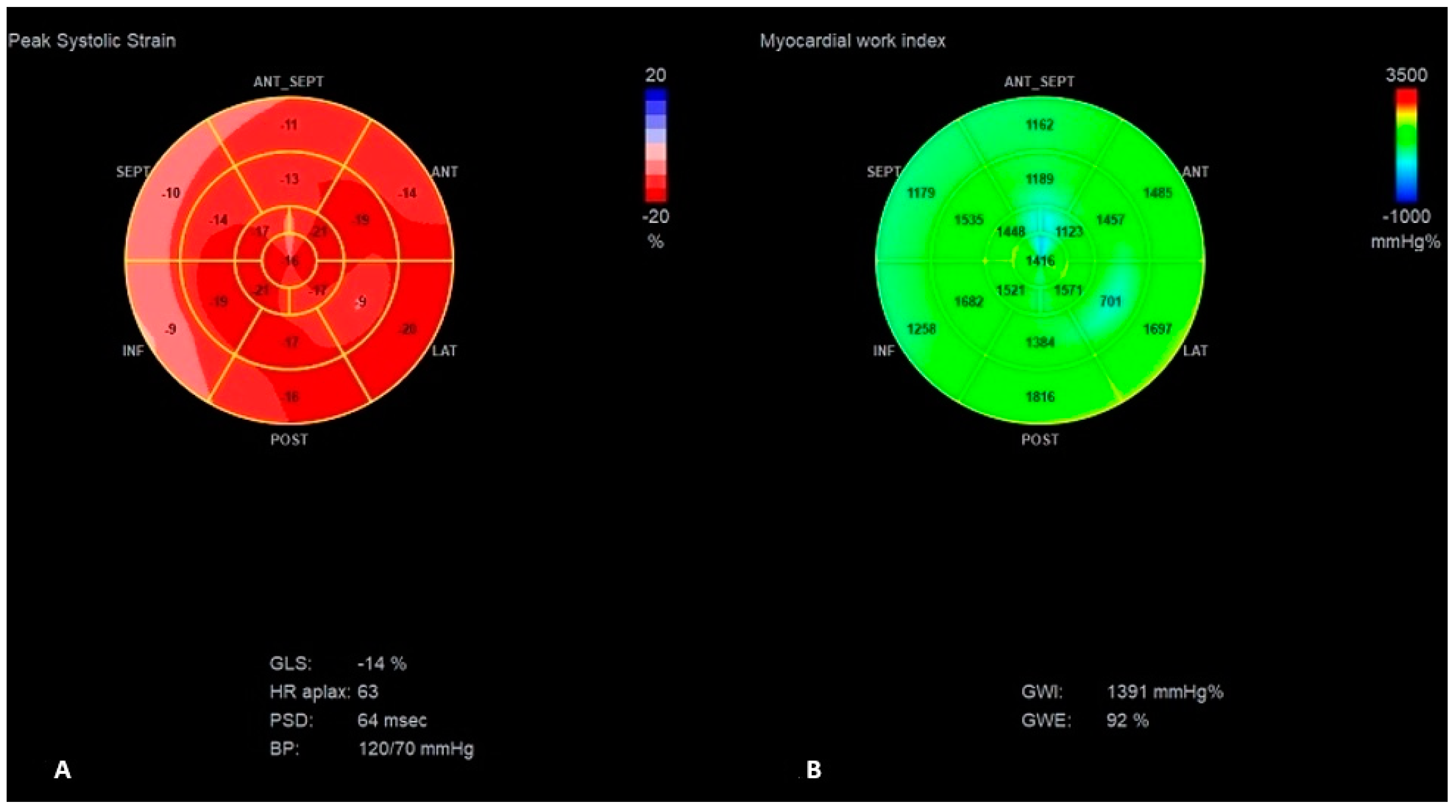
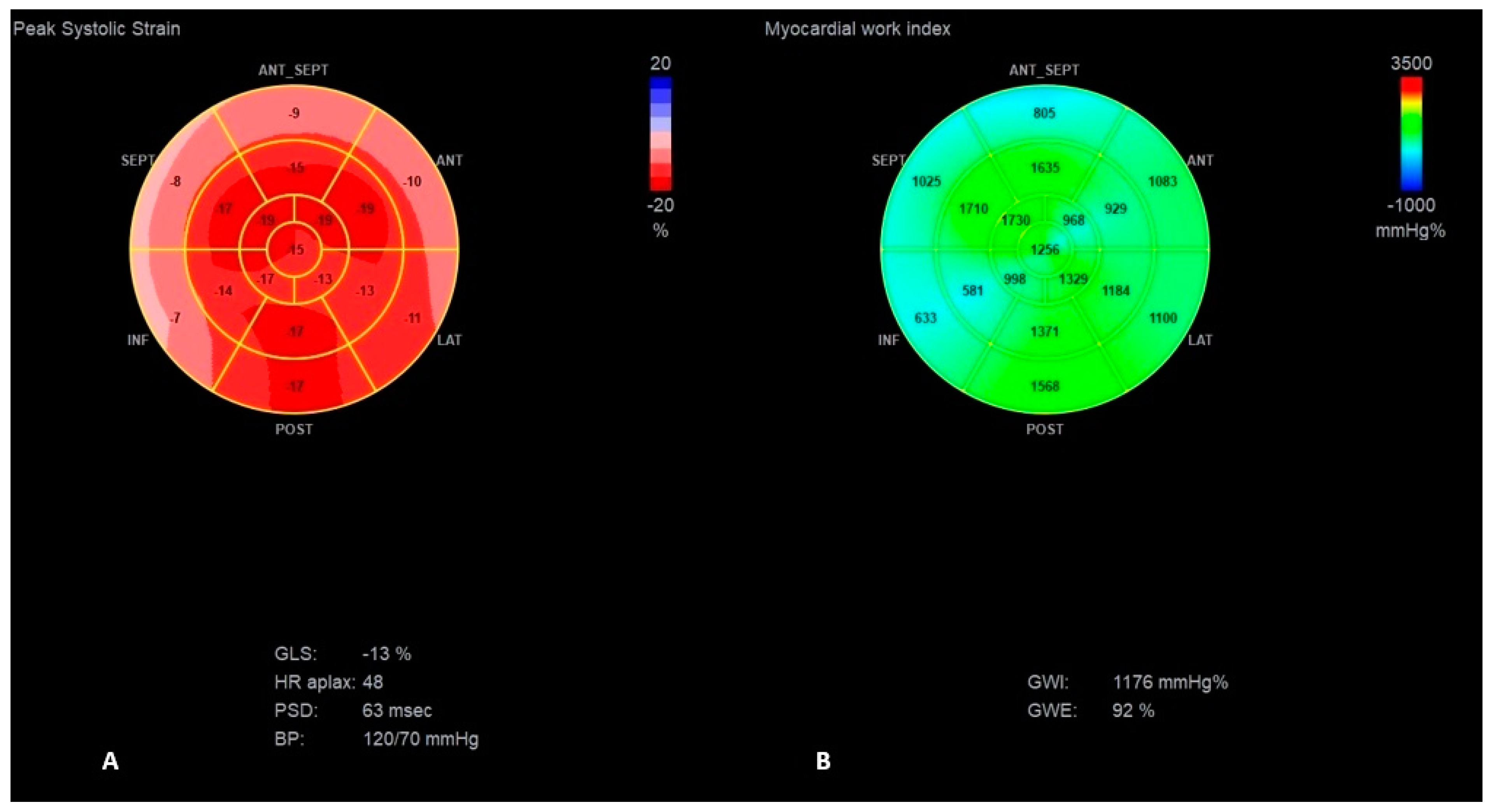
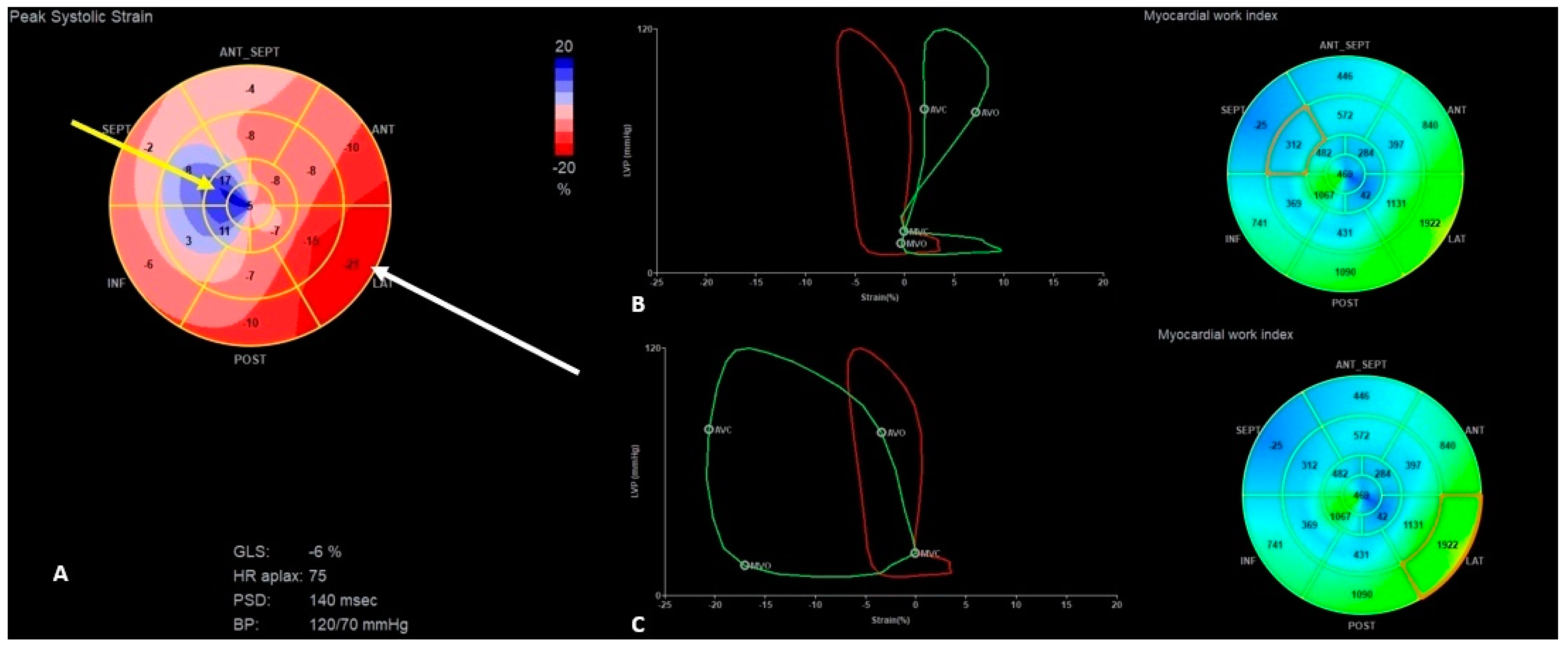
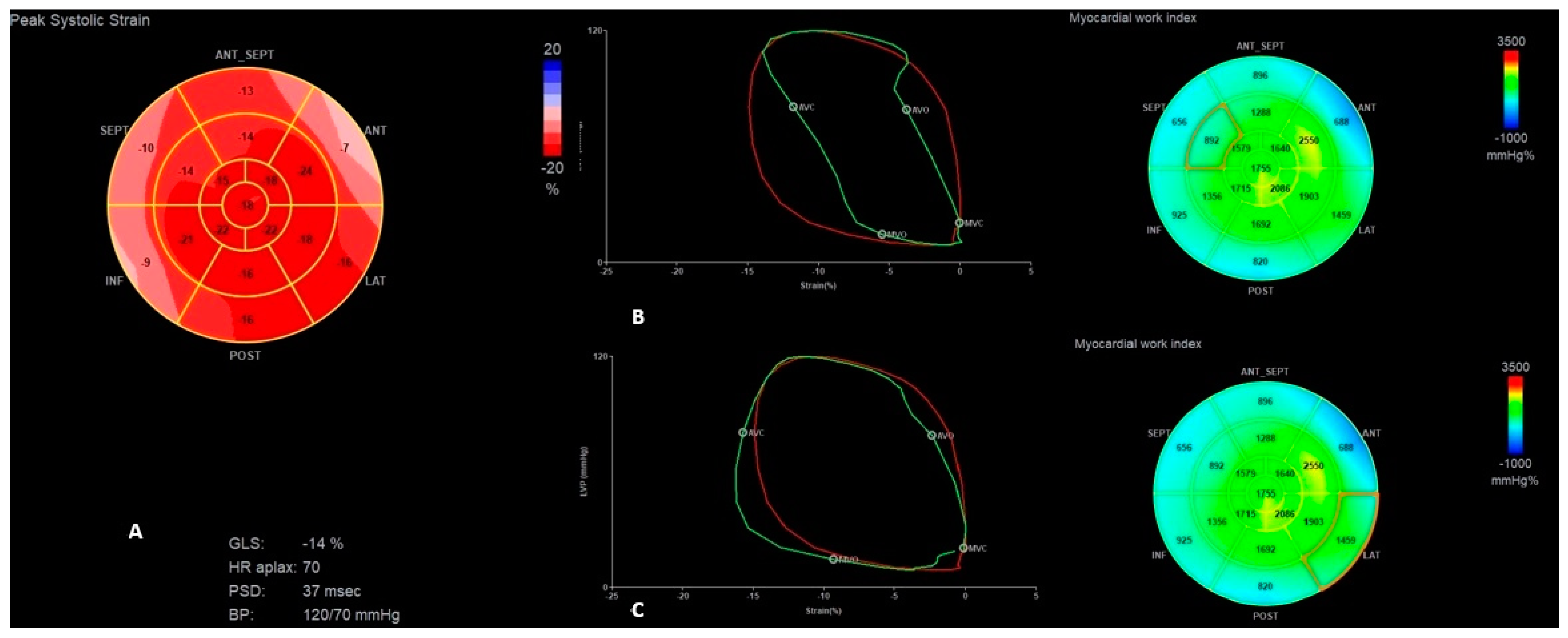

| Parameter | Normal Range | Baseline Hospitalization | First HF Exacerbation | Second HF Exacerbation | 6 Months Follow-Up |
|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 12–15 | 16 | 15.7 | 15.2 | 15.9 |
| BNP (pg/mL) | <89 | 138 | 333 | 220 | 212 |
| hsTnI (ng/mL) | <0.0156 | 0.0200 | 0.011 | 0.012 | 0.012 |
| Potassium (mmol/L) | 3.5–5.1 | 4.4 | 5.3 | 5.5 | 5.0 |
| Creatinine (mg/dL) | 0.73–1.18 | 1.14 | 1.19 | 1.22 | 1.17 |
| GFR (%) | 70–99 | 62 | 58 | 56 | 60 |
| Glucose (mg/dL) | 70–99 | 92 | 98 | 88 | 93 |
| Parameter | Baseline Examination | First HF Exacerbation | Second HF Exacerbation | Examination After CRT-P Implantation | |||
|---|---|---|---|---|---|---|---|
| Intrinsic Rhythm | VVI Pacing | Intrinsic Rhythm | VVI Pacing | Intrinsic Rhythm | BiV VVI Pacing | 6 mo. Follow-Up | |
| LVEF (%) | 55 | 40 | 45 | 34 | 45 | 45 | 50 |
| IVMD (ms) | 10 | 42 | 7 | 52 | 3 | 3 | 5 |
| STE parameters | |||||||
| GLS (%) | −14 | −10 | −13 | −6 | −11 | −15 | −15 |
| PSD (ms) | 63 | 85 | 63 | 140 | 53 | 40 | 61 |
| GWE (%) | 92 | 77 | 92 | 79 | 85 | 90 | 89 |
| GWW (mmHg%) | 138 | 414 | 113 | 263 | 162 | 180 | 220 |
| GCW (mmHg%) | 1757 | 1405 | 1446 | 1044 | 1235 | 1825 | 1866 |
| GWI (mmHg%) | 1391 | 752 | 1176 | 613 | 1241 | 1441 | 1436 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wabich, E.; Daniłowicz-Szymanowicz, L.; Budrejko, S.; Kochańska, A.; Kozłowski, D.; Kempa, M. Advanced Speckle-Tracking Echocardiography Could Play a Crucial Role in the Diagnosis of Post-Implanted Cardiomyopathy Associated with a Leadless Pacemaker System. J. Clin. Med. 2024, 13, 7692. https://doi.org/10.3390/jcm13247692
Wabich E, Daniłowicz-Szymanowicz L, Budrejko S, Kochańska A, Kozłowski D, Kempa M. Advanced Speckle-Tracking Echocardiography Could Play a Crucial Role in the Diagnosis of Post-Implanted Cardiomyopathy Associated with a Leadless Pacemaker System. Journal of Clinical Medicine. 2024; 13(24):7692. https://doi.org/10.3390/jcm13247692
Chicago/Turabian StyleWabich, Elżbieta, Ludmiła Daniłowicz-Szymanowicz, Szymon Budrejko, Anna Kochańska, Dariusz Kozłowski, and Maciej Kempa. 2024. "Advanced Speckle-Tracking Echocardiography Could Play a Crucial Role in the Diagnosis of Post-Implanted Cardiomyopathy Associated with a Leadless Pacemaker System" Journal of Clinical Medicine 13, no. 24: 7692. https://doi.org/10.3390/jcm13247692
APA StyleWabich, E., Daniłowicz-Szymanowicz, L., Budrejko, S., Kochańska, A., Kozłowski, D., & Kempa, M. (2024). Advanced Speckle-Tracking Echocardiography Could Play a Crucial Role in the Diagnosis of Post-Implanted Cardiomyopathy Associated with a Leadless Pacemaker System. Journal of Clinical Medicine, 13(24), 7692. https://doi.org/10.3390/jcm13247692






