Methods of Quantitative Assessment of the Response of Dilated Skin Blood Vessels to High-Energy Light Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Design
- Skin examination using a mexameter by Mexameter® Courage-Khazaka Electronic (Germany).
- Hyperspectral imaging using a Specim IQ (Finland) hyperspectral camera with a spectral range of 400–1000 nm, together with a dedicated lighting system with flat spectral characteristics.
- Analysis of total reflectance using the hemispherical directional reflectometer from Solar 410, Surface Optics Corporation (USA), operating in the spectral range of 335–2500 nm.
2.2.1. Mexametric Measurement
2.2.2. Hyperspectral Imaging
2.2.3. Hemispheric Directional Reflectance (HDR)
2.3. The Erythema Reduction Treatments
2.4. Statistic Analysis
3. Results
3.1. Mexametric Analysis
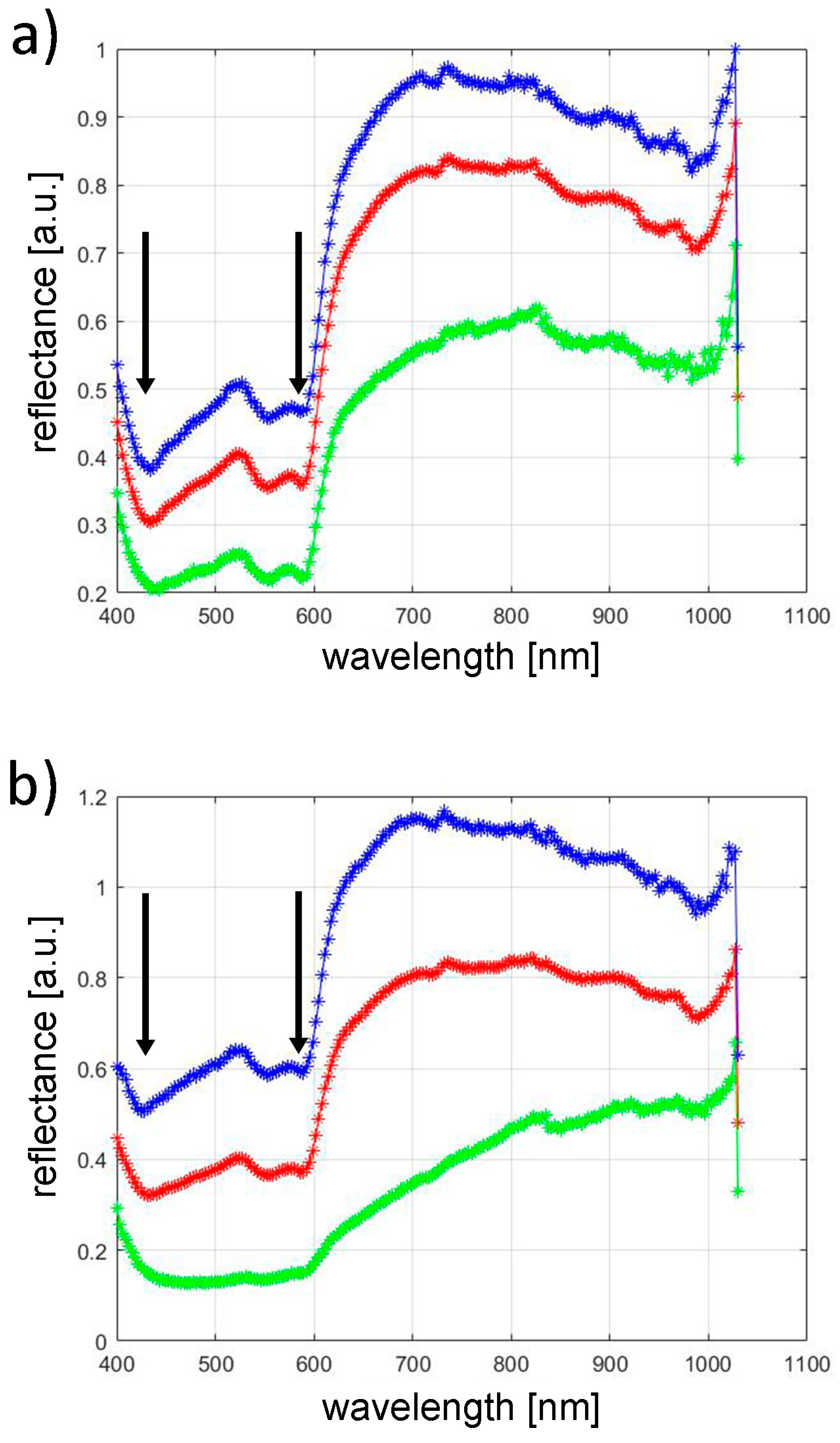
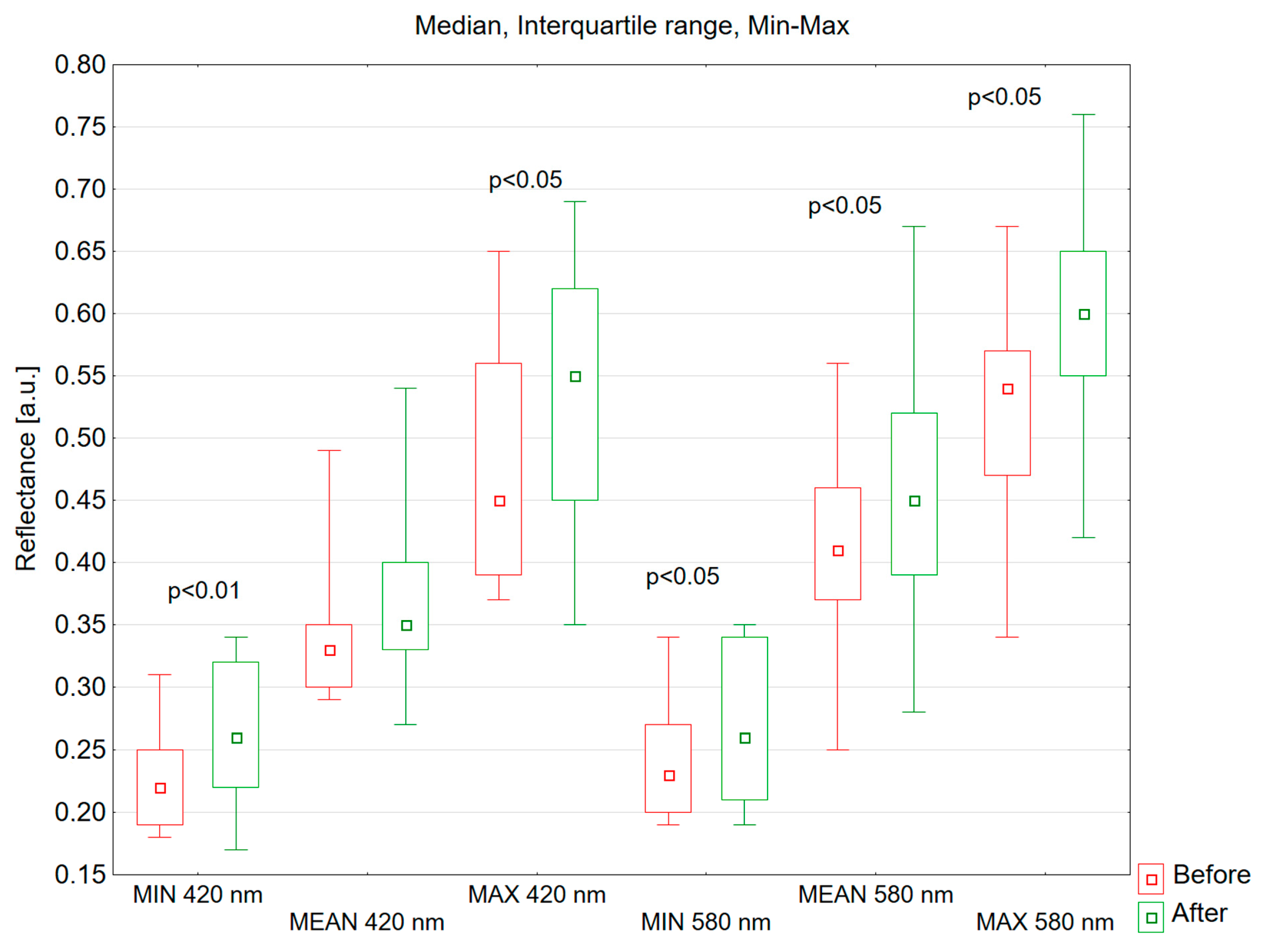
3.2. Hyperspectral Analysis
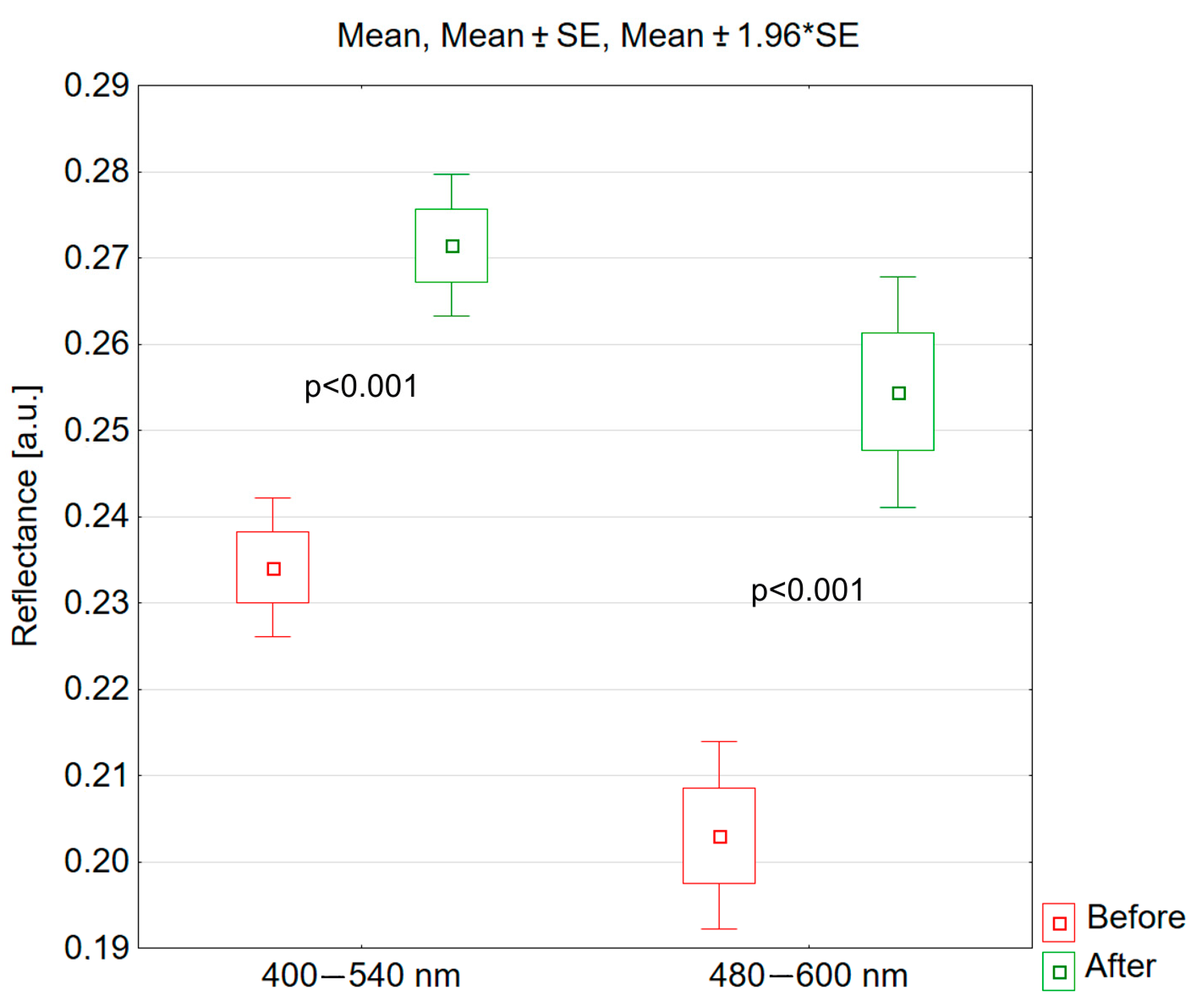
3.3. Hemispheric Directional Reflectance
4. Discussion
5. Conclusions
- Mexametric measurements do not allow for a quantitative assessment of the response of skin blood vessels to high-energy light.
- Hyperspectral imaging is an effective method for the quantitative assessment of vascular lesions.
- The most effective assessment of vascular changes using hyperspectral imaging is obtained at wavelengths of 420 nm and 580 nm.
- The results of measurements using hemispheric directional reflectance confirm the results obtained from hyperspectral imaging and allow for a quick, accurate, and repeatable assessment of vascular lesions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chomiczewska, D.; Kieć-Świerczyńska, M.; Kręcisz, B. Irritant contact dermatitis. Part III. Non-invasive methods to assess biophysical properties of the skin. Med. Pr. 2010, 61, 457–466. [Google Scholar] [PubMed]
- Aoki, K.C.; Barrant, M.; Gai, M.J.; Handal, M.; Xu, V.; Mayrovitz, H.N. Impacts of Skin Color and Hypoxemia on Noninvasive Assessment of Peripheral Blood Oxygen Saturation: A Scoping Review. Cureus 2023, 15, e46078. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. Cosmeceuticals E-Book: Procedures in Cosmetic Dermatology Series. In Evaluating Cosmeceutical Efficacy; Grove, G., Damia, J., Houser, T., Zerweck, C., Eds.; Clinical Tree: Minneapolis, MN, USA, 2015; pp. 21–27. [Google Scholar]
- Ratajczak-Stefańska, V.; Maleszka, R.; Boer, M.; Kiedrowicz, M. Erythematotelangiectatic skin—Diagnostics difficulties. Ann. Acad. Medicae. Stetin. 2009, 55, 58–65. [Google Scholar]
- Kollias, N.; Stamatas, G.N. Optical non-invasive approaches to diagnosis of skin diseases. J. Investig. Dermatol. Symp. Proc. 2002, 7, 64–67. [Google Scholar] [CrossRef]
- Stamatas, G.N.; Zmudzka, B.Z.; Kollias, N.; Beer, J.Z. Non-invasive measurements of skin pigmentation in situ. Pigment. Cell Res. 2004, 17, 618–626. [Google Scholar] [CrossRef]
- Qu, S.; Xu, Y.; Huang, S.; Sun, M.; Wang, C.; Shang, Y. Recent advancements in optical frequency-domain reflectometry: A review. IEEE Sens. J. 2022, 23, 1707–1723. [Google Scholar] [CrossRef]
- Mlosek, K.R.; Malinowska, S.; Serafin-Król, M.; Górski, G.; Ciostek, P.; Jakubowski, W. Ultrasound imaging of laser closing of broken capillary vessels—Own experiences. Acta Bio-Opt. Inform. Medica 2011, 4, 59–61. [Google Scholar]
- Srinivas, C.R.; Kumaresan, M. Lasers for vascular lesions: Standard guidelines of care. Indian. J. Dermatol. Venereol. Leprol. 2011, 77, 349–368. [Google Scholar]
- Dwiyana, R.F.; Lestari, E.R.; Effendi, R.M.R.A.; Gondokaryono, S.P.; Diana, I.A.; Usman, H.A.; Anandita, R. Effectiveness of Pulsed Dye Laser Treatment in Tufted Angioma: A Promising Intervention. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2885–2891. [Google Scholar] [CrossRef]
- Pytrus-Sędłak, B.; Drozdowski, P.; Zub, K. Intense Pulsed Light (IPL) in aesthetic dermatology. Esthet. Dermatol. 2009, 11, 385–390. [Google Scholar]
- Cozzi, C.; Mocchi, R.; D’Este, E.; Caravello, S.; Campoli, M.; Minuti, A.; Herrera, M.; Galadari, H.; Borodina, M.; Rauso, R.; et al. Rendu-Osler’s disease: The effectiveness of mixed technology laser (Alexandrite laser combined with Nd:YAG laser). J. Appl. Cosmetol. 2023, 41, 12–18. [Google Scholar] [CrossRef]
- Zawodny, P.; Malec, W.; Gill, K.; Skonieczna-Żydecka, K.; Sieńko, J. Assessment of the Effectiveness of Treatment of Vascular Lesions within the Facial Skin with a Laser with a Wavelength of 532 nm Based on Photographic Diagnostics with the Use of Polarized Light. Sensors 2023, 23, 1010. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Cao, Y.; Wang, P.; Zhang, H.; Chen, Q.; Yang, Y.; Zeng, Q.; Zhang, L.; Wang, X. 5-Aminolevulinic acid photodynamic therapy versus minocycline for moderate-to-severe rosacea: A single-center, randomized, evaluator-blind controlled study. J. Am. Acad. Dermatol. 2023, 89, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, L.; Zhang, Y.; Gao, X.; Wu, Y.; Chen, H. Topical photodynamic therapy with 5-aminolevulinic acid in Chinese patients with Rosacea. J. Cosmet. Laser Ther. 2019, 21, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yin, R.; Lan, T.; Hamblin, M.R. Photodynamic therapy for rosacea in Chinese patients. Photodiagnosis Photodyn. Ther. 2018, 24, 82–87. [Google Scholar] [CrossRef]
- Wen, X.; Li, Y.; Hamblin, M.R. Photodynamic therapy in dermatology beyond non-melanoma cancer: An update. Photodiagnosis Photodyn. Ther. 2017, 19, 140–152. [Google Scholar] [CrossRef]
- Li, A.; Fang, R.; Mao, X.; Sun, Q. Photodynamic therapy in the treatment of rosacea: A systematic review. Photodiagnosis Photodyn. Ther. 2022, 38, 102875. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Shi, L.; Yang, J.; Zhang, L.; Zhang, Y.; Yan, G.; Zhang, H.; Yang, J.; Wang, P.; Zhang, G.; Zhou, Z.; et al. Adverse reactions of ALA-PDT for the treatment of cutaneous diseases: A retrospective study. Photodiagnosis Photodyn. Ther. 2022, 38, 102783. [Google Scholar] [CrossRef]
- Bao, N.; Gu, T.; Zeng, J.; Wu, Y.; Sun, Y.; Gao, X.; Chen, H. Combined therapy of 5-aminolevulinic acid photodynamic therapy and intense pulsed light for rosacea. Lasers Med. Sci. 2022, 38, 17. [Google Scholar] [CrossRef]
- Kołodziejczak, A.M.; Rotsztejn, H. Mexametric and cutometric assessment of the signs of aging of the skin area around the eyes after the use of non-ablative fractional laser, non-ablative radiofrequency and intense pulsed light. Dermatol. Ther. 2017, 30, 12470. [Google Scholar] [CrossRef] [PubMed]
- Saknite, I.; Zavorins, A.; Jakovels, D.; Spigulis, J.; Kisis, J. Comparison of single-spot technique and RGB imaging for erythema index estimation. Physiol. Meas. 2016, 37, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Clarys, P.; Alewaeters, K.; Lambrech, R.; Barel, O.A. Skin color measurment: Comparision between three instruments: The Chromameter, the DermaSpectrometer, and the Mexameter. Skin Res. Technol. 2000, 6, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Na, J.I.; Huh, C.H.; Youn, S.W.; Park, K.C. Application of a pigment measuring device—Mexameter, for the differential diagnosis of vitiligo and nevus depigmentosus. Skin Res. Technol. 2006, 12, 298–302. [Google Scholar] [CrossRef]
- Matias, A.R.; Ferreira, M.; Costa, P.; Neto, P. Skin colour, skin redness and melanin biometric measurments: Comparision study between Antera® 3D, Mexameter® and Colorimeter. Skin Res. Technol. 2015, 1, 346–362. [Google Scholar] [CrossRef]
- Shirazi, B.R.; Valentine, R.J.; Lang, J.A. Reproducibility and normalization of reactive hyperemia using laser speckle contrast imaging. PLoS ONE 2021, 16, e0244795. [Google Scholar] [CrossRef]
- Steinhoff, M.; Schmelz, M.; Schauber, J. Facial erythema of rosacea—Aetiology, different pathophysiologies and treatment options. Acta Derm.-Venereol. 2016, 96, 579–586. [Google Scholar] [CrossRef]
- Cline, A.; McGregor, S.P.; Feldman, S.R. Medical management of facial redness in rosacea. Dermatol. Clin. 2018, 36, 151–159. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. Management of facial erythema of rosacea: What is the role of topical α-adrenergic receptor agonist therapy? J. Am. Acad. Dermatol. 2013, 69, S44–S56. [Google Scholar] [CrossRef]
- Wilkin, J.K. Why is flushing limited to a mostly facial cutaneous distribution? J. Am. Acad. Dermatol. 1988, 19, 309–313. [Google Scholar] [CrossRef]
- Liang, Y.; Li, L. The Combination of Red and Blue Light, Radiofrequency and Intense Pulsed Light for the Treatment of Facial Postacne Erythema. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Yepuri, V.; Patil, A.D.; Fritz, K.; Salavastru, C.; Kroumpouzos, G.; Nisticò, S.P.; Piccolo, D.; Sadek, A.; Badawi, A.; Kassir, M.; et al. Light-Based Devices for the Treatment of Facial Erythema and Telangiectasia. Dermatol. Ther. 2020, 11, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Vissing, A.E.; Dierickx, C.; Karmisholt, K.E.; Haedersdal, M. Topical brimonidine reduces IPL-induced erythema without affecting efficacy: A randomized controlled trial in patients with facial telangiectasias. Lasers Surg. Med. 2018, 50, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, K.; Akasaka, K.; Akasaka, T.; Amano, H. Successful treatment of erythematotelangiectatic rosacea with intense pulsed light: Report of 13 cases. J. Dermatol. 2018, 45, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Henseler, H. Measurement of the effects of the IMAGE MD® skin care regimen on skin surface features via modern imaging technology with the Visia® complexion analysis camera system. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2022, 11, Doc09. [Google Scholar]
- Xu, Y.; Ma, R.; Juliandri, J.; Wang, X.; Xu, B.; Wang, D.; Lu, Y.; Zhou, B.; Luo, D. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: A randomized, self-controlled, split-face study. Medicine 2017, 96, 6897. [Google Scholar] [CrossRef]
- An, J.H.; Lee, H.J.; Yoon, M.S.; Kim, D.H. Anti-Wrinkle Efficacy of Cross-Linked Hyaluronic Acid-Based Microneedle Patch with Acetyl Hexapeptide-8 and Epidermal Growth Factor on Korean Skin. Ann. Dermatol. 2019, 31, 263–271. [Google Scholar] [CrossRef]
- Liu, G.; Jia, W.; Nelson, J.S.; Chen, Z. In vivo, high-resolution, three-dimensional imaging of port wine stain microvasculature in human skin. Lasers Surg. Med. 2013, 45, 628–632. [Google Scholar] [CrossRef]
- Waibel, J.S.; Holmes, J.; Rudnick, A.; Woods, D.; Kelly, K.M. Angiographic optical coherence tomography imaging of hemangiomas and port wine birthmarks. Lasers Surg. Med. 2018, 50, 718–726. [Google Scholar] [CrossRef]
- Frigerio, A.; Bhama, P.K.; Tan, O.T. Quantitative three-dimensional assessment of port-wine stain clearance after laser treatments. Lasers Surg. Med. 2013, 45, 633–638. [Google Scholar] [CrossRef]
- Ratanapokasatit, Y.; Sirithanabadeekul, P. The Efficacy and Safety of Epidermal Growth Factor Combined with Fractional Carbon Dioxide Laser for Acne Scar Treatment: A Split-Face Trial. J. Clin. Aesthet. Dermatol. 2022, 15, 44–48. [Google Scholar] [PubMed]
- Bloemen, M.C.; van Gerven, M.S.; van der Wal, M.B.; Verhaegen, P.D.; Middelkoop, E. An objective device for measuring surface roughness of skin and scars. J. Am. Acad. Dermatol. 2011, 64, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Abdlaty, R.; Fang, Q. Skin erythema assessment techniques. Clin. Dermatol. 2021, 39, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Sprigle, S.; Linden, M.; Riordan, B. Analysis of localized erythema using clinical indicators and spectroscopy. Ostomy Wound Manag. 2003, 49, 42–52. [Google Scholar]
- Troilius, A.M.; Ljunggren, B. Evaluation of port wine stains by laser Doppler perfusion imaging and reflectance photometry before and after pulsed dye laser treatment. Acta Derm.-Venereol. 1996, 76, 291–294. [Google Scholar] [CrossRef]
- Troilius, A.; Ljunggren, B. Reflectance spectrophotometry in the objective assessment of dye laser-treated port-wine stains. Br. J. Dermatol. 1995, 132, 245–250. [Google Scholar] [CrossRef]



| Volunteer | First Treatment | Second Treatment | Third Treatment | |||
|---|---|---|---|---|---|---|
| Energy Density (J/cm2) | ||||||
| First Pass | Second Pass | First Pass | Second Pass | First Pass | Second Pass | |
| Mean | 12.39 | 9.81 | 13.26 | 11.21 | 14.24 | 11.95 |
| SD | 0.96 | 0.39 | 0.71 | 0.69 | 0.74 | 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deda, A.; Lipka-Trawińska, A.; Błońska-Fajfrowska, B.; Odrzywołek, W.; Lebiedowska, A.; Hartman-Petrycka, M.; Wcisło-Dziadecka, D.; Wilczyński, S. Methods of Quantitative Assessment of the Response of Dilated Skin Blood Vessels to High-Energy Light Treatments. J. Clin. Med. 2024, 13, 7547. https://doi.org/10.3390/jcm13247547
Deda A, Lipka-Trawińska A, Błońska-Fajfrowska B, Odrzywołek W, Lebiedowska A, Hartman-Petrycka M, Wcisło-Dziadecka D, Wilczyński S. Methods of Quantitative Assessment of the Response of Dilated Skin Blood Vessels to High-Energy Light Treatments. Journal of Clinical Medicine. 2024; 13(24):7547. https://doi.org/10.3390/jcm13247547
Chicago/Turabian StyleDeda, Anna, Aleksandra Lipka-Trawińska, Barbara Błońska-Fajfrowska, Wiktoria Odrzywołek, Agata Lebiedowska, Magdalena Hartman-Petrycka, Dominika Wcisło-Dziadecka, and Sławomir Wilczyński. 2024. "Methods of Quantitative Assessment of the Response of Dilated Skin Blood Vessels to High-Energy Light Treatments" Journal of Clinical Medicine 13, no. 24: 7547. https://doi.org/10.3390/jcm13247547
APA StyleDeda, A., Lipka-Trawińska, A., Błońska-Fajfrowska, B., Odrzywołek, W., Lebiedowska, A., Hartman-Petrycka, M., Wcisło-Dziadecka, D., & Wilczyński, S. (2024). Methods of Quantitative Assessment of the Response of Dilated Skin Blood Vessels to High-Energy Light Treatments. Journal of Clinical Medicine, 13(24), 7547. https://doi.org/10.3390/jcm13247547







