A Review of the Literature on Videoscopic and Robotic Inguinal–Iliac–Obturator Lymphadenectomy in Patients with Cutaneous Melanoma
Abstract
1. Introduction
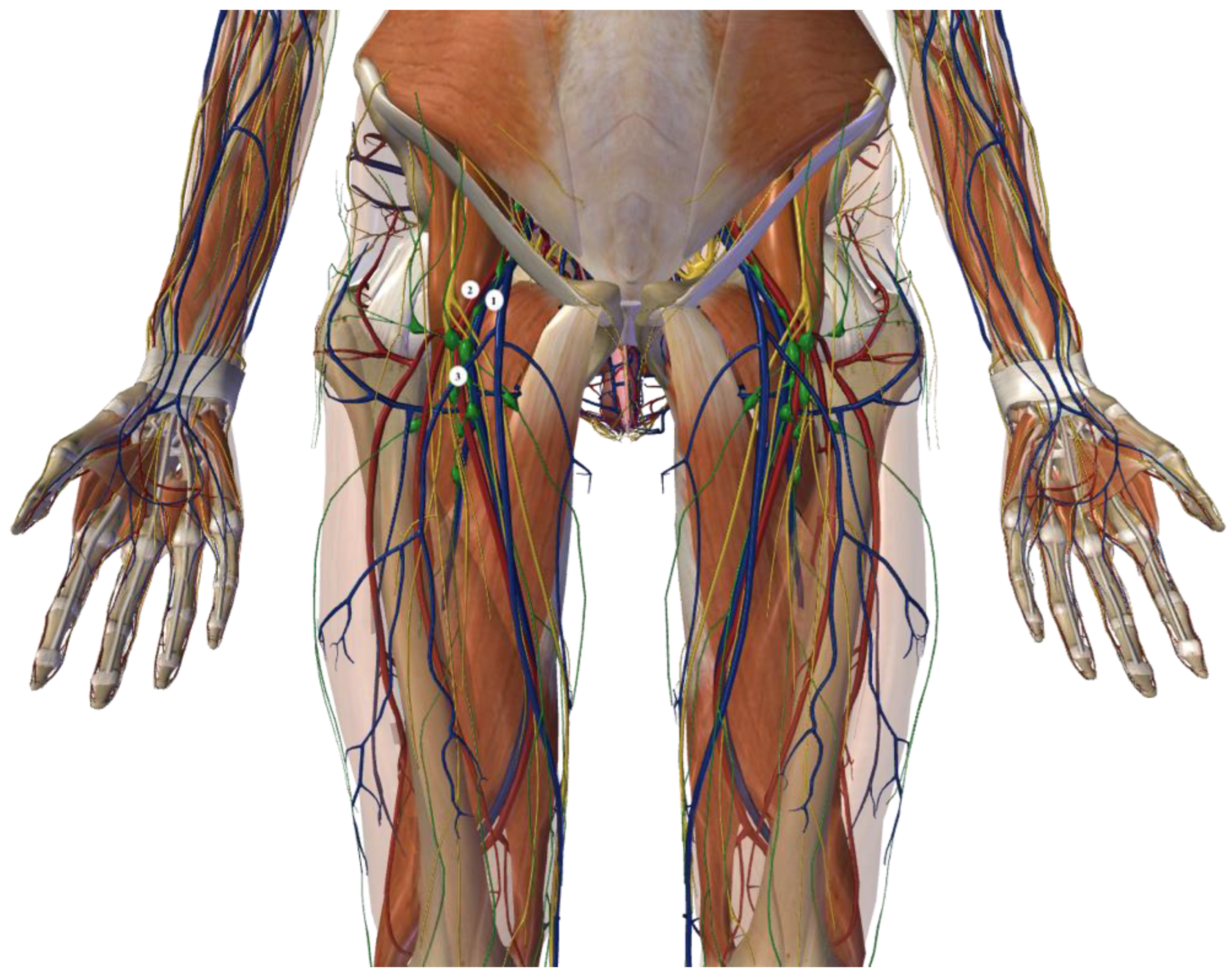
1.1. Open Inguinal–Iliac–Obturator Lymph Node Dissection Technique
1.2. Videoscopic Inguinal–Iliac–Obturator Lymph Node Dissection Technique
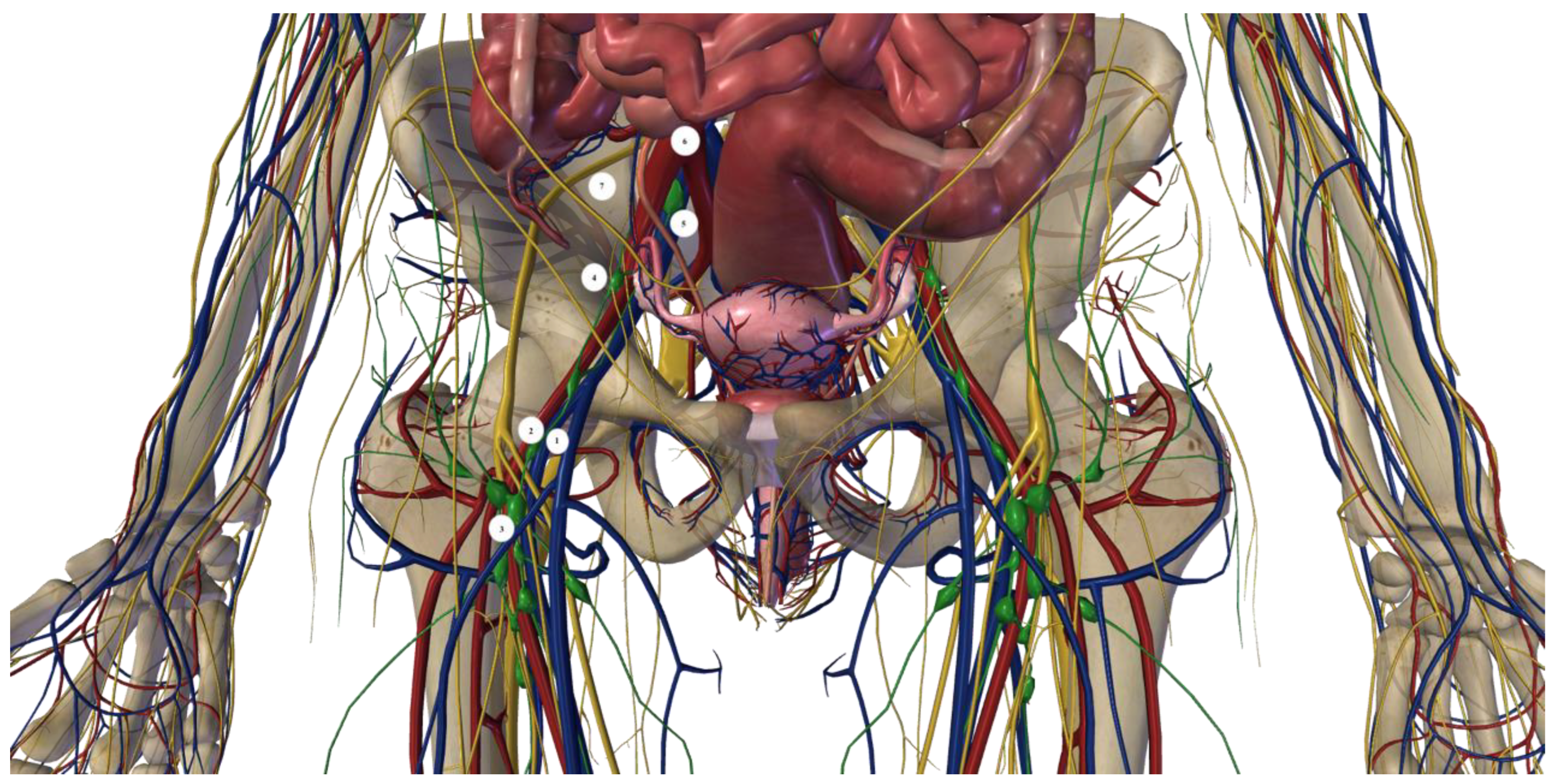
1.3. Robotic Inguinal–Iliac–Obturator Lymph Node Dissection Technique
2. Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AIOM. Guidelines; AIOM: Milan, Italy, 2021. [Google Scholar]
- Swetter, S.M.; Johnson, D.; Albertini, M.R.; Barker, C.A.; Bateni, S.; Baumgartner, J.; Bhatia, S.; Bichakjian, C.; Boland, G.; Chandra, S.; et al. NCCN Guidelines® Insights: Melanoma: Cutaneous, Version 2.2024. J. Natl. Compr. Cancer Netw. 2024, 22, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, R.; Sayegh, A.S.; Medina, L.G.; Perez, L.C.; La Riva, A.; Eppler, M.B.; Gaona, J.; Tobias-Machado, M.; E Spiess, P.; A Pettaway, C.; et al. Complications and adverse events in lymphadenectomy of the inguinal area: Worldwide expert consensus. BJS Open 2024, 8, zrae056. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Francone, E.; Reina, S.; Spagnolo, F.; Di Maira, L.; Cafiero, F.; Solari, N. Combined robotic inguinal and iliac-obturator lymphadenectomy for stage III skin cancers: Surgical technique and preliminary results. Int. J. Med Robot. 2022, 18, e2391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nabavizadeh, R.; Petrinec, B.; Nabavizadeh, B.; Singh, A.; Rawal, S.; Master, V. Inguinal lymph node dissection in the era of minimally invasive surgical technology. Urol. Oncol. 2020, 41, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Roshan, A.; Roshan, A.; Roshan, A.; Shah, B.; Shah, B.; Shah, B.; Anderson, K.D.; Anderson, K.D.; Anderson, K.D.; Murphy, S.; et al. Robot-Assisted Pelvic Dissection for Enlarged Lymph Nodes in Melanoma Improves Recovery with Equivalent Oncological Outcomes to Open Pelvic Dissection. Ann. Surg. Oncol. 2024, 31, 2727–2736. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miura, J.T.; Dossett, L.A.; Thapa, R.; Kim, Y.; Potdar, A.; Daou, H.; Sun, J.; Sarnaik, A.A.; Zager, J.S. Robotic-Assisted Pelvic Lymphadenectomy for Metastatic Melanoma Results in Durable Oncologic Outcomes. Ann. Surg. Oncol. 2019, 27, 196–202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dossett, L.A.; Castner, N.B.; Pow-Sang, J.M.; Abbott, A.M.; Sondak, V.K.; Sarnaik, A.A.; Zager, J.S. Robotic-Assisted Transperitoneal Pelvic Lymphadenectomy for Metastatic Melanoma: Early Outcomes Compared with Open Pelvic Lymphadenectomy. J. Am. Coll. Surg. 2016, 222, 702–709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hyde, G.A.; Jung, N.L.; Valle, A.A.; Bhattacharya, S.D.; Keel, C.E. Robotic inguinal lymph node dissection for melanoma: A novel approach to a complicated problem. J. Robot. Surg. 2018, 12, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Elia, R.; Clemente, E.T.; Vestita, M.; Nacchiero, E. Robotic inguinal lymph node dissection for melanoma: A novel approach to a complicated problem. J. Robot. Surg. 2019, 13, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Sotelo, R.; Rodriguez, O.; Sánchez, R.; Rosciano, J.; Medina, L.; Vegas, L. Robot-assisted video endoscopic inguinal lymphadenectomy for melanoma. J. Robot. Surg. 2016, 10, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, A.; Cona, C.; Tonello, M.; Pilati, P.; Rossi, C.R. Oncological outcome of videoscopic groin dissection for lymph node metastasis from melanoma. Surg. Endosc. 2020, 35, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- Vrielink, O.M.; Faut, M.; Deckers, E.A.; van Leeuwen, B.L.; Been, L.B. Evaluation of the videoscopic inguinal lymphadenectomy in melanoma patients. Eur. J. Surg. Oncol. (EJSO) 2019, 45, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, A.; Pasquali, S.; Cona, C.; Ciccarese, A.A.; Saadeh, L.; Campana, L.G.; Meroni, M.; Rossi, C.R. Videoscopic ilioinguinal lymphadenectomy for groin lymph node metastases from melanoma. Br. J. Surg. 2016, 103, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Solari, N.; Gipponi, M.; Franco, D.S.; Rè, F.; Sonia, O.; Bertoglio, S.; Cafiero, F. Videoscopic Inguinal-iliac-obturator Lymph-node Dissection: New Videoscopic Technique for Regional Lymphadenectomy in Patients with Melanoma. Anticancer Res. 2016, 36, 6579–6584. [Google Scholar] [CrossRef] [PubMed]
- Delman, K.A.; Kooby, D.A.; Rizzo, M.; Ogan, K.; Master, V. Initial experience with videoscopic inguinal lymphadenectomy. Ann. Surg. Oncol. 2010, 18, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Kelley, J.; Wright, G.P. Starting a minimally invasive inguinal lymphadenectomy program: Initial learning experience and outcomes. Surgery 2022, 173, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.E.; Brown, R.E.; Roach, B.A.; Quillo, A.R.; Martin, R.C.; Scoggins, C.R.; Stromberg, A.J.; McMasters, K.M. Addition of an iliac/obturator lymph node dissection does not improve nodal recurrence or survival in melanoma. J. Am. Coll. Surg. 2014, 219, 101–108. [Google Scholar] [CrossRef]
- Verver, D.; Madu, M.F.; Ophuis, C.M.C.O.; Faut, M.; de Wilt, J.H.W.; Bonenkamp, J.J.; Grünhagen, D.J.; van Akkooi, A.C.J.; Verhoef, C.; van Leeuwen, B.L. Optimal extent of completion lymphadenectomy for patients with melanoma and a positive sentinel node in the groin. Br. J. Surg. 2017, 105, 96–105. [Google Scholar] [CrossRef]
- Arié, A.; Yamamoto, T. Lymphedema secondary to melanoma treatments: Diagnosis, evaluation, and treatments. Glob. Health Med. 2020, 2, 227–234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stuiver, M.M.; Westerduin, E.; ter Meulen, S.; Vincent, A.D.; Nieweg, O.E.; Wouters, M.W. Surgical wound complications after groin dissection in melanoma patients-a historical cohort study and risk factor analysis. Eur. J. Surg. Oncol. 2014, 40, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.E. Treatment of refractory donor-site seromas with percutaneous instillation of fibrin sealant. Plast. Reconstr. Surg. 2006, 117, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Teiche, P.E.; Pauer, W.; Schmid, N. Use of talcum in sclerotherapy of pelvic lymphoceles. Tech. Urol. 1999, 5, 52–53. [Google Scholar] [PubMed]
- Hackert, T.; Werner, J.; Loos, M.; Büchler, M.W.; Weitz, J. Successful doxycycline treatment of lymphatic fistulas: Report of five cases and review of the literature. Langenbeck’s Arch. Surg. 2006, 391, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Ali-Khan, A.S.; Orlando, A.; Kenealy, J. Erythromycin sclerotherapy in the management of seroma. J. Plast. Reconstr. Aesthet.Surg. 2009, 62, e55–e58. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.; Sminia, P.; McBride, W.H.; Stranzl, H.; Prettenhofer, U.; Fruhwirth, J.; Poschauko, J. Lymphatic fistulas: Obliteration by low-dose radiotherapy. Strahlenther. Onkol. 2005, 181, 660–664. [Google Scholar] [CrossRef] [PubMed]


| Author | N of Patients | Histology | Lymph Node Yield, Median | Positive Lymph Nodes Median | Hospital Days | Surgery Time (Min; Median) | Complications by Clavien–Dindo Score 1 | Complications by Clavien–Dindo Score 2 | Complications by Clavien–Dindo Score > 3 |
|---|---|---|---|---|---|---|---|---|---|
| Miura [7] | 22 | Melanoma | 11 (8.2–12) | 1 (0.1) | N/A | 199 (168–229.2) | 40.9% | 4.6% | 0% |
| Roshan [6] | 14 | Melanoma | 6.0 (3.75–9.0) | 2 (1.0–3.0) | 1.98 (1.39–3.50) | 126 (97.8–161) | 35.7% | 50% | 7.1% |
| Hyde [9] | 4 | Melanoma | 5.5 (1–14) | N/A | 2 (1–3) | 192 (120–231) | 0% | 50% | 0% |
| Sanchez [11] | 1 | Melanoma | 8 | 0 | 2 | 130 | 0% | 0% | 0% |
| Francone [4] | 10 | Melanoma (8) and MMC (2) | 15.2 (5–27) | N/A | 2 | 330 | 30% | 10% | N/A |
| Dossett [8] | 13 | Melanoma | 11 (5–16) | 0 (0–5) | 2 (1–3) | 227 | 31% | 0% | 0% |
| Author | N of Patients | Histology | Lymph Node Yeld, Median | Positive Lymph Nodes, Median | Hospital Days | Surgery Time (Min, Median) | Conversion to Open Procedure | Complications by Clavien–Dindo Score 1 | Complications by Clavien–Dindo Score 2 | Complications by Clavien–Dindo Score > 3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sommariva [12] | 48 | Melanoma | N/A | 40 (80%) | 6 (5–7) | 255 (231–278) | 6 (11%) | 15 (30%) | 4 (8%) | 3 (6%) |
| Solari [15] | 24 | Melanoma | 24.1 (8–38) | 1.58 (6.58%) | 2 (1–3) | 302 | N/A | 16.5% | N/A | N/A |
| Sommariva [14] | 24 | Melanoma | 21 (15–25) | 1 (1–2) | 7 (5–8) | 270 (245–300) | 4 | 11 | 5 | 1 |
| Vrielink [13] | 20 | Melanoma | 9.0 (1–19) | N/A | 4.0 (2.0–5.0) | 110 (79.0–165-0) | N/A | 5 (31.3%) | 6 (37.7) | 5 (31.3) |
| Khan [17] | 26 | Melanoma; MCC; penile cancer | 9.8 +/− 3.7 | 1 (0–9) | N/A | 119 (89–160) | 0 | 4 | 7 | 6 |
| Delman [16] | 45 | Melanoma; MCC; penile cancer | 11 (4–24) | N/A | 3.1 | 165 (75–245) | 2 | 8 | N/A | N/A |
| Author | N of Patients | Histology | Lymph Node Yeld, Median | Positive Lymph Nodes, Median | Hospital Days | Surgery Time (Min, Median) | Complications by Clavien–Dindo 1 | Complications by Clavien–Dindo 2 | Complications by Clavien–Dindo > 3 |
|---|---|---|---|---|---|---|---|---|---|
| Miura [7] | 30 | Melanoma | 9 (8–13) | 2 (0–4) | 4 | 214.5 (151.5–250.2) | 10 (24.4) | 0 | |
| Roshan [6] | 8 | Melanoma | 6.5 (6.0–12.5) | 2.5 (1.25–3.75) | 5.34 (3.77–6.94) | 174 (158–216) | 1 (12.5) | 4 (50) | 2 (25) |
| Dossett [8] | 25 | Melanoma | 10 (5–16) | 1 (0–8) | 4 (1–7) | 230 | 11 (44) | 1 (4) | 1 (4) |
| Solari [15] | 20 | Melanoma | 15.5 (11–28) | 3.15 (15.75%) | 6 (4–8) | 190 | 6 (30%) | 3 (13) | 0 |
| Author | Median FU (Months) | RFS (Months) | OS (Months) | Development of Distant Disease | Basin Recurrence |
|---|---|---|---|---|---|
| Miura [7] | 37.2 | 14.4 (rPLND) vs. 9.6 (oPLND) | 42.6 (rPLND) vs. 50 (oPLND) | rPLND: 40.9% vs. oPLND: 43.9% | rPLND: 4.5% vs. oPLND: 7.3% |
| Roshan [6] | oPLND: 25.7 vs. rPLND: 21.1 | N/A | N/A | rPLND: 71.4% vs. oPLND: 75.0% | rPLND: 42.9% vs. oPLND: 37.5% |
| Dossett [8] | oPLND: 25.7 vs. rPLND: 21 | N/A | N/A | rPLND: 23% vs. oPLND: 44% | rPLND: 0% vs. oPLND: 4% |
| Sommariva [12] | 28.0 | 9 (4–17.8) | N/A | VIL: 27.5 % | VIL: 23.5% |
| Sommariva [14] | 18.0 | N/A | N/A | VIL: 13% | VIL: 8.7% |
| LN Retrieval, n | LOS (Days) | Surgery Time, Min | Clavien–Dindo 1,% | Clavien–Dindo 2, % | Clavien–Dindo 3, % | |
|---|---|---|---|---|---|---|
| Robotic | 5–15 | 2 | 126–330 | 31–41 | 5–50 | 7 |
| Videoscopic | 9–24 | 3–7 | 165–270 | 17–31 | 8–38 | 6–31 |
| Open | 6–15 | 4–6 | 174–215 | 13–44 | 4–50 | 4–25 |
| Robotic | Videoscopic | Open |
|---|---|---|
| Lower LOS | Lower LOS than open | Higher LOS |
| Lower C-D3 complication rates | Lower C-D 2 complication rates than robotic | Higher C-D 3 complication rates |
| Lower hospital cost | Lower surgical time than robotic | Higher hospital cost |
| Higher C-D 1 complication rates | Higher hospital cost than robotic | Lower surgical time than robotic |
| Higher surgical time | Higher C-D 3 complication |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matteucci, M.; Bruzzone, P.; Pinto, S.; Covarelli, P.; Boselli, C.; Popivanov, G.I.; Cirocchi, R. A Review of the Literature on Videoscopic and Robotic Inguinal–Iliac–Obturator Lymphadenectomy in Patients with Cutaneous Melanoma. J. Clin. Med. 2024, 13, 7305. https://doi.org/10.3390/jcm13237305
Matteucci M, Bruzzone P, Pinto S, Covarelli P, Boselli C, Popivanov GI, Cirocchi R. A Review of the Literature on Videoscopic and Robotic Inguinal–Iliac–Obturator Lymphadenectomy in Patients with Cutaneous Melanoma. Journal of Clinical Medicine. 2024; 13(23):7305. https://doi.org/10.3390/jcm13237305
Chicago/Turabian StyleMatteucci, Matteo, Paolo Bruzzone, Sabrina Pinto, Piero Covarelli, Carlo Boselli, Georgi I. Popivanov, and Roberto Cirocchi. 2024. "A Review of the Literature on Videoscopic and Robotic Inguinal–Iliac–Obturator Lymphadenectomy in Patients with Cutaneous Melanoma" Journal of Clinical Medicine 13, no. 23: 7305. https://doi.org/10.3390/jcm13237305
APA StyleMatteucci, M., Bruzzone, P., Pinto, S., Covarelli, P., Boselli, C., Popivanov, G. I., & Cirocchi, R. (2024). A Review of the Literature on Videoscopic and Robotic Inguinal–Iliac–Obturator Lymphadenectomy in Patients with Cutaneous Melanoma. Journal of Clinical Medicine, 13(23), 7305. https://doi.org/10.3390/jcm13237305






