Effects of Alpha-Lytic Therapy Withdrawal on Choroidal Parameters in Intraoperative Floppy Iris Syndrome High-Risk Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Clinical and Instrumental Examination
2.3. OCT Analysis: Choroidal Parameters
2.4. Statistical Analysis
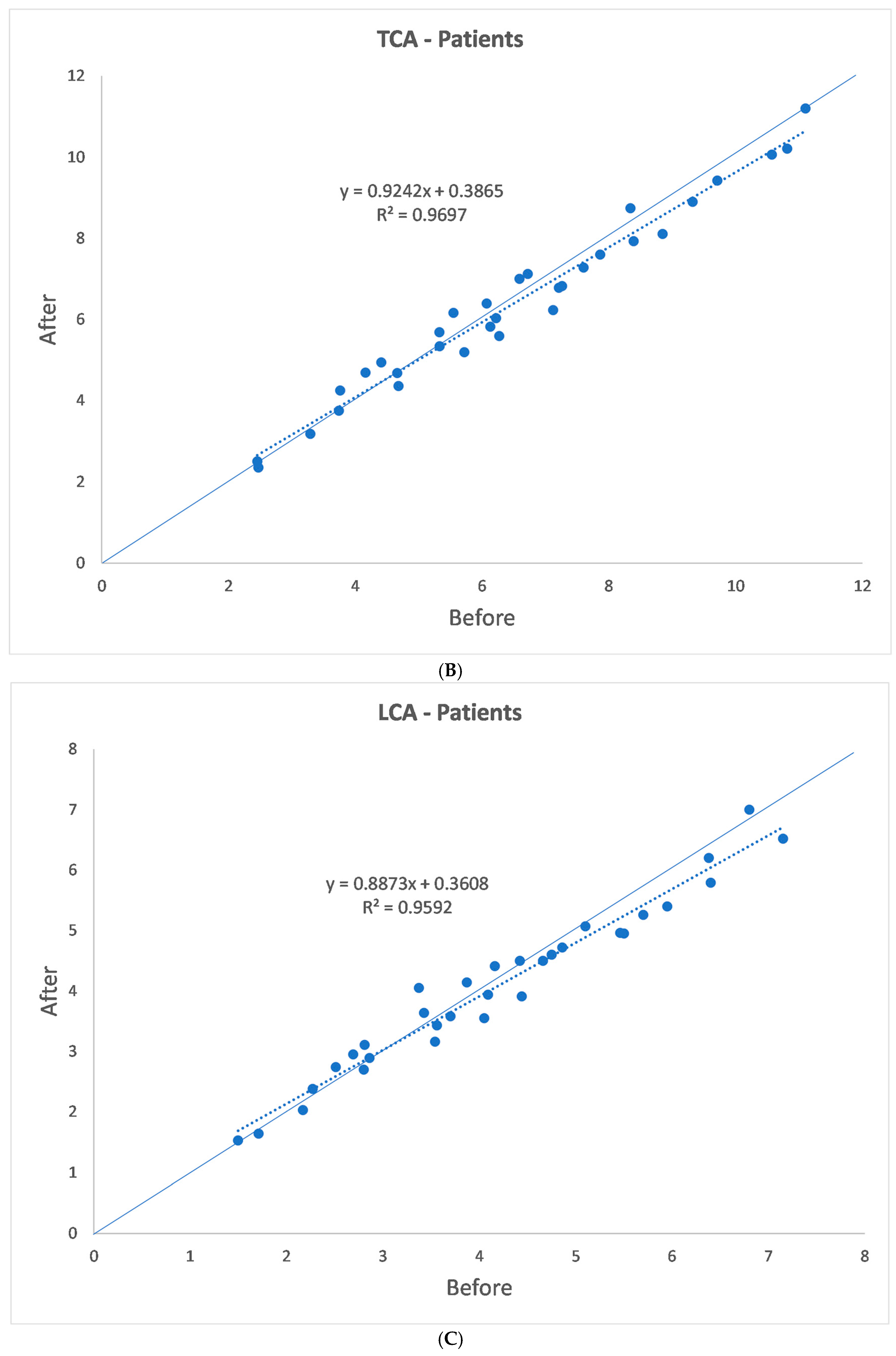
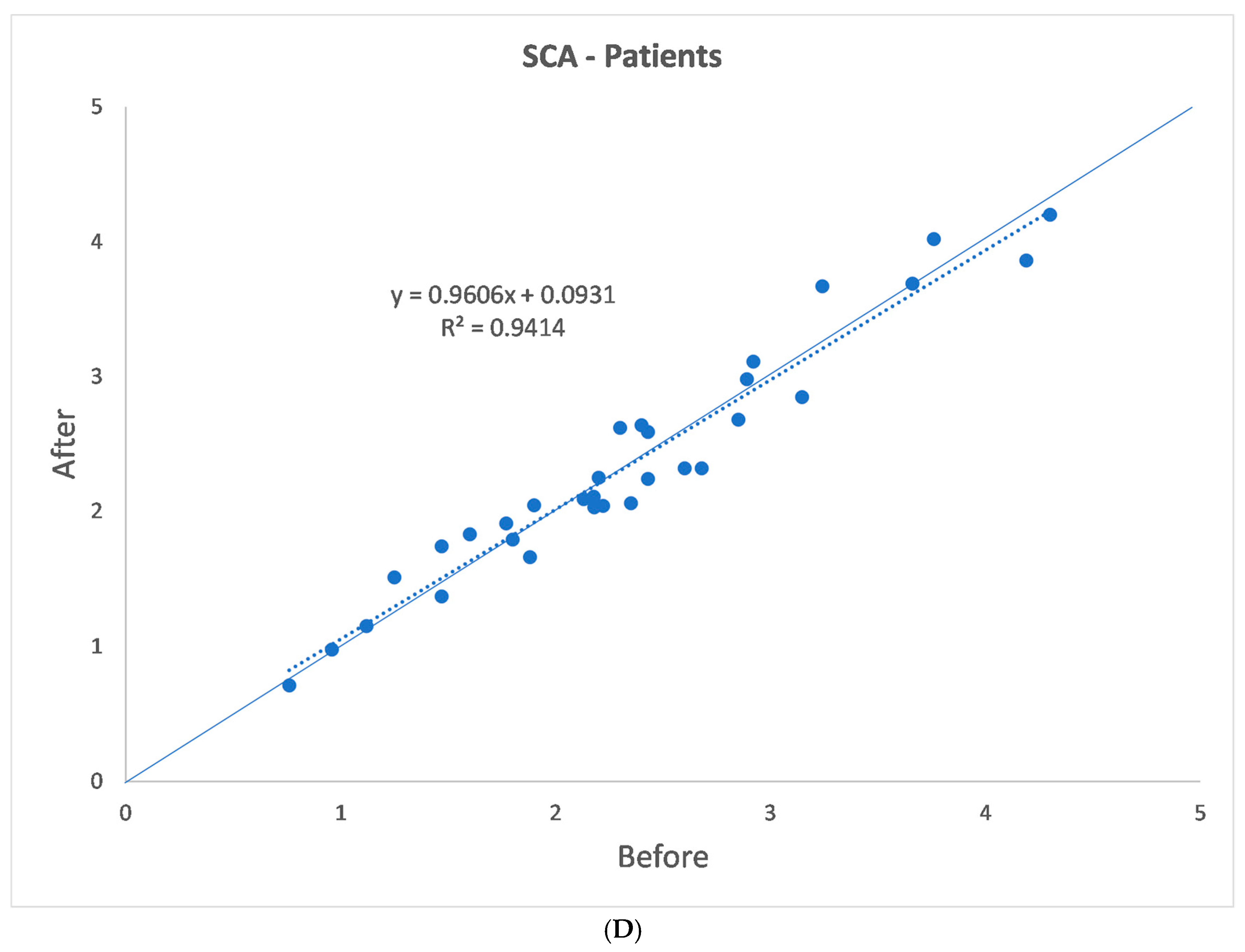
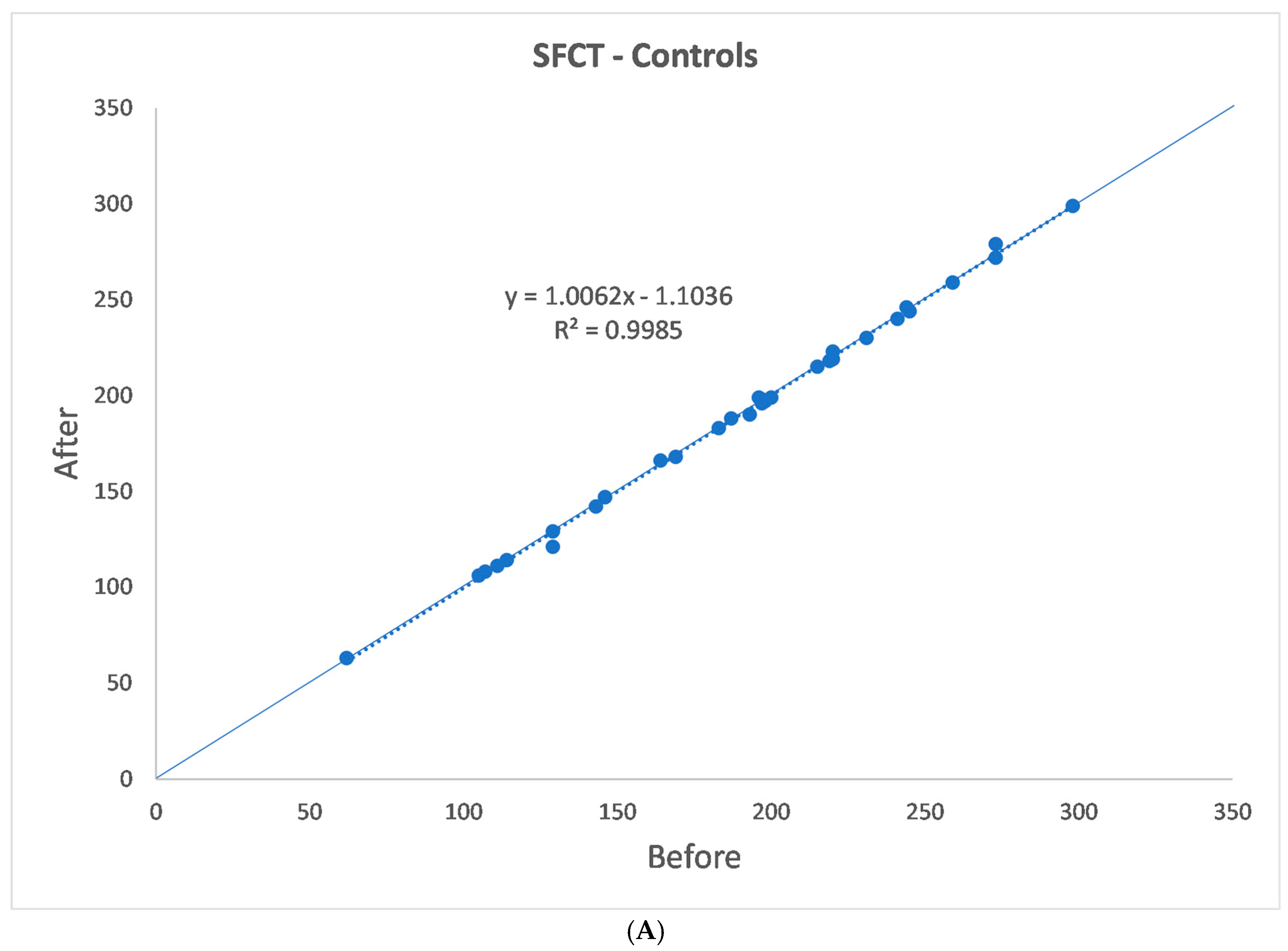
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, D.F.; Campbell, J.R. Intraoperative floppy iris syndrome associated with tamsulosin. J. Cataract. Refract. Surg. 2005, 31, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Kahn, T.; Bosch, J.; Levitt, M.F.; Goldstein, M.H. Effect of sodium nitrate loading on electrolyte transport by the renal tubule. Am. J. Physiol. 1975, 229, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Haridas, A.; Syrimi, M.; Al-Ahmar, B.; Hingorani, M. Intraoperative floppy iris syndrome (IFIS) in patients receiving tamsulosin or doxazosin—A UK-based comparison of incidence and complication rates. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Taben, R.; Thakuria, P.; Jones, M.; Lohman, L.E. Intraoperative Floppy-Iris Syndrome After One Day of Tamsulosin Therapy. Aktuelle Urol. 2022, 53, 246–253. [Google Scholar]
- Shah, N.; Tendulkar, M.; Brown, R. Should we anticipate intraoperative floppy iris syndrome (IFIS) even with very short history of tamsulosin? Eye 2009, 23, 740. [Google Scholar] [CrossRef]
- Sari, E.; Sogutlu Sari, E.; Yazici, A.; Koç, A.; Bulbul, E.; Koytak, A.; Ermis, S.S.; Erol, M.K. The Effect of Systemic Tamsulosin Hydrochloride on Choroidal Thickness Measured by Enhanced Depth Imaging Spectral Domain Optical Coherence Tomography. Curr. Eye Res. 2015, 40, 1068–1072. [Google Scholar] [CrossRef]
- Dogan, M.; Kutluksaman, B.; Keles, I.; Karalar, M.; Halat, A.O. The Effects of Systemic Alfuzosin and Tamsulosin Hydrochloride on Choroidal Thickness and Pupil Diameter Sizes in Cases with Benign Prostatic Hyperplasia. Curr. Eye Res. 2017, 42, 1638–1643. [Google Scholar] [CrossRef]
- Michel, M.C.; Okutsu, H.; Noguchi, Y.; Suzuki, M.; Ohtake, A.; Yuyama, H.; Yanai-Inamura, H.; Ukai, M.; Watanabe, M.; Someya, A.; et al. In vivo studies on the effects of α1-adrenoceptor antagonists on pupil diameter and urethral tone in rabbits. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2006, 372, 346–353. [Google Scholar] [CrossRef]
- Schwinn, D.A.; Afshari, N.A. α1-Adrenergic Antagonists and Floppy Iris Syndrome: Tip of the Iceberg? Ophthalmology 2005, 112, 2059–2060. [Google Scholar] [CrossRef]
- Libíková, H.; Pogády, J.; Wiedermann, V.; Breier, S. Search for herpetic antibodies in the cerebrospinal fluid in senile dementia and mental retardation. Acta Virol. 1975, 19, 493–495. [Google Scholar]
- Kerimoglu, H.; Zengin, N.; Ozturk, B.; Gunduz, K. Unilateral chemosis, acute onset myopia and choroidal detachment following the use of tamsulosin. Acta Ophthalmol. 2010, 88, e20–e21. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, B.L.; Petrovic, V.; Lee, S.E.; Flach, A.; McCaffery, S.; O’Brien, J.M. Choroidal Detachment Following the Use of Tamsulosin (Flomax). Am. J. Ophthalmol. 2007, 143, 351–353. [Google Scholar] [CrossRef] [PubMed]
- De Bernardo, M.; Altieri, V.; Coppola, A.; Gioia, M.; Rosa, N. Choroidal evaluation in patients under alpha-lytic therapy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-A.; Oh, S.Y. Choroidal thickness in healthy children. Retina 2013, 33, 1971–1976. [Google Scholar] [CrossRef]
- Bidaut-Garnier, M.; Schwartz, C.; Puyraveau, M.; Montard, M.; Delbosc, B.; Saleh, M. Choroidal thickness measurement in children using optical coherence tomography. Retina 2014, 34, 768–774. [Google Scholar] [CrossRef]
- He, X.; Jin, P.; Zou, H.; Li, Q.; Jin, J.; Lu, L.; Zhao, H.; He, J.; Xu, X.; Wang, M.; et al. Choroidal thickness in healthy chinese children aged 6 to 12: The Shanghai Children Eye Study. Retina 2017, 37, 368–375. [Google Scholar] [CrossRef]
- Entezari, M.; Karimi, S.; Ramezani, A.; Nikkhah, H.; Fekri, Y.; Kheiri, B. Choroidal thickness in healthy subjects. J. Ophthalmic Vis. Res. 2018, 13, 39. [Google Scholar]
- Tuncer, I.; Karahan, E.; Zengin, M.O.; Atalay, E.; Polat, N. Choroidal thickness in relation to sex, age, refractive error, and axial length in healthy Turkish subjects. Int. Ophthalmol. 2015, 35, 403–410. [Google Scholar] [CrossRef]
- Wang, W.; He, M.; Zhong, X. Sex-Dependent Choroidal Thickness Differences in Healthy Adults: A Study Based on Original and Synthesized Data. Curr. Eye Res. 2018, 43, 796–803. [Google Scholar] [CrossRef]
- Spaide, R.F.; Koizumi, H.; Pozonni, M.C. Enhanced Depth Imaging Spectral-Domain Optical Coherence Tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef]
- Tan, C.S.; Ouyang, Y.; Ruiz, H.; Sadda, S.R. Diurnal Variation of Choroidal Thickness in Normal, Healthy Subjects Measured by Spectral Domain Optical Coherence Tomography. Invest. Ophthalmol. Vis. Sci. 2012, 53, 261. [Google Scholar] [CrossRef] [PubMed]
- Gioia, M.; De Bernardo, M.; Capasso, L.; Rosa, N. Correspondence. Retina 2021, 41, e70. [Google Scholar] [CrossRef] [PubMed]
- De Bernardo, M.; Vitiello, L.; Battipaglia, M.; Mascolo, F.; Iovino, C.; Capasso, L.; Ciacci, C.; Rosa, N. Choroidal structural evaluation in celiac disease. Sci. Rep. 2021, 11, 16398. [Google Scholar] [CrossRef] [PubMed]
- Gioia, M.; De Bernardo, M.; Capasso, L.; Rosa, N. Correspondence. Retina 2021, 41, e59–e60. [Google Scholar] [CrossRef]
- Gioia, M.; De Bernardo, M.; Rosa, N.; Capasso, L. Comment on: Evolution of Dome-Shaped Macula Is due to Differential Elongation of the Eye Predominant in the Peri-dome Region. Am. J. Ophthalmol. 2021, 226, 270–275. [Google Scholar] [CrossRef]
- Gioia, M.; De Bernardo, M.; Rosa, N.; Capasso, L. Comment on: Choroidal Structural Analysis in Alzheimer Disease, Mild Cognitive Impairment, and Cognitively Healthy Controls. Am. J. Ophthalmol. 2021, 225, 207–208. [Google Scholar] [CrossRef]
- Capasso, L.; Gioia, M.; De Bernardo, M.; Rosa, N. Choroidal Thickness and microRNA146 in Lupus Nephritis Patients [Letter]. Clin. Ophthalmol. 2020, 14, 2773–2774. [Google Scholar] [CrossRef]
- De Bernardo, M.; Salerno, G.; Gioia, M.; Capasso, L.; Russillo, M.C.; Picillo, M.; Erro, R.; Amboni, M.; Barone, P.; Rosa, N.; et al. Intraocular pressure and choroidal thickness postural changes in multiple system atrophy and Parkinson’s disease. Sci. Rep. 2021, 11, 8936. [Google Scholar] [CrossRef]
- Gioia, M.; De Bernardo, M.; Luigi, C.; Rosa, N. Correspondence. Retina 2021, 41, e3. [Google Scholar] [CrossRef]
- Sonoda, S.; Sakamoto, T.; Yamashita, T.; Uchino, E.; Kawano, H.; Yoshihara, N.; Terasaki, H.; Shirasawa, M.; Tomita, M.; Ishibashi, T. Luminal and Stromal Areas of Choroid Determined by Binarization Method of Optical Coherence Tomographic Images. Am. J. Ophthalmol. 2015, 159, 1123–1131. [Google Scholar] [CrossRef]
- Agrawal, R.; Gupta, P.; Tan, K.-A.; Cheung, C.M.G.; Wong, T.-Y.; Cheng, C.-Y. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci. Rep. 2016, 6, 21090. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Salman, M.; Tan, K.A.; Karampelas, M.; Sim, D.A.; Keane, P.A.; Pavesio, C. Choroidal Vascularity Index (CVI)—A Novel Optical Coherence Tomography Parameter for Monitoring Patients with Panuveitis? PLoS ONE 2016, 11, e0146344. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Karaca, U.; Kaya, E.; Ayyildiz, O.; Ozge, G.; Kucukevcilioglu, M.; Usta, G.; Mutlu, F.M. Evaluation of static and dynamic Pupillometry changes in men using Silodosin for benign prostatic hypertrophy. BMC Ophthalmol. 2021, 21, 125. [Google Scholar] [CrossRef]
- Safir, M.; Hecht, I.; Hartstein, M.E.; Mahler, O.; Einan-Lifshitz, A.; Pras, E. Preoperative ocular characteristics predicting the development of intraoperative floppy iris syndrome regardless of alpha-antagonist exposure status. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 1209–1214. [Google Scholar] [CrossRef]
- Vural, G.S.; Vural, M. Predictive value of pupillography on intraoperative floppy iris syndrome in preoperative period. Int. J. Ophthalmol. 2021, 14, 1018–1024. [Google Scholar] [CrossRef]
- Casuccio, A.; Cillino, G.; Pavone, C.; Spitale, E.; Cillino, S. Pharmacologic pupil dilation as a predictive test for the risk for intraoperative floppy-iris syndrome. J. Cataract. Refract. Surg. 2011, 8, 1447–1454. [Google Scholar] [CrossRef]
- Enright, J.M.; Karacal, H.; Tsai, L.M. Floppy iris syndrome and cataract surgery. Curr. Opin. Ophthalmol. 2017, 28, 29–34. [Google Scholar] [CrossRef]
- Shtein, R.M.; Hussain, M.T.; Cooney, T.M.; Elner, V.M.; Hood, C.T. Effect of tamsulosin on iris vasculature and morphology. J. Cataract. Refract. Surg. 2014, 40, 793–798. [Google Scholar] [CrossRef]
- Santaella, R.M.; Destafeno, J.J.; Stinnett, S.S.; Proia, A.D.; Chang, D.F.; Kim, T. The Effect of α1-Adrenergic Receptor Antagonist Tamsulosin (Flomax) on Iris Dilator Smooth Muscle Anatomy. Ophthalmology 2010, 117, 1743–1749. [Google Scholar] [CrossRef]
- Rosa, N.; Gioia, M.; Orlando, R.; De Luca, M.; D’Aniello, E.; Fioretto, I.; Sannino, C.; De Bernardo, M. Impact of Brightness on Choroidal Vascularity Index. J. Clin. Med. 2024, 13, 1020. [Google Scholar] [CrossRef]
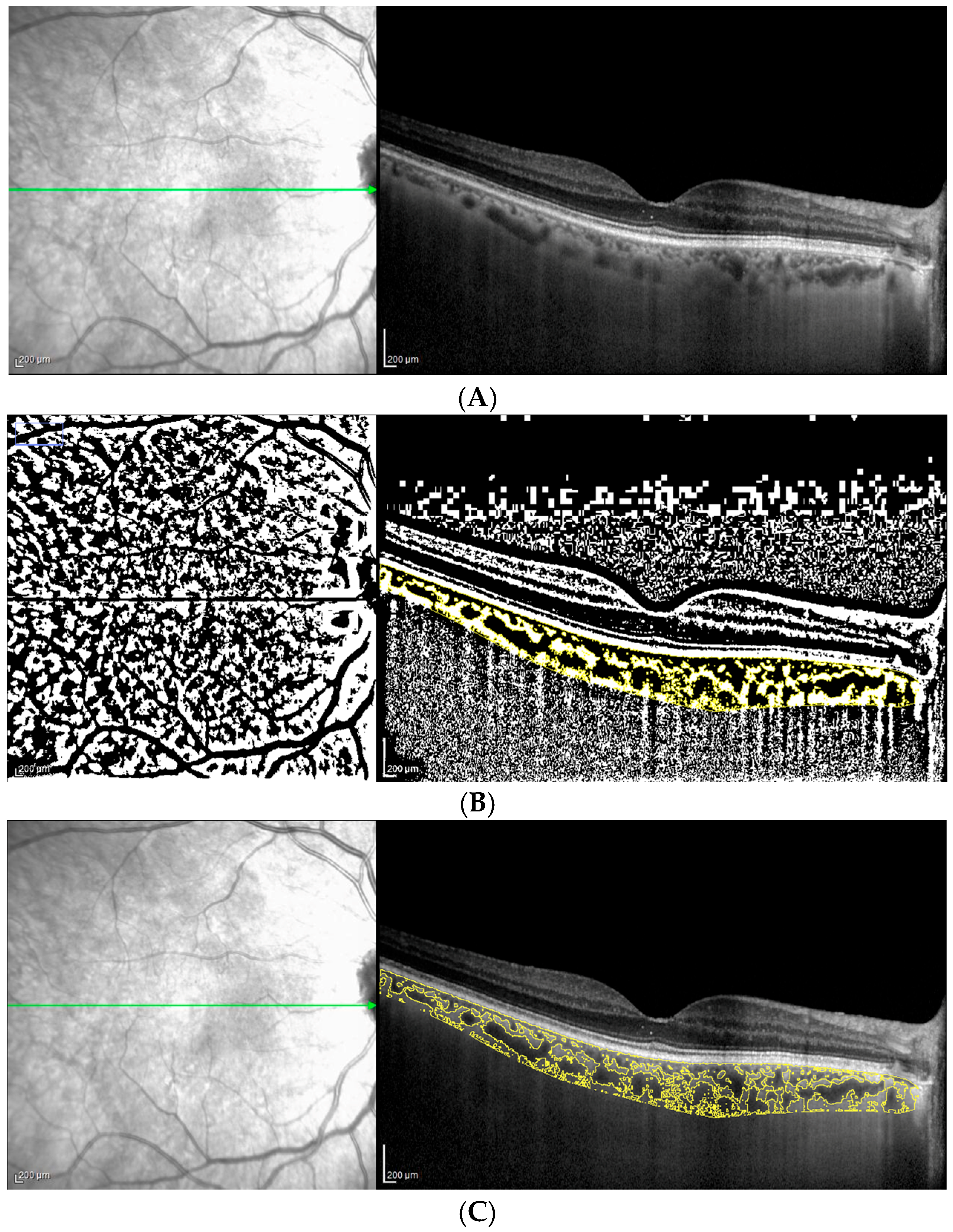

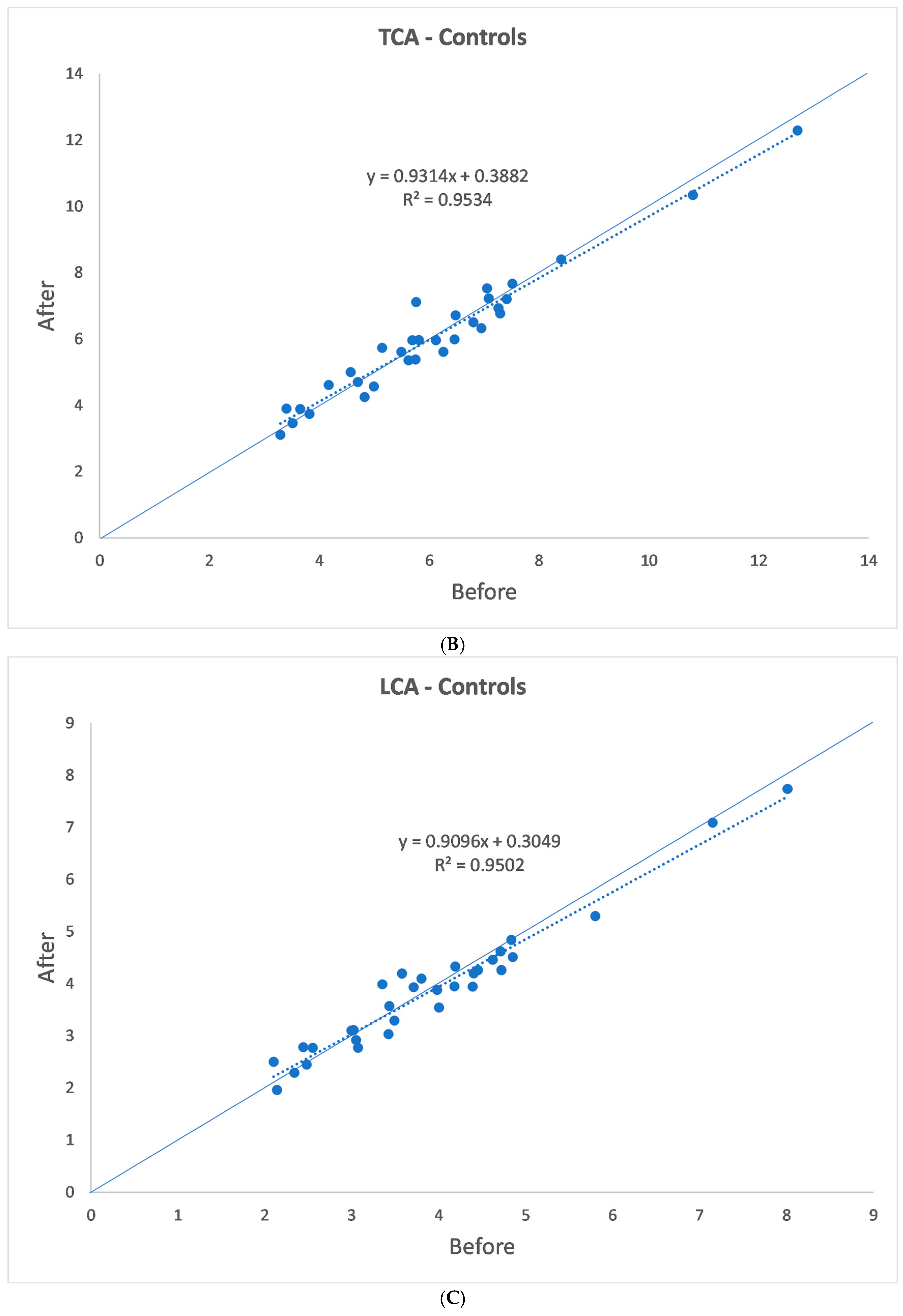
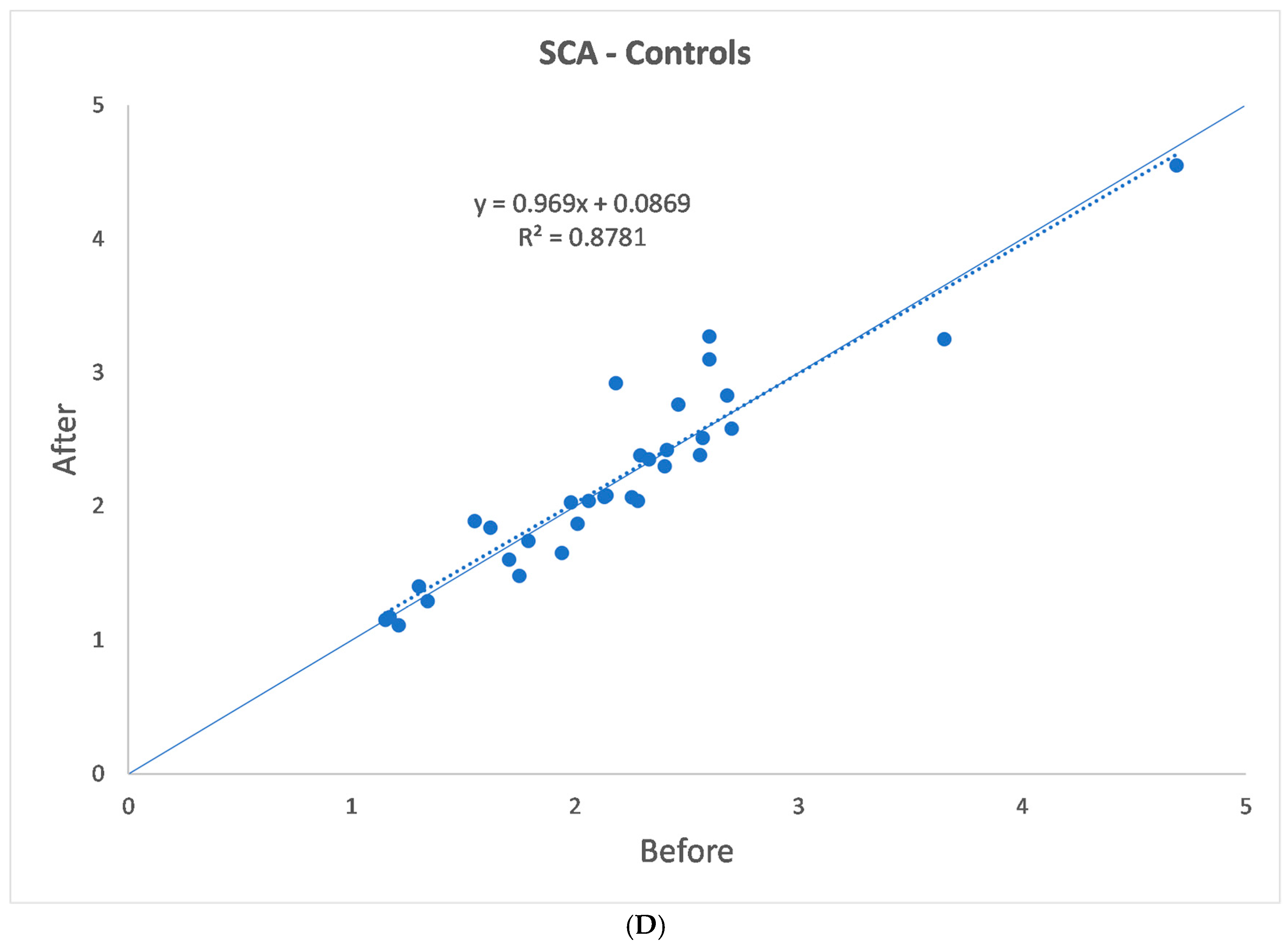
| (a) | |||||
| Before | |||||
| SFCT | TCA | LCA | SCA | CVI | |
| Mean | 213.84 µm | 6.49 mm2 | 4.16 mm2 | 2.35 mm2 | 63.92% |
| SD | 79.65 µm | 2.34 mm2 | 1.51 mm2 | 0.88 mm2 | 2.77% |
| Median | 220.00 µm | 6.25 mm2 | 4.10 mm2 | 2.25 mm2 | 64.00% |
| Min | 62.00 µm | 2.50 mm2 | 1.50 mm2 | 0.80 mm2 | 59.80% |
| Max | 387.00 µm | 11.10 mm2 | 7.20 mm2 | 4.30 mm2 | 69.50% |
| After | |||||
| SFCT | TCA | LCA | SCA | CVI | |
| Mean | 205.91 µm | 6.39 mm2 | 4.04 mm2 | 2.34 mm2 | 63.47% |
| SD | 80.24 µm | 2.19 mm2 | 1.37 mm2 | 0.87 mm2 | 2.55% |
| Median | 214.50 µm | 6.20 mm2 | 4.00 mm2 | 2.15 mm2 | 63.35% |
| Min | 53.00 µm | 2.40 mm2 | 1.70 mm2 | 0.70 mm2 | 57.30% |
| Max | 392.00 µm | 11.20 mm2 | 7.00 mm2 | 4.20 mm2 | 69.80% |
| p | 0.007 | 0.181 | 0.045 | 0.806 | 0.248 |
| (b) | |||||
| Before | |||||
| SFCT | TCA | LCA | SCA | CVI | |
| Mean | 187.94 µm | 6.09 mm2 | 3.92 mm2 | 2.18 mm2 | 64.23% |
| SD | 57.10 µm | 2.02 mm2 | 1.33 mm2 | 0.71 mm2 | 1.97% |
| Median | 196.50 µm | 5.80 mm2 | 3.75 mm2 | 2.15 mm2 | 64.70% |
| Min | 62.00 µm | 3.30 mm2 | 2.10 mm2 | 1.20 mm2 | 59.50% |
| Max | 298.00 µm | 12.70 mm2 | 8.00 mm2 | 4.70 mm2 | 69.00% |
| After | |||||
| SFCT | TCA | LCA | SCA | CVI | |
| Mean | 188.00 µm | 6.06 mm2 | 3.87 mm2 | 2.20 mm2 | 63.94% |
| SD | 57.50 µm | 1.92 mm2 | 1.23 mm2 | 0.74 mm2 | 3.27% |
| Median | 196.50 µm | 6.00 mm2 | 3.90 mm2 | 2.10 mm2 | 64.00% |
| Min | 63.00 µm | 3.10 mm2 | 2.00 mm2 | 1.10 mm2 | 56.30% |
| Max | 299.00 µm | 12.30 mm2 | 7.70 mm2 | 4.60 mm2 | 71.50% |
| p | 0.875 | 0.654 | 0.359 | 0.424 | 0.545 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gioia, M.; De Bernardo, M.; De Luca, M.; Rosa, N. Effects of Alpha-Lytic Therapy Withdrawal on Choroidal Parameters in Intraoperative Floppy Iris Syndrome High-Risk Patients. J. Clin. Med. 2024, 13, 7543. https://doi.org/10.3390/jcm13247543
Gioia M, De Bernardo M, De Luca M, Rosa N. Effects of Alpha-Lytic Therapy Withdrawal on Choroidal Parameters in Intraoperative Floppy Iris Syndrome High-Risk Patients. Journal of Clinical Medicine. 2024; 13(24):7543. https://doi.org/10.3390/jcm13247543
Chicago/Turabian StyleGioia, Marco, Maddalena De Bernardo, Martina De Luca, and Nicola Rosa. 2024. "Effects of Alpha-Lytic Therapy Withdrawal on Choroidal Parameters in Intraoperative Floppy Iris Syndrome High-Risk Patients" Journal of Clinical Medicine 13, no. 24: 7543. https://doi.org/10.3390/jcm13247543
APA StyleGioia, M., De Bernardo, M., De Luca, M., & Rosa, N. (2024). Effects of Alpha-Lytic Therapy Withdrawal on Choroidal Parameters in Intraoperative Floppy Iris Syndrome High-Risk Patients. Journal of Clinical Medicine, 13(24), 7543. https://doi.org/10.3390/jcm13247543







