Long-Term Survival of Methotrexate as First-Line Therapy in Rheumatoid Arthritis, Psoriatic Arthritis and Undifferentiated Arthritis
Abstract
:1. Introduction
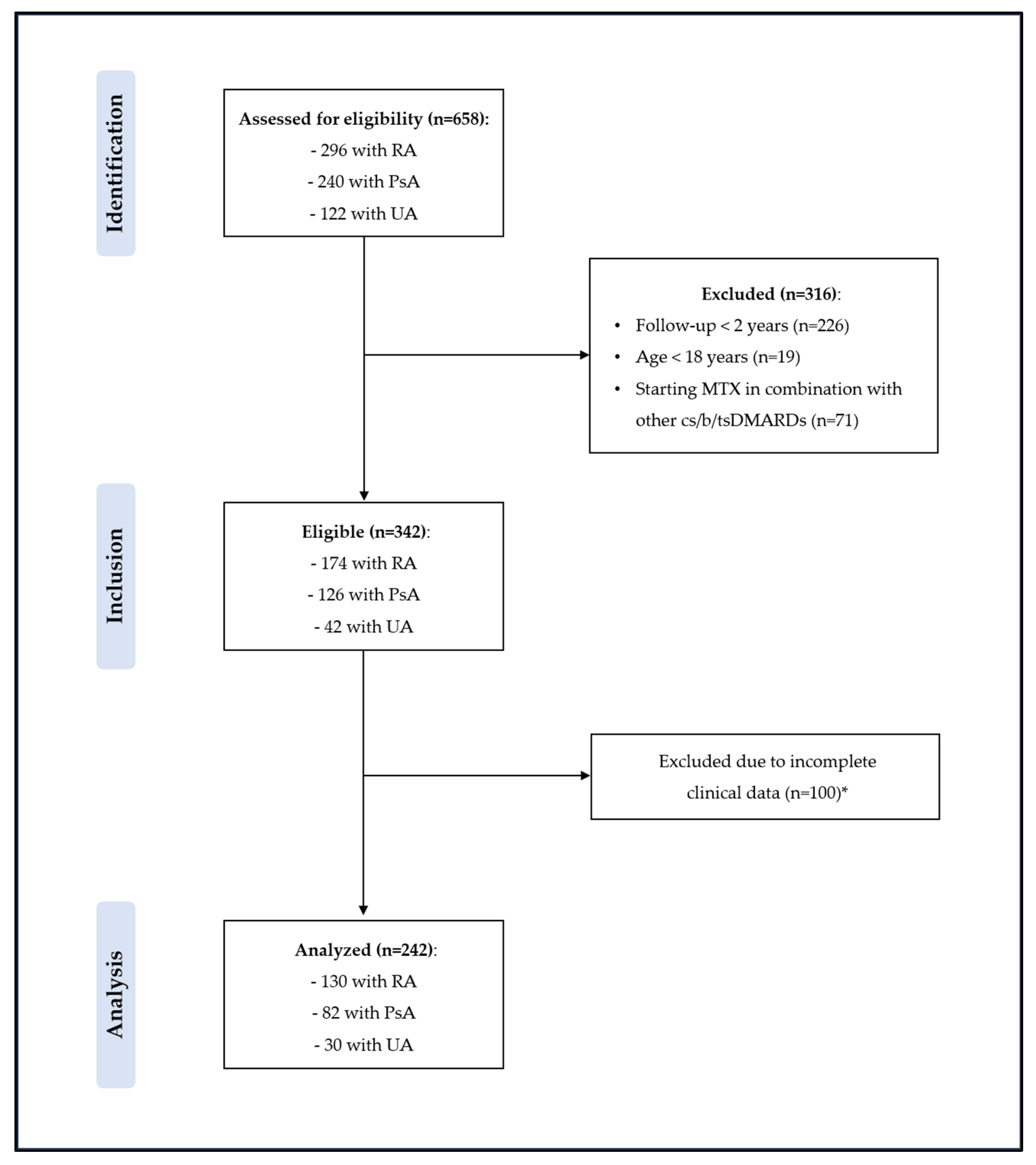
2. Methods
2.1. Study Protocol
2.2. Study Procedures
2.3. Statistical Analysis
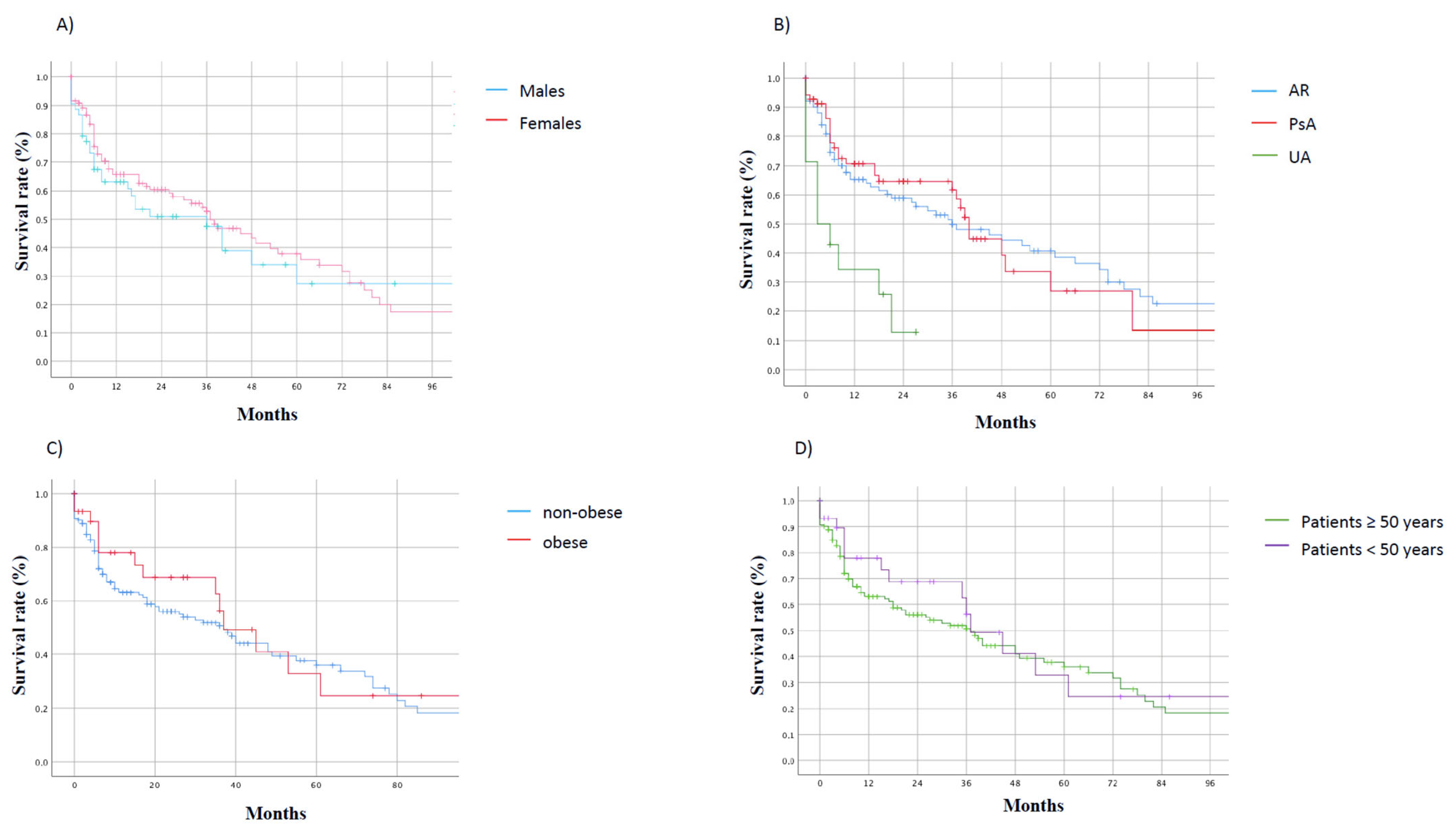
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gossec, L.; Kerschbaumer, A.; Ferreira, R.J.; Aletaha, D.; Baraliakos, X.; Bertheussen, H.; Boehncke, W.-H.; Esbensen, B.A.; McInnes, I.B.; McGonagle, D.; et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update. Ann. Rheum. Dis. 2024, 83, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Coates, L.C.; Soriano, E.R.; Corp, N.; Bertheussen, H.; Duffin, K.C.; Campanholo, C.B.; Chau, J.; Eder, L.; Fernández-Ávila, D.G.; Garg, A.; et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): Updated treatment recommendations for psoriatic arthritis 2021. Nat. Rev. Rheumatol. 2022, 18, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, F.; Fabre, S.; Pers, Y.-M. Remission-induction therapies for early rheumatoid arthritis: Evidence to date and clinical implications. Ther. Adv. Musculoskelet. Dis. 2016, 8, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Gladman, D.D.; Collier, D.H.; Ritchlin, C.T.; Helliwell, P.S.; Liu, L.; Kricorian, G.; Chung, J.B. Etanercept and Methotrexate as Monotherapy or in Combination for Psoriatic Arthritis: Primary Results From a Randomized, Controlled Phase III Trial. Arthritis Rheumatol. 2019, 71, 1112–1124. [Google Scholar] [CrossRef]
- Lubrano, E.; Perrotta, F.M.; Kavanaugh, A. An overview of low disease activity and remission in psoriatic arthritis. Clin. Exp. Rheumatol. 2015, 33, S51–S54. [Google Scholar]
- Cutolo, M.; Sulli, A.; Pizzorni, C.; Seriolo, B.; Straub, R.H. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann. Rheum. Dis. 2001, 60, 729–735. [Google Scholar] [CrossRef]
- Thompson, R.N.; Watts, C.; Edelman, J.; Esdaile, J.; Russel, A.S. Acontrolledtwo- centre trial of parenteral methotrexate therapy for refractory rheumatoid arthritis. J. Rheumatol. 1984, 11, 760–762. [Google Scholar]
- Andersen, P.A.; West, S.G.; O’Dell, J.R.; Via, C.S.; Claypool, R.G.; Kotzin, B.L. Weekly pulse methotrexate in rheumatoid arthritis. Ann. Intern. Med. 1985, 103, 489–496. [Google Scholar] [CrossRef]
- Weinblatt, M.E.; Cobby, J.S.; Fox, D.A. EYcacy of low-dose methotrexate in rheumatoid arthritis. N. Engl. J. Med. 1985, 312, 818–822. [Google Scholar] [CrossRef]
- Kingsley, G.H.; Kowalczyk, A.; Taylor, H.; Ibrahim, F.; Packham, J.C.; McHugh, N.J.; Mulherin, D.M.; Kitas, G.D.; Chakravarty, K.; Tom, B.D.M.; et al. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology 2012, 51, 1368–1377. [Google Scholar] [CrossRef]
- Lie, E.; van der Heijde, D.; Uhlig, T.; Heiberg, M.S.; Koldingsnes, W.; Rødevand, E.; Kaufmann, C.; Mikkelsen, K.; Kvien, T.K. Effec-tiveness and retention rates of methotrexate in psoriatic arthritis in comparison with meth-otrexate-treated patients with rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Dhir, V.; Prasad, C.B.; Kumar, S.; Kaul, K.K.; Dung, N.; Naidu, G.S.R.S.N.K.; Sharma, S.K.; Sharma, A.; Jain, S. Long-term persistence of oral methotrexate and associated factors in rheumatoid arthritis: A retrospective cohort study. Rheumatol. Int. 2023, 43, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Tishler, M.; Caspi, D.; Yaron, M. Long-term experience with low dose methotrexate in rheu-matoid arthritis. Rheumatol. Int. 1993, 13, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Ortendahl, M.; Schettler, J.D.; Fries, J.F. Factors influencing length of time taking methotrexate in rheumatoid arthritis. J. Rheumatol. 2000, 27, 1139–1147. [Google Scholar]
- Papadopoulos, N.G.; Alamanos, Y.; Papadopoulos, I.A.; Tsifetaki, N.; Voulgari, P.V.; Drosos, A.A. Disease modifying antirheumatic drugs in early rheumatoid arthritis: A longterm observa-tional study. J. Rheumatol. 2002, 29, 261–266. [Google Scholar]
- Hoekstra, M.; Laar, M.A.F.J.V.D.; Moens, H.J.B.; Kruijsen, M.W.M.; Haagsma, C.J. Longterm observational study of methotrexate use in a Dutch cohort of 1022 patients with rheumatoid arthritis. J. Rheumatol. 2003, 30, 2325–2329. [Google Scholar]
- Yazici, Y.; Sokka, T.; Kautiainen, H.; Swearingen, C.; Kulman, I.; Pincus, T. Long term safety of methotrexate in routine clinical care: Discontinuation is unusual and rarely the result of laboratory abnormalities. Ann. Rheum. Dis. 2005, 64, 207–211. [Google Scholar] [CrossRef]
- Ideguchi, H.; Ohno, S.; Ishigatsubo, Y. Risk factors associated with the cumulative survival of low-dose methotrexate in 273 Japanese patients with rheumatoid arthritis. J. Clin. Rheumatol. 2007, 13, 73–78. [Google Scholar] [CrossRef]
- Anaya, J.M.; Jorgensen, C.; Daurès, J.P.; Combe, B.; Sany, J. Therapeutic maintenance dose with methotrexate in rheumatoid polyarthritis. Prospective study of 191 cases. Rev. Rhum. Mal. Osteoartic 1992, 59, 181–187. [Google Scholar]
- Ricci, M.; De Marco, G.; Desiati, F.; Mazzocchi, D.; Rotunno, L.; Battafarano, N.; Marchesoni, A. Long-term survival of methotrexate in psoriatic arthritis. Reumatismo 2009, 61, 125–131. [Google Scholar] [CrossRef]
- Kay, J.; Upchurch, K.S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology 2012, 51 (Suppl. S6), vi5–vi9. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H.; CASPAR Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Bologna, C.; Viu, P.; Jorgensen, C.; Sany, J. Effect of age on the efficacy and tolerance of meth-otrexate in rheumatoid arthritis. Br. J. Rheumatol. 1996, 35, 453–457. [Google Scholar] [CrossRef]
- George, M.D.; Baker, J.F.; Ogdie, A. Comparative Persistence of Methotrexate and Tumor Necrosis Factor Inhibitors in Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis. J. Rheumatol. 2019, 47, 826–834. [Google Scholar] [CrossRef]

| RA | PsA | UA | |
|---|---|---|---|
| Participants | 130 | 82 | 30 |
| Age (years), median (IQR) | 59 (51–69) | 57 (46–65) | 53 (46–61) |
| Male sex, n. (%) | 25 (24.7) | 122 (50.8) | 48 (39.3) |
| Smoking, n. (%) | 45 (34.6) | 24 (29.2) | 10 (30) |
| BMI (kg/m2), median (IQR) | 25.7 (23.1–29.3) | 26.9 (23.8–30.9) | 25.1 (21.9–27.5) |
| Obesity, n (%) | 13 (12.9) | 14 (20.2) | 1 (7.1) |
| SJC, median (IQR) | 5 (3–8) | 2 (1–4) | 2 (1–3) |
| TJC, median (IQR) | 11 (5–17) | 4 (2–6) | 3 (1–5) |
| Concomitant treatment at baseline | |||
| 22 (16.9) | 20 (24.3) | 10 (33.3) |
| 70 (53.8) | 4 (4.8) | 9 (30) |
| 4 (0–4) | 4 (4–4) | 4 (4–8) |
| 6 (3–24) | 3 (3–6) | 3 (3–6) |
| Predictive Factors | Hazard Ratio (95% Confidence Interval) | p-Value |
|---|---|---|
| Age | 1.03 (0.78–2.12) | 0.42 |
| Sex (male) | 1.23 (0.81–2.40) | 0.22 |
| Smoking habit | 1.52 (0.92–3.34) | 0.13 |
| BMI | 1.12 (0.86–2.56) | 0.65 |
| Concomitant treatment with steroids | 0.89 (0.74–1.18) | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrotta, F.M.; Ambrosino, P.; Lubrano, E. Long-Term Survival of Methotrexate as First-Line Therapy in Rheumatoid Arthritis, Psoriatic Arthritis and Undifferentiated Arthritis. J. Clin. Med. 2024, 13, 7540. https://doi.org/10.3390/jcm13247540
Perrotta FM, Ambrosino P, Lubrano E. Long-Term Survival of Methotrexate as First-Line Therapy in Rheumatoid Arthritis, Psoriatic Arthritis and Undifferentiated Arthritis. Journal of Clinical Medicine. 2024; 13(24):7540. https://doi.org/10.3390/jcm13247540
Chicago/Turabian StylePerrotta, Fabio Massimo, Pasquale Ambrosino, and Ennio Lubrano. 2024. "Long-Term Survival of Methotrexate as First-Line Therapy in Rheumatoid Arthritis, Psoriatic Arthritis and Undifferentiated Arthritis" Journal of Clinical Medicine 13, no. 24: 7540. https://doi.org/10.3390/jcm13247540
APA StylePerrotta, F. M., Ambrosino, P., & Lubrano, E. (2024). Long-Term Survival of Methotrexate as First-Line Therapy in Rheumatoid Arthritis, Psoriatic Arthritis and Undifferentiated Arthritis. Journal of Clinical Medicine, 13(24), 7540. https://doi.org/10.3390/jcm13247540







