68Ga-DOTATOC Uptake by Stellate Ganglia, Mimicking a Right Cervical Metastasis of Neuroendocrine Tumors: A Case Report
Abstract
1. Introduction
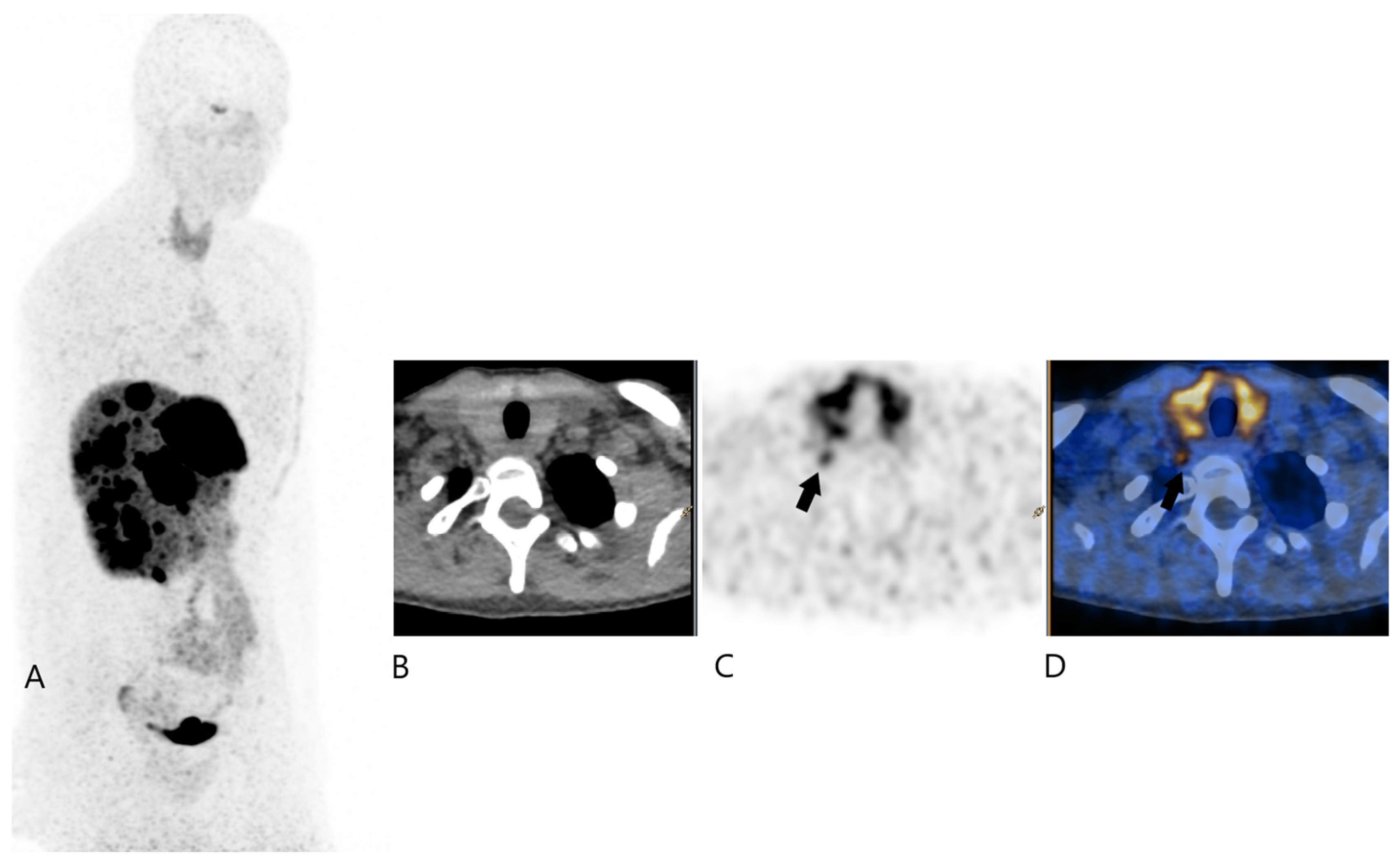
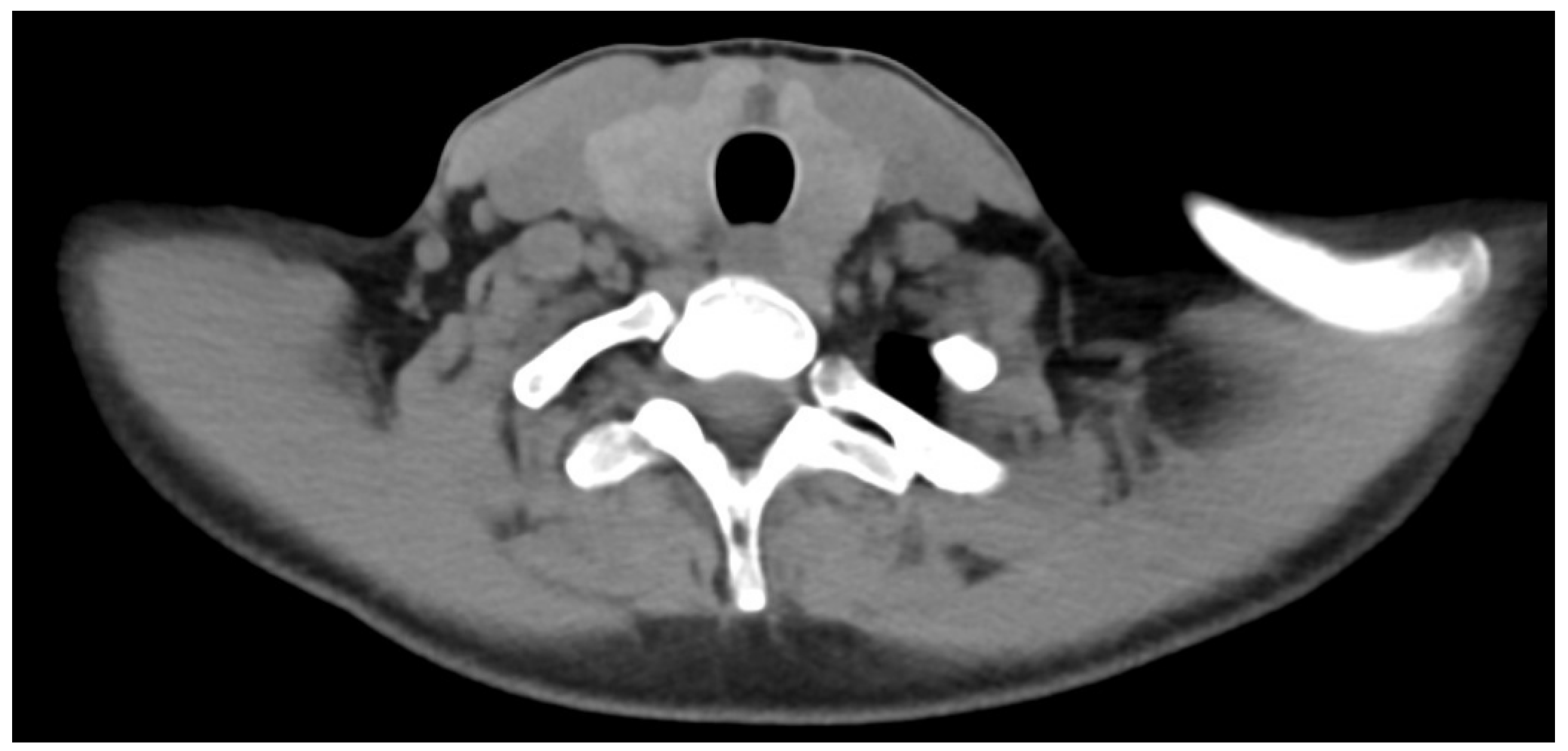
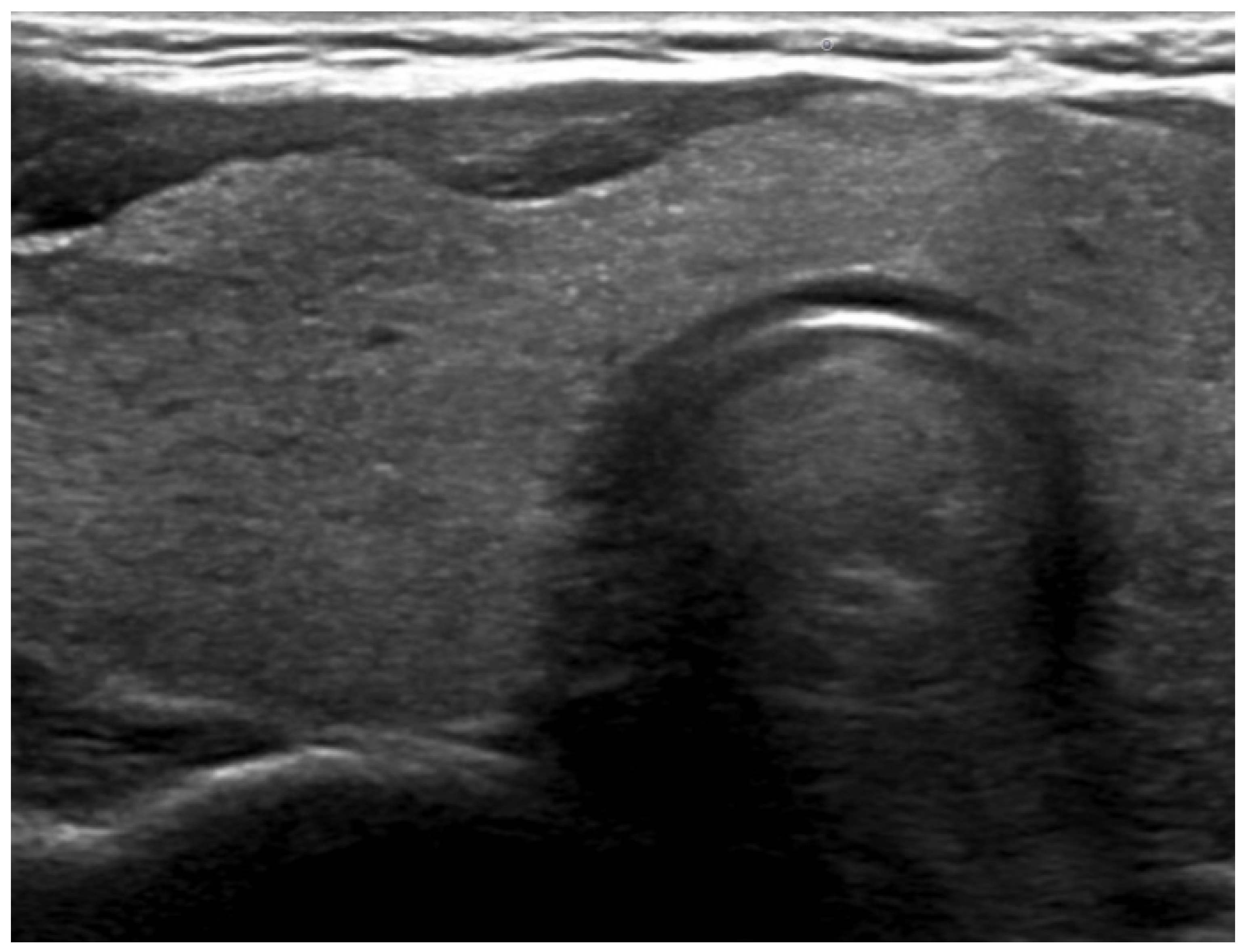
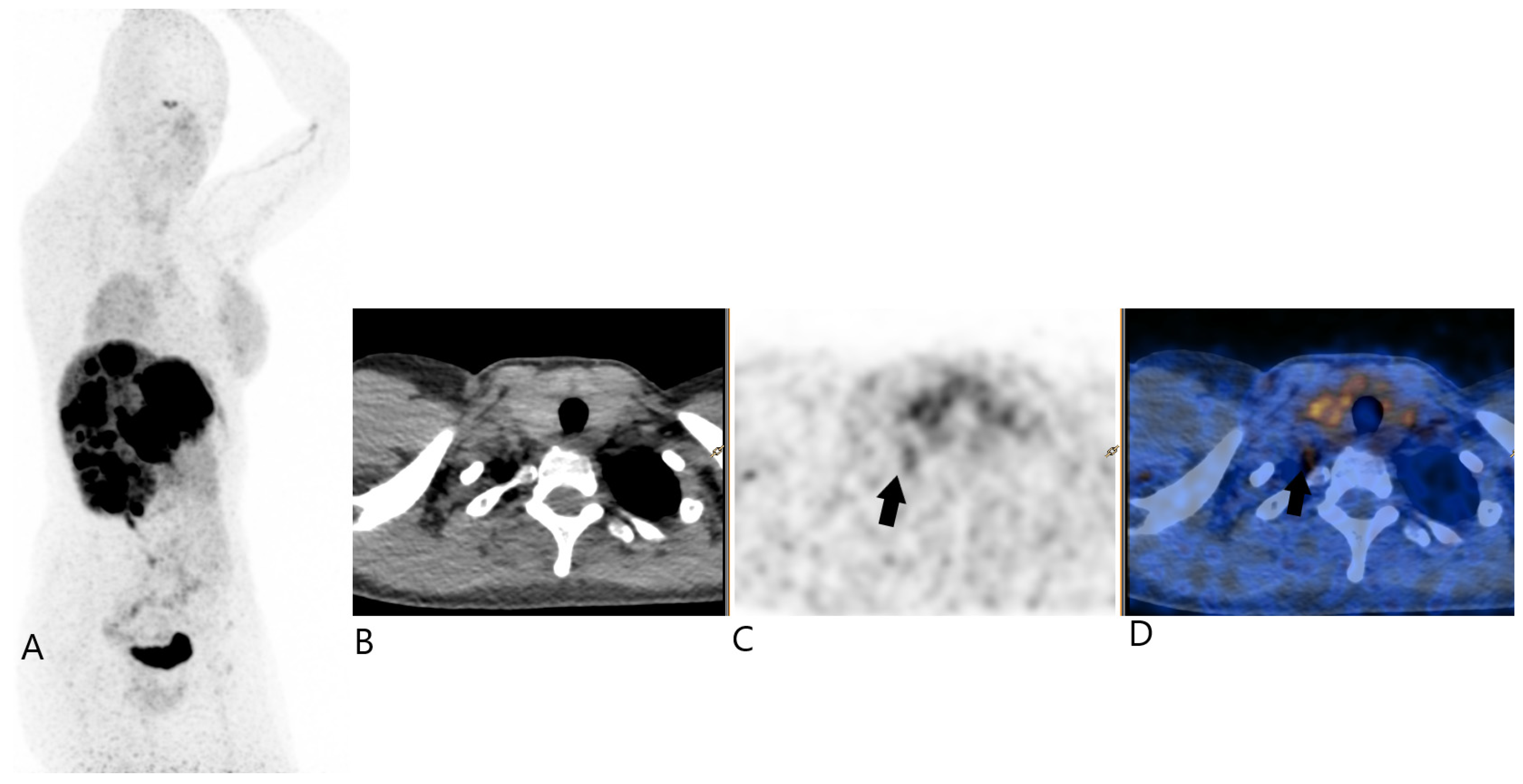
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Panzuto, F.; Boninsegna, L.; Fazio, N.; Campana, D.; Pia Brizzi, M.; Capurso, G.; Scarpa, A.; De Braud, F.; Dogliotti, L.; Tomassetti, P.; et al. Metastatic and locally advanced pancreatic endocrine carcinomas: Analysis of factors associated with disease progression. J Clin. Oncol. 2011, 29, 2372–2377. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.; Campana, D.; Polverari, G.; Peterle, C.; Diodato, S.; Ricci, C.; Allegri, V.; Casadei, R.; Tomassetti, P.; Fanti, S. Prognostic Value of 68Ga-DOTANOC PET/CT SUVmax in Patients with Neuroendocrine Tumors of the Pancreas. J. Nucl. Med. 2015, 56, 1843–1848. [Google Scholar] [CrossRef] [PubMed]
- Öberg, K. Gallium-68 somatostatin receptor PET/CT: Is it time to replace (111)Indium DTPA octreotide for patients with neuroendocrine tumors? Endocrine 2012, 42, 3–4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schreiter, N.F.; Brenner, W.; Nogami, M.; Buchert, R.; Huppertz, A.; Pape, U.F.; Prasad, V.; Hamm, B.; Maurer, M.H. Cost comparison of 111In-DTPA-octreotide scintigraphy and 68Ga-DOTATOC PET/CT for staging enteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 72–82. [Google Scholar] [CrossRef]
- Graham, M.M.; Gu, X.; Ginader, T.; Breheny, P.; Sunderland, J.J. 68Ga-DOTATOC Imaging of Neuroendocrine Tumors: A Systematic Review and Metaanalysis. J Nucl Med. 2017, 58, 1452–1458, Erratum in J. Nucl. Med. 2017, 58, 1707. [Google Scholar] [CrossRef]
- Ohnona, J.; Nataf, V.; Gauthe, M.; Balogova, S.; Belissant Benesty, O.; Zhang-Yin, J.; Talbot, J.N.; Montravers, F. Prognostic value of functional tumor burden on 68Ga-DOTATOC PET/CT in patients with pancreatic neuro-endocrine tumors. Neoplasma 2019, 66, 140–148. [Google Scholar] [CrossRef]
- Bélissant Benesty, O.; Nataf, V.; Ohnona, J.; Michaud, L.; Zhang-Yin, J.; Bertherat, J.; Chanson, P.; Reznik, Y.; Talbot, J.N.; Montravers, F. 68Ga-DOTATOC PET/CT in detecting neuroendocrine tumours responsible for initial or recurrent paraneoplastic Cushing’s syndrome. Endocrine 2020, 67, 708–717. [Google Scholar] [CrossRef]
- Marcer, N.; Bergmann, M.; Klie, A.; Moor, B.; Djonov, V. An anatomical investigation of the cervicothoracic ganglion. Clin. Anat. 2012, 25, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.L.; Agur, A.M.R.; Dalley, A.F., II. Clinically Oriented Anatomy; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2017; ISBN 9781496347213. [Google Scholar]
- Available online: https://radiopaedia.org/articles/stellate-ganglion (accessed on 20 September 2024).
- Ikeda, S.R.; Schofield, G.G. Somatostatin blocks a calcium current in rat sympathetic ganglion neurones. J. Physiol. 1989, 409, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.C. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999, 20, 157–198. [Google Scholar] [CrossRef] [PubMed]
- Häppölä, O.; Lakomy, M.; Majewski, M.; Wasowicz, K.; Yanaihara, N. Distribution of neuropeptides in the porcine stellate ganglion. Cell Tissue Res. 1993, 274, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Maslyukov, P.M.; Shilkin, V.V.; Timmermans, J.P. Immunocytochemical characteristics of neurons in the stellate ganglion of the sympathetic trunk in mice during postnatal ontogenesis. Neurosci. Behav. Physiol. 2006, 36, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Roudenok, V.; Kühnel, W. Distribution of vasoactive intestinal polypeptide-, calcitonin gene-related peptide-, somatostatin- and neurofilament-immunoreactivities in sympathetic ganglia of human fetuses and premature neonates. Ann. Anat. 2001, 183, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Berg, Z.; Koppula, B.R. 68Ga-DOTATATE Uptake by Cervicothoracic (Stellate) Ganglia. Clin. Nucl. Med. 2019, 44, 810–811. [Google Scholar] [CrossRef]
- Malan, N.; Vangu, M.D. Normal Variants, Pitfalls and Artifacts in Ga-68 DOTATATE PET/CT Imaging. Front. Nucl. Med. 2022, 2, 825486. [Google Scholar] [CrossRef]
- Sheikhbahaei, S.; Afshar-Oromieh, A.; Eiber, M.; Solnes, L.B.; Javadi, M.S.; Ross, A.E.; Pienta, K.J.; Allaf, M.E.; Haberkorn, U.; Pomper, M.G.; et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2117–2136. [Google Scholar] [CrossRef]
- Mamlins, E.; Schmitt, D.; Beu, M.; Mattes-György, K.; Henke, J.M.; Antke, C.; Novruzov, E.; Cardinale, J.; Kirchner, J.; Niegisch, G.; et al. PSMA-1007 Uptake in Ganglia of the Sympathetic Trunk and Its Intra-individual Reproducibility. Mol. Imaging Biol. 2023, 25, 554–559. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang-Yin, J.T.; Panagiotidis, E. 68Ga-DOTATOC Uptake by Stellate Ganglia, Mimicking a Right Cervical Metastasis of Neuroendocrine Tumors: A Case Report. J. Clin. Med. 2024, 13, 7413. https://doi.org/10.3390/jcm13237413
Zhang-Yin JT, Panagiotidis E. 68Ga-DOTATOC Uptake by Stellate Ganglia, Mimicking a Right Cervical Metastasis of Neuroendocrine Tumors: A Case Report. Journal of Clinical Medicine. 2024; 13(23):7413. https://doi.org/10.3390/jcm13237413
Chicago/Turabian StyleZhang-Yin, Jules Tianyu, and Emmanouil Panagiotidis. 2024. "68Ga-DOTATOC Uptake by Stellate Ganglia, Mimicking a Right Cervical Metastasis of Neuroendocrine Tumors: A Case Report" Journal of Clinical Medicine 13, no. 23: 7413. https://doi.org/10.3390/jcm13237413
APA StyleZhang-Yin, J. T., & Panagiotidis, E. (2024). 68Ga-DOTATOC Uptake by Stellate Ganglia, Mimicking a Right Cervical Metastasis of Neuroendocrine Tumors: A Case Report. Journal of Clinical Medicine, 13(23), 7413. https://doi.org/10.3390/jcm13237413





