Assessment of Facial Pain After Internal Carotid Artery Stenting: The Role of External Carotid Artery Overstenting
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Studied Population
2.2. Pain Measurement
2.3. Equipment
2.4. Analyzed Data
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Group
3.2. Perioperative Data
3.3. Perioperative Facial Pain
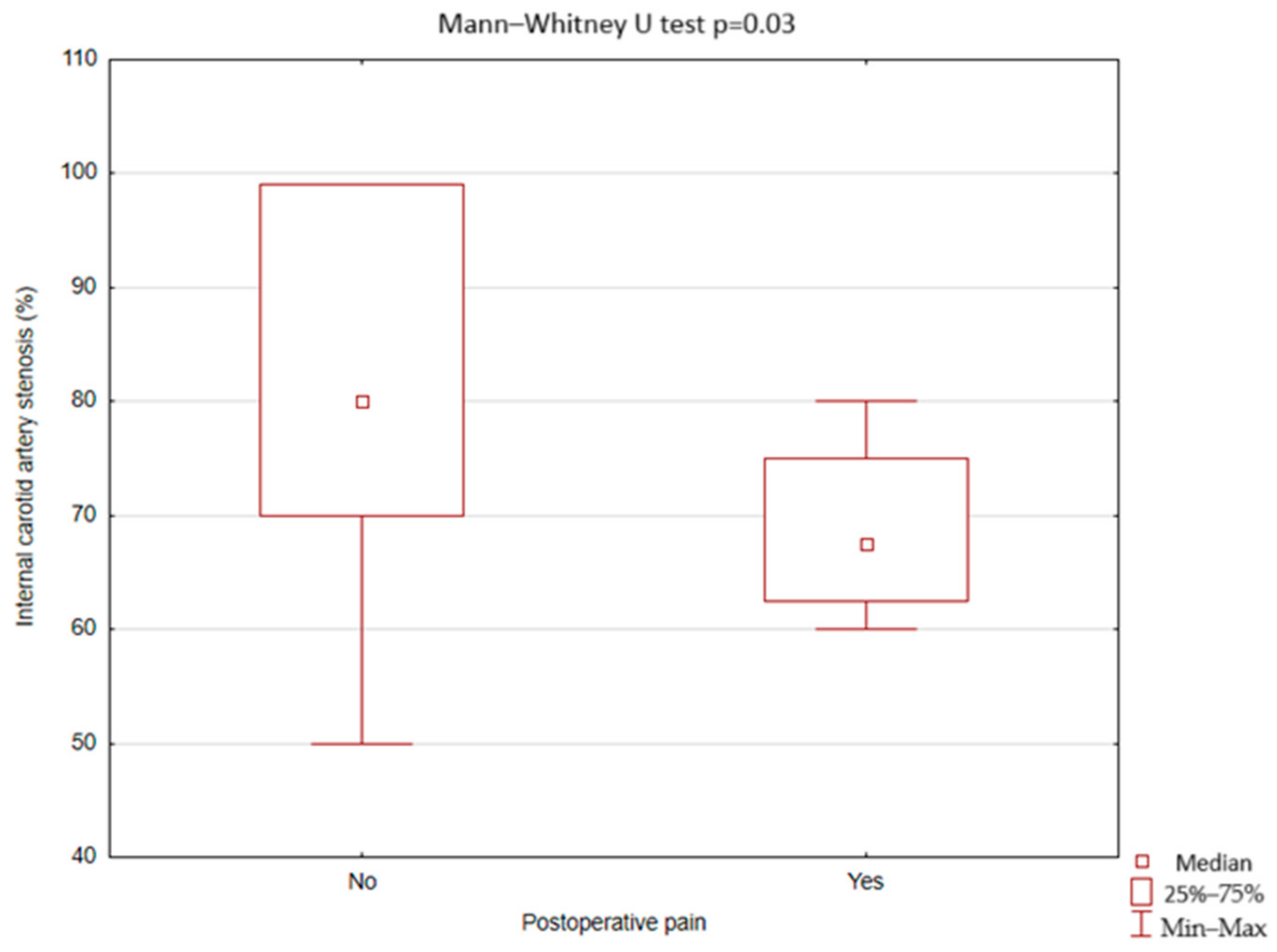
3.4. Postoperative Facial Pain
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sethi, D.; Gofur, E.M.; Munakomi, S. Anatomy, Head and Neck: Carotid Arteries. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Nguyen, J.D.; Duong, H. Anatomy, Head and Neck, Anterior: Common Carotid Arteries. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lin, P.H.; Barshes, N.R.; Annambhotla, S.; Huynh, T.T. Prospective Randomized Trials of Carotid Artery Stenting versus Carotid Endarterectomy: An Appraisal of the Current Literature. Vasc. Endovasc. Surg. 2008, 42, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd Edition. Cephalalgia 2018, 38, 1–211. Available online: https://pubmed.ncbi.nlm.nih.gov/29368949/ (accessed on 28 November 2024).
- Mertens, E. Modifying factors on perception and expression of pain. Pflege Z. 2007, 60, 312. [Google Scholar] [PubMed]
- Stępień, A. Neuralgie i Nerwobóle Twarzy. Pol. Przegląd Neurol. 2007, 3, 262–271. [Google Scholar]
- Lerman, S.F.; Rudich, Z.; Brill, S.; Shalev, H.; Shahar, G. Longitudinal Associations between Depression, Anxiety, Pain, and Pain-Related Disability in Chronic Pain Patients. Psychosom. Med. 2015, 77, 333–341. [Google Scholar] [CrossRef]
- Ziegeler, C.; Brauns, G.; May, A. Characteristics and Natural Disease History of Persistent Idiopathic Facial Pain, Trigeminal Neuralgia, and Neuropathic Facial Pain. Headache 2021, 61, 1441–1451. [Google Scholar] [CrossRef]
- Graff-Radford, S.B. Facial Pain. Neurologist 2009, 15, 171–177. [Google Scholar] [CrossRef]
- Chen, H.; Kougias, P.; Lin, P.H.; Bechara, C.F. Jaw Claudication in the Era of Carotid Stenting. J. Vasc. Surg. 2011, 54, 526–528. [Google Scholar] [CrossRef]
- de Borst, G.J.; Vos, J.A.; Reichmann, B.; Hellings, W.E.; de Vries, J.P.P.M.; Suttorp, M.J.; Moll, F.L.; Ackerstaff, R.G.A. The Fate of the External Carotid Artery after Carotid Artery Stenting. A Follow-up Study with Duplex Ultrasonography. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 657–663. [Google Scholar] [CrossRef]
- Greil, O.; Pflugbeil, G.; Weigand, K.; Weiss, W.; Liepsch, D.; Maurer, P.C.; Berger, H. Changes in Carotid Artery Flow Velocities after Stent Implantation: A Fluid Dynamics Study with Laser Doppler Anemometry. J. Endovasc. Ther. Off. J. Int. Soc. Endovasc. Spec. 2003, 10, 275–284. [Google Scholar] [CrossRef]
- Woo, E.Y.; Karmacharya, J.; Velazquez, O.C.; Carpenter, J.P.; Skelly, C.L.; Fairman, R.M. Differential Effects of Carotid Artery Stenting versus Carotid Endarterectomy on External Carotid Artery Patency. J. Endovasc. Ther. Off. J. Int. Soc. Endovasc. Spec. 2007, 14, 208–213. [Google Scholar] [CrossRef]
- Lewis, R.R.; Beasley, M.G.; MacLean, K.S. Occlusion of External Carotid Artery Causing Intermittent Claudication of the Masseter—PubMed. Br. Med. J. 1978, 2, 1611. Available online: https://pubmed.ncbi.nlm.nih.gov/728751/ (accessed on 28 November 2024). [CrossRef] [PubMed]
- Herishanu, Y.; Bendheim, P.; Dolberg, M. External Carotid Occlusive Disease as a Cause of Facial Pain. J. Neurol. Neurosurg. Psychiatry 1974, 37, 963–965. [Google Scholar] [CrossRef] [PubMed]
- Venna, N.; Goldman, R.; Tilak, S.; Sabin, T.D. Temporal Arteritis-like Presentation of Carotid Atherosclerosis. Stroke 1986, 17, 325–327. [Google Scholar] [CrossRef]
- Schiller, A.; Schwarz, U.; Schuknecht, B.; Mayer, D.; Hess, K.; Baumgartner, R.W. Successful Treatment of Cold-Induced Neck Pain and Jaw Claudication with Revascularization of Severe Atherosclerotic External Carotid Artery Stenoses. J. Endovasc. Ther. Off. J. Int. Soc. Endovasc. Spec. 2007, 14, 304–306. [Google Scholar] [CrossRef]
- Argentino, C.; Iadecola, C.; Pistolese, G.R.; Faraglia, V. Ischaemic Intermittent Claudication of the Masticatory Muscles: Two Case Reports. Ital. J. Neurol. Sci. 1980, 1, 271–274. [Google Scholar] [CrossRef]
- Shinozaki, N.; Ogata, N.; Ikari, Y. Plaque Protrusion Detected by Intravascular Ultrasound during Carotid Artery Stenting. J. Stroke Cerebrovasc. Dis. 2014, 23, 2622–2625. [Google Scholar] [CrossRef]
- Kotsugi, M.; Takayama, K.; Myouchin, K.; Wada, T.; Nakagawa, I.; Nakagawa, H.; Taoka, T.; Kurokawa, S.; Nakase, H.; Kichikawa, K. Carotid Artery Stenting: Investigation of Plaque Protrusion Incidence and Prognosis. JACC Cardiovasc. Interv. 2017, 10, 824–831. [Google Scholar] [CrossRef]
- Tigkiropoulos, K.; Nikas, S.; Manolis, A.-P.; Sidiropoulou, K.; Stavridis, K.; Karamanos, D.; Lazaridis, I.; Saratzis, N. One-Year Outcomes of CGuard Double Mesh Stent in Carotid Artery Disease: A Systematic Review and Meta-Analysis. Medicina 2024, 60, 286. [Google Scholar] [CrossRef]
- Mazurek, A.; Malinowski, K.; Rosenfield, K.; Capoccia, L.; Speziale, F.; de Donato, G.; Setacci, C.; Wissgott, C.; Sirignano, P.; Tekieli, L.; et al. Clinical Outcomes of Second- versus First-Generation Carotid Stents: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 4819. [Google Scholar] [CrossRef]
- AbuRahma, A.F.; Avgerinos, E.D.; Chang, R.W.; Darling, R.C.; Duncan, A.A.; Forbes, T.L.; Malas, M.B.; Murad, M.H.; Perler, B.A.; Powell, R.J.; et al. Society for Vascular Surgery Clinical Practice Guidelines for Management of Extracranial Cerebrovascular Disease. J. Vasc. Surg. 2022, 75, 4S–22S. [Google Scholar] [CrossRef] [PubMed]
- Fearn, S.J.; Picton, A.J.; Mortimer, A.J.; Parry, A.D.; McCollum, C.N. The Contribution of the External Carotid Artery to Cerebral Perfusion in Carotid Disease. J. Vasc. Surg. 2000, 31, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Diethrich, E.B.; Liddicoat, J.E.; McCutchen, J.J.; De Bakey, M.E. Surgical Significance of the External Carotid Artery in the Treatment of Cerebrovascular Insufficiency. J. Cardiovasc. Surg. 1968, 9, 213–223. [Google Scholar]
- Sillesen, H. The Haemodynamic Value of External Carotid Artery Collateral Blood Supply in Carotid Artery Disease. Eur. J. Vasc. Surg. 1988, 2, 309–313. [Google Scholar] [CrossRef]
- Willfort-Ehringer, A.; Ahmadi, R.; Gruber, D.; Gschwandtner, M.E.; Haumer, A.; Heinz, G.; Lang, W.; Ehringer, H. Effect of Carotid Artery Stenting on the External Carotid Artery. J. Vasc. Surg. 2003, 38, 1039–1044. [Google Scholar] [CrossRef]
- Kaszczewski, P.; Elwertowski, M.; Leszczyński, J.; Ostrowski, T.; Gałązka, Z. Volumetric Flow Assessment in Doppler Ultrasonography in Risk Stratification of Patients with Internal Carotid Stenosis and Occlusion. J. Clin. Med. 2022, 11, 531. [Google Scholar] [CrossRef]
- Kaszczewski, P.; Elwertowski, M.; Leszczyński, J.; Ostrowski, T.; Kaszczewska, J.; Brzeziński, T.; Jarosz, D.; Świeczkowski-Feiz, S.; Gałązka, Z. Volumetric Flow Assessment in Extracranial Arteries in Patients with 70–99% Internal Carotid Artery Stenosis. Diagnostics 2022, 12, 2216. [Google Scholar] [CrossRef]
- Giurgea, G.-A.; Haumer, M.; Mlekusch, I.; Sabeti-Sandor, S.; Dick, P.; Schillinger, M.; Minar, E.; Mlekusch, W. Stent-Induced Flow Disturbances in the Ipsilateral External Carotid Artery Following Internal Carotid Artery Stenting: A Temporary Cause of Jaw Claudication. Wien. Klin. Wochenschr. 2017, 129, 487–490. [Google Scholar] [CrossRef]
- Domanin, M.; Isalberti, M.; Romagnoli, S.; Rolli, A.; Sommaruga, S. Acute Hemifacial Ischemia as a Late Complication of Carotid Stenting. J. Vasc. Surg. Cases Innov. Tech. 2017, 3, 83–86. [Google Scholar] [CrossRef]
- Kim, E.S.H.; Marycz, D.M.; Archinal, D.; Gornik, H.L.; Shishehbor, M.H.; Bartholomew, J.R. Presence of External Carotid Artery Plaque Independently Predicts Mortality in Patients without Internal Carotid Artery Atherosclerosis. Vasc. Med. 2014, 19, 351–355. [Google Scholar] [CrossRef]
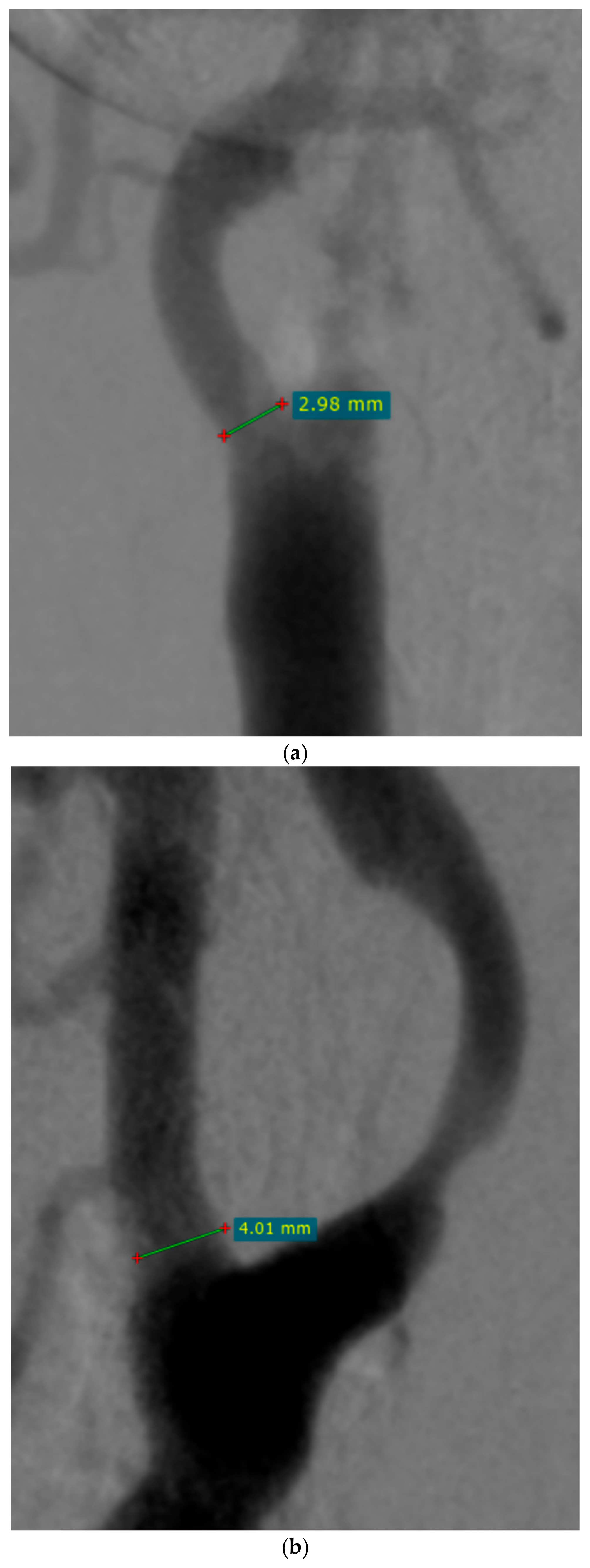
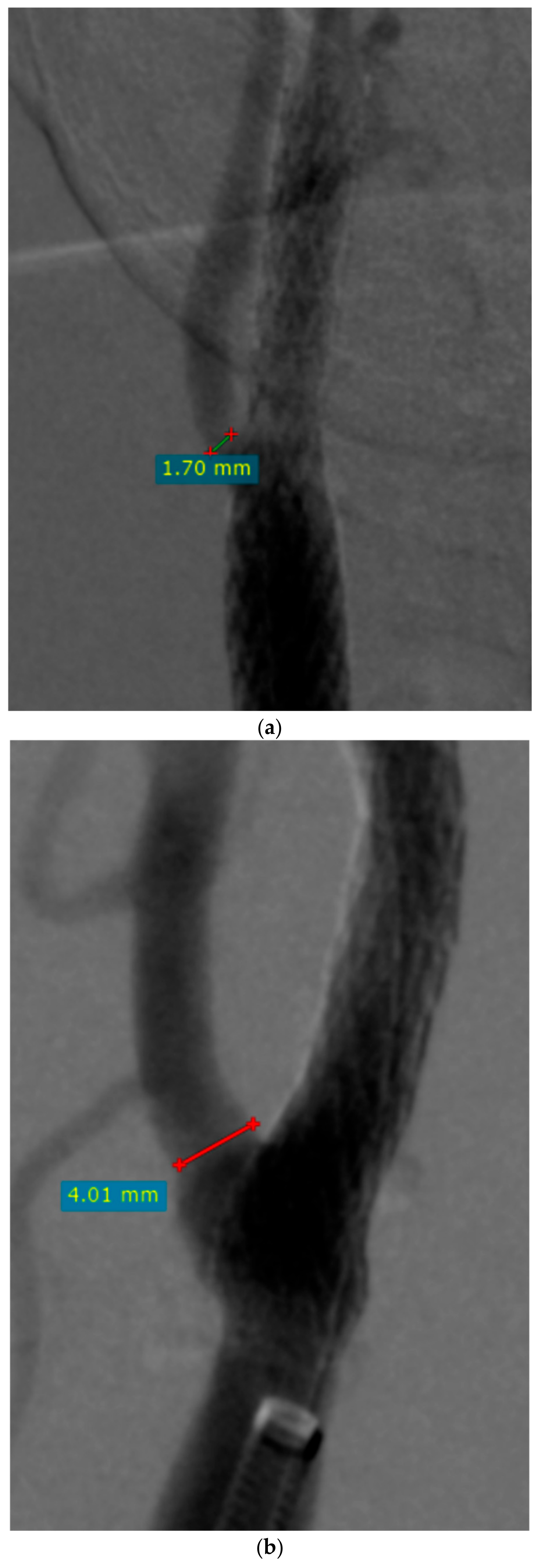

| Variable | n (%), Mean/Median (Range, SD/IQR) |
|---|---|
| Age (years) | 69 (53–93, SD 7.53) |
| Gender | |
| Male | 39 (70.9%) |
| Female | 16 (29.1%) |
| Current cigarette smoking | 22 (40.7%) |
| Presence of comorbidities (yes) | 50 (92.6%) |
| Arterial hypertension | 50 (92.6%) |
| Dyslipidemia | 38 (70.4%) |
| Diabetes mellitus | 20 (37%) |
| Clinical symptoms (yes) | 43 (78.2%) |
| Dizziness | 23 (41.8% |
| Stroke | 20 (37%) |
| Headache | 11 (20%) |
| Syncope | 6 (10.9%) |
| Tinnitus | 5 (9.1%) |
| Variable | n (%), Mean/Median (Range, SD/IQR) |
|---|---|
| ICA stenosis (%) | 80 (50–99) IQR 24.5 |
| Side of the procedure | |
| Left ICA | 39 (70.9%) |
| Right ICA | 16 (29.1%) |
| Stent used | |
| 1st generation | 20 (37%) |
| 2nd generation | 35 (63.6%) |
| Stent length | |
| 40 mm | 39 (70.9%) |
| 30 mm | 15 (27.3%) |
| 60 mm | 1 (1.8%) |
| Stent width | |
| 8 mm | 22 (40%) |
| 9 mm | 15 (27.3%) |
| 7 mm | 12 (21.8%) |
| 10 mm | 6 (10.9%) |
| Covering of ECA orifice by a carotid stent | |
| Yes | 53 (96.4%) |
| No | 2 (3.6%) |
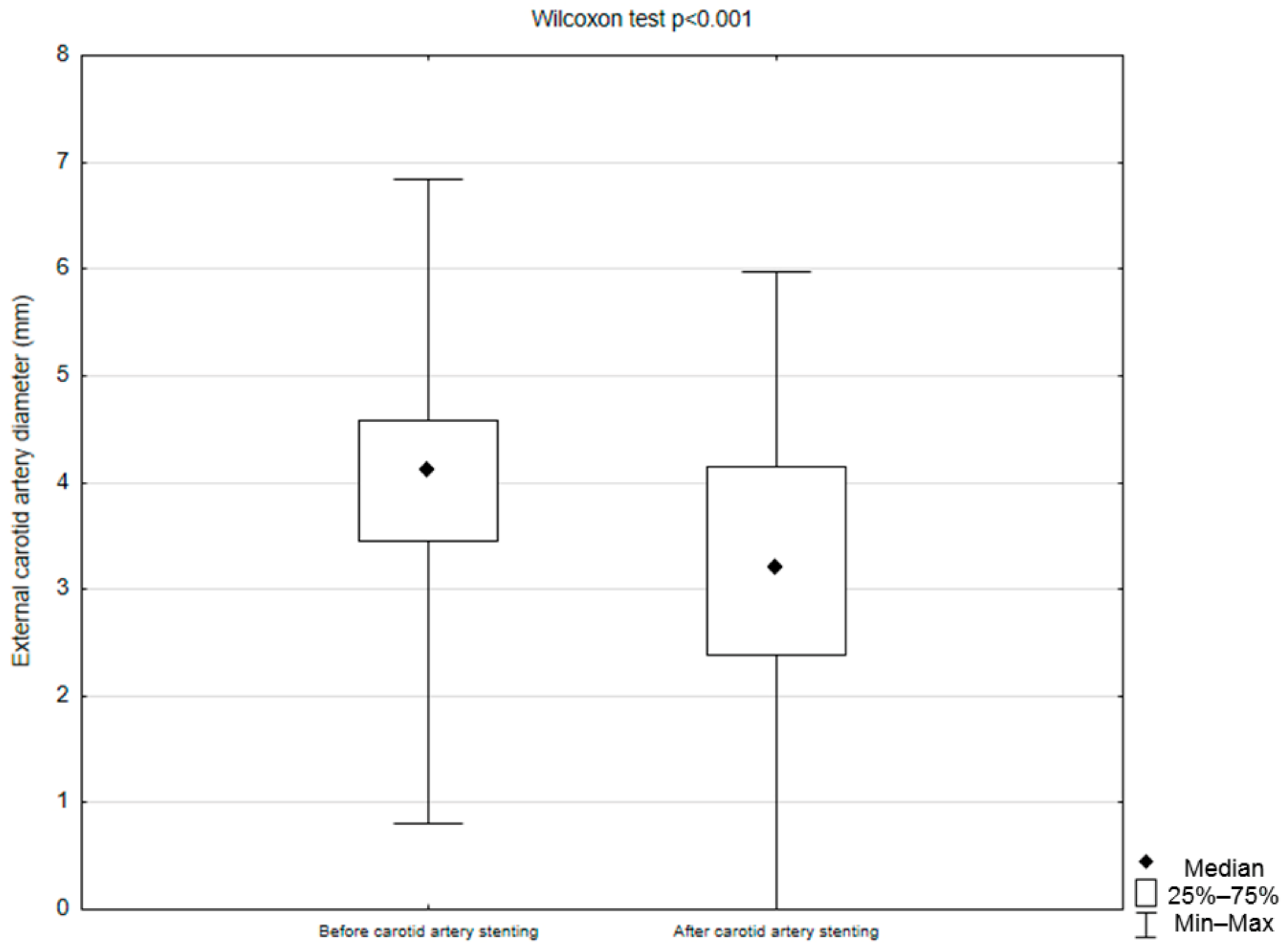
| ECA diameter before CAS (mm) | 4.11 (0–6.84) IQR 1.22 |
| ECA diameter after CAS (mm) | 3.16 (0–5.97) IQR 1.78 |
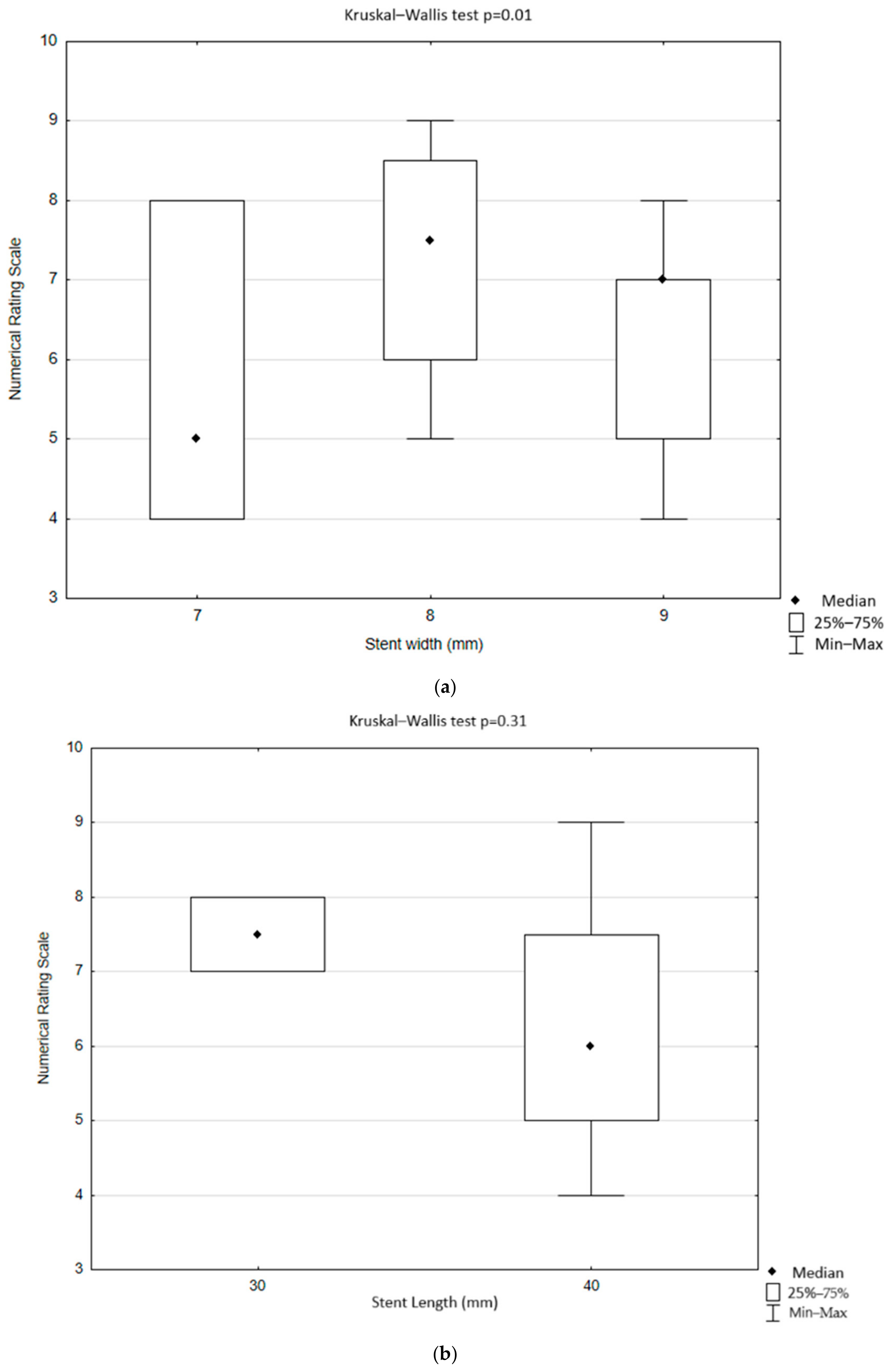
| Presence of Pain During the Procedure | Stent Width 7 mm n = 12 | Stent Width 8 mm n = 22 | Stent Width 9 mm n = 15 | Stent Width 10 mm n = 6 | Total n = 55 | p |
|---|---|---|---|---|---|---|
| No | 9 (75%) | 17 (77.17%) | 8 (53.33%) | 6 (100%) | 40 (72.73%) | 0.15 |
| Yes | 3 (25%) | 5 (22.73%) | 7 (46.67%) | 0 (0%) | 15 (27.27%) |
| Presence of Pain During the Procedure | Stent Length 30 mm n = 15 | Stent Width 40 mm n = 39 | Stent Width 60 mm n = 1 | Total n = 55 | p |
|---|---|---|---|---|---|
| No | 13 (86.67%) | 26 (66.67%) | 1 (100%) | 40 (72.73%) | 0.27 |
| Yes | 2 (13.33%) | 13 (33.33%) | 0 (0%) | 15 (27.27%) |
| Presence of Pain During the Procedure | Stent Width 7 mm n = 12 | Stent Width 8 mm n = 22 | Stent Width 9 mm n = 15 | Stent Width 10 mm n = 6 | Total n = 55 | p |
|---|---|---|---|---|---|---|
| No | 12 (91.67%) | 22 (100%) | 12 (80%) | 6 (100%) | 51 (92.73%) | 0.12 |
| Yes | 1 (8.33%) | 0 (0%) | 3 (20%) | 0 (0%) | 4 (7.27%) |
| Presence of Pain During the Procedure | Stent Length 30 mm n = 15 | Stent Width 40 mm n = 39 | Stent Width 60 mm n = 1 | Total n = 55 | p |
|---|---|---|---|---|---|
| No | 14 (93.33%) | 36 (92.31%) | 1 (100%) | 51 (92.73%) | 0.87 |
| Yes | 1 (6.67%) | 3 (7.69%) | 0 (0%) | 4 (7.27%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łyko-Morawska, D.; Szkółka, Ł.; Serafin, M.; Senderek, E.; Kiczmer, P.; Górska, M.; Kuczmik, W. Assessment of Facial Pain After Internal Carotid Artery Stenting: The Role of External Carotid Artery Overstenting. J. Clin. Med. 2024, 13, 7666. https://doi.org/10.3390/jcm13247666
Łyko-Morawska D, Szkółka Ł, Serafin M, Senderek E, Kiczmer P, Górska M, Kuczmik W. Assessment of Facial Pain After Internal Carotid Artery Stenting: The Role of External Carotid Artery Overstenting. Journal of Clinical Medicine. 2024; 13(24):7666. https://doi.org/10.3390/jcm13247666
Chicago/Turabian StyleŁyko-Morawska, Dorota, Łukasz Szkółka, Michał Serafin, Emila Senderek, Paweł Kiczmer, Magdalena Górska, and Wacław Kuczmik. 2024. "Assessment of Facial Pain After Internal Carotid Artery Stenting: The Role of External Carotid Artery Overstenting" Journal of Clinical Medicine 13, no. 24: 7666. https://doi.org/10.3390/jcm13247666
APA StyleŁyko-Morawska, D., Szkółka, Ł., Serafin, M., Senderek, E., Kiczmer, P., Górska, M., & Kuczmik, W. (2024). Assessment of Facial Pain After Internal Carotid Artery Stenting: The Role of External Carotid Artery Overstenting. Journal of Clinical Medicine, 13(24), 7666. https://doi.org/10.3390/jcm13247666







