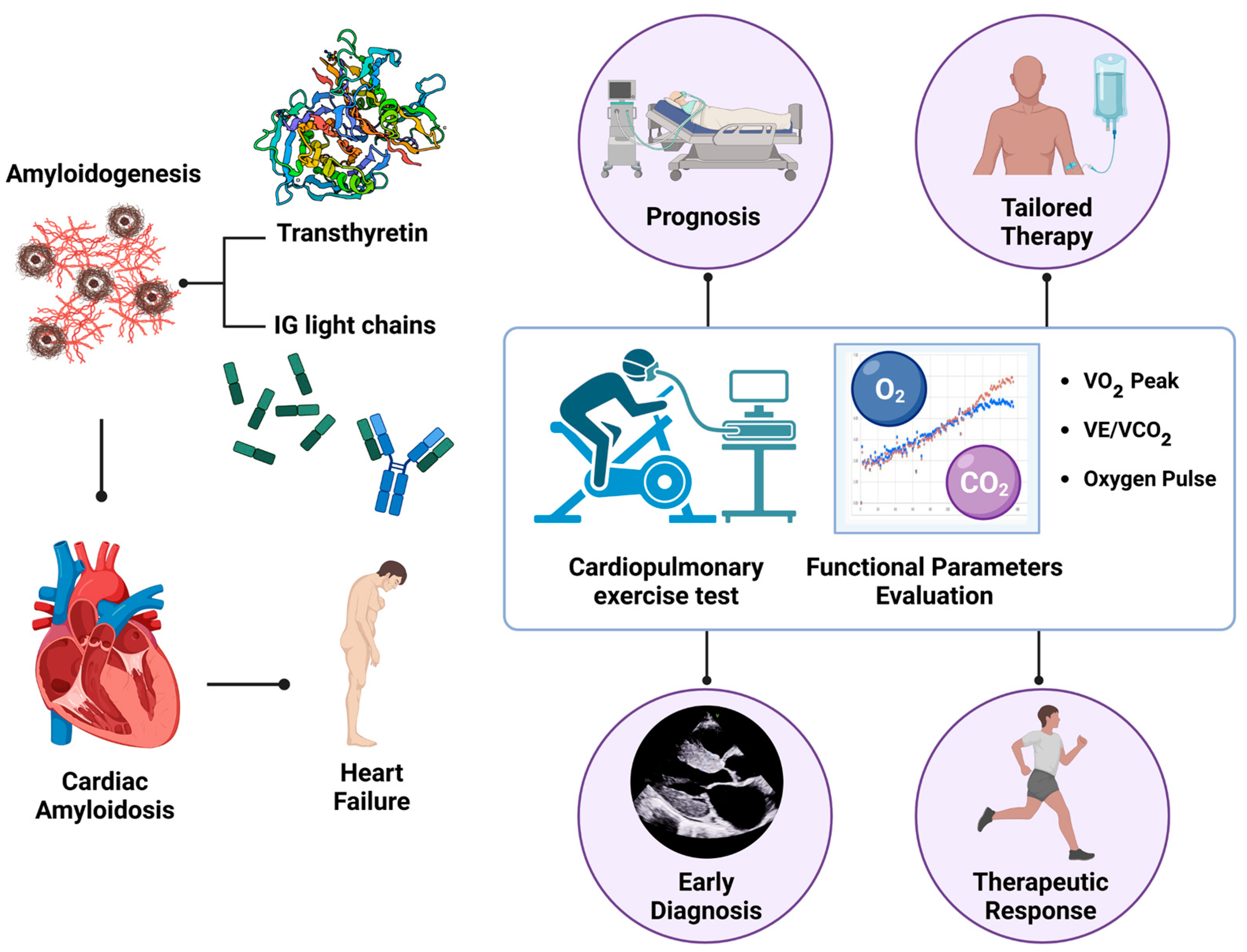
Advancing Cardiac Amyloidosis Care Through Insights from Cardiopulmonary Exercise Testing
Abstract
1. Introduction
2. Cardiac Amyloidosis: From Diagnosis to Risk Stratification
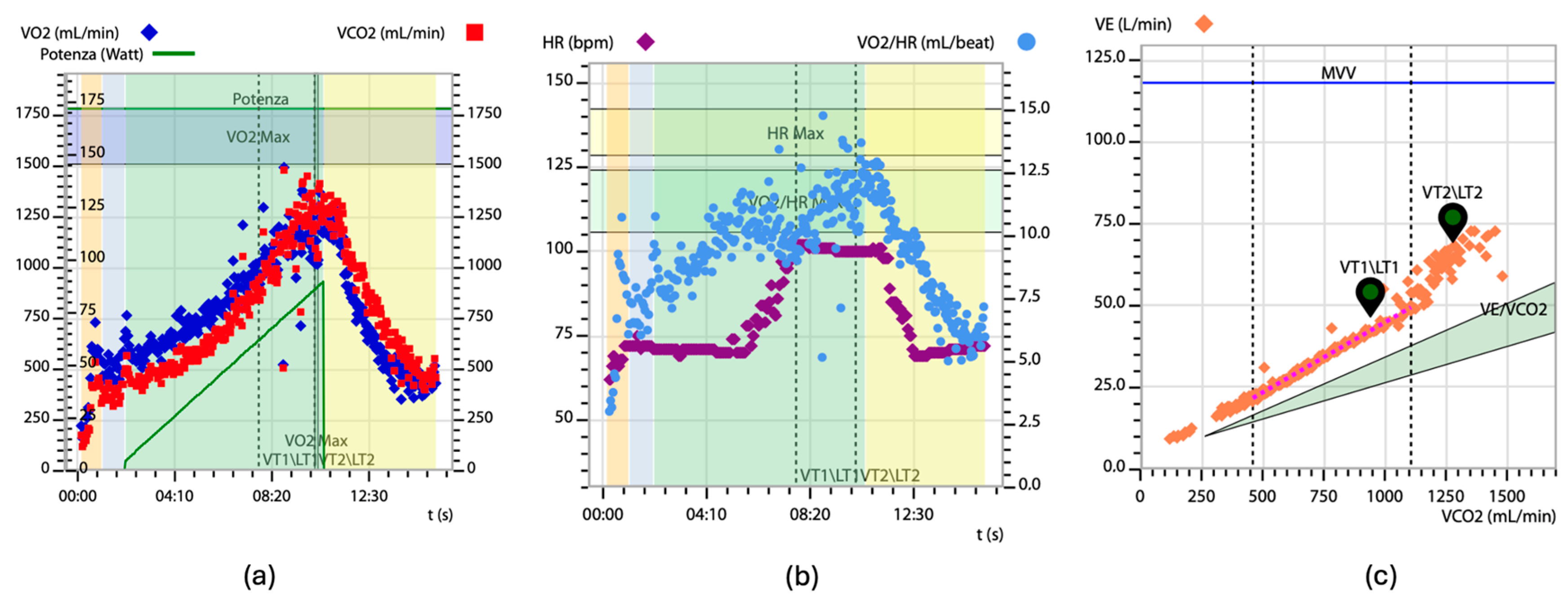
3. Cardiopulmonary Exercise Testing in Evaluating Heart Failure
4. Cardiopulmonary Exercise Testing in Cardiac Amyloidosis: Specific Features and Insights
5. Predictive Significance of VO2 Peak in Patients with Cardiac Amyloidosis
6. VE/VCO2 Slope and Its Prognostic Value in Patients with Cardiac Amyloidosis
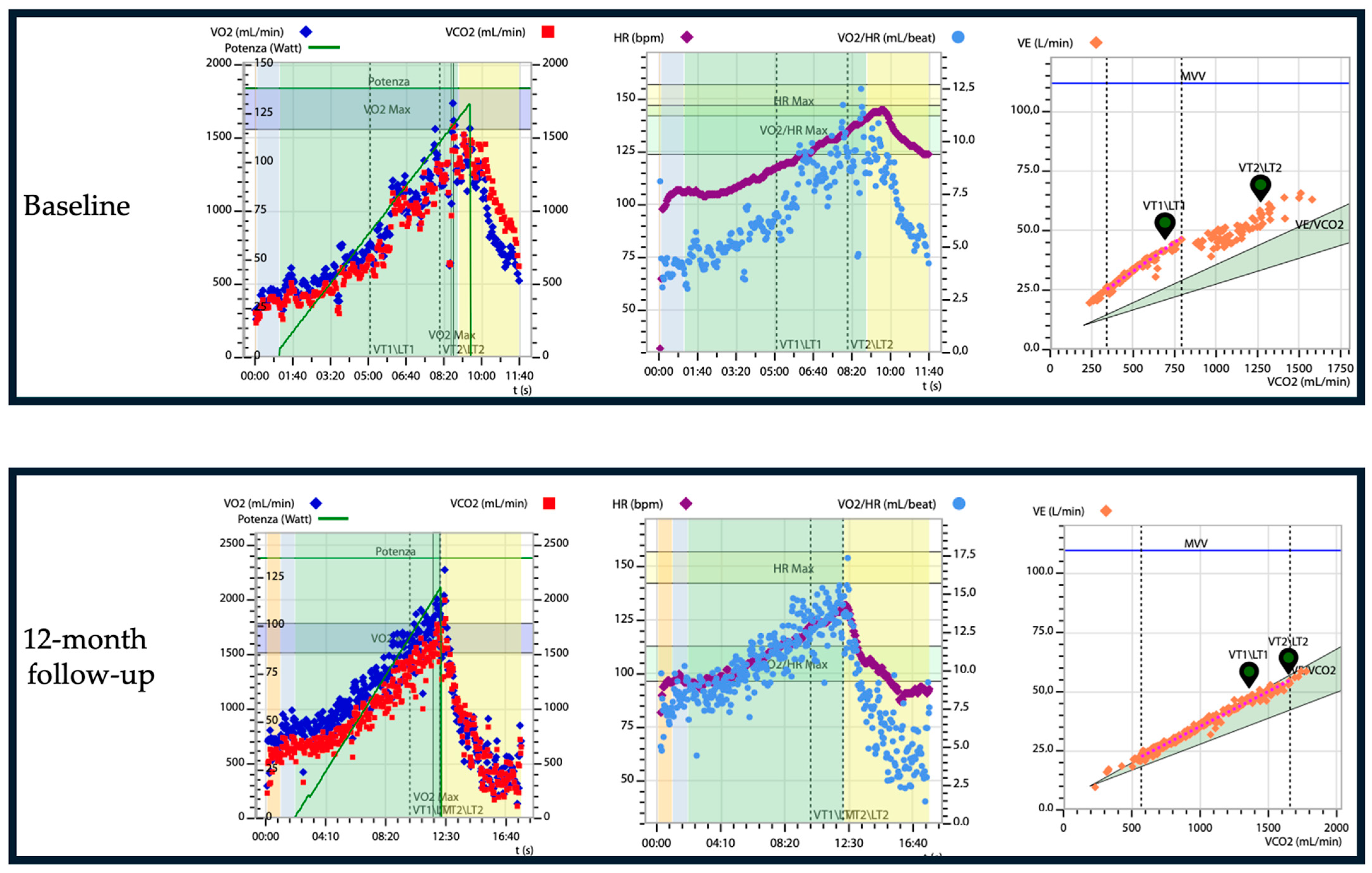
7. Impact of Cardiac Amyloidosis Treatment on Physical Performance: The Role of CPET Evaluation
8. Future Perspectives and Clinical Applications of CPET in Cardiac Amyloidosis
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saito, Y.; Nakamura, K.; Ito, H. Molecular Mechanisms of Cardiac Amyloidosis. Int. J. Mol. Sci. 2022, 23, 25. [Google Scholar] [CrossRef] [PubMed]
- Clerc, O.F.; Cuddy, S.A.M.; Jerosch-Herold, M.; Benz, D.C.; Katznelson, E.; Canseco Neri, J.; Taylor, A.; Kijewski, M.F.; Bianchi, G.; Ruberg, F.L.; et al. Myocardial Characteristics, Cardiac Structure, and Cardiac Function in Systemic Light-Chain Amyloidosis. JACC Cardiovasc. Imaging 2024, 17, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Oghina, S.; Bougouin, W.; Bézard, M.; Kharoubi, M.; Komajda, M.; Cohen-Solal, A.; Mebazaa, A.; Damy, T.; Bodez, D. The Impact of Patients with Cardiac Amyloidosis in HFpEF Trials. JACC Heart Fail. 2021, 9, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Skow, R.J.; Sarma, S.; MacNamara, J.P.; Bartlett, M.F.; Wakeham, D.J.; Martin, Z.T.; Samels, M.; Nandadeva, D.; Brazile, T.L.; Ren, J.; et al. Identifying the Mechanisms of a Peripherally Limited Exercise Phenotype in Patients with Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2024, 17, e011693. [Google Scholar] [CrossRef]
- Juarez, M.; Castillo-Rodriguez, C.; Soliman, D.; Del Rio-Pertuz, G.; Nugent, K. Cardiopulmonary Exercise Testing in Heart Failure. J. Cardiovasc. Dev. Dis. 2024, 11, 70. [Google Scholar] [CrossRef]
- Gargiulo, P.; Olla, S.; Boiti, C.; Contini, M.; Perrone-Filardi, P.; Agostoni, P. Predicted Values of Exercise Capacity in Heart Failure: Where We Are, Where to Go. Heart Fail. Rev. 2014, 19, 645–653. [Google Scholar] [CrossRef][Green Version]
- Di Bella, G.; Pizzino, F.; Minutoli, F.; Zito, C.; Donato, R.; Dattilo, G.; Oreto, G.; Baldari, S.; Vita, G.; Khandheria, B.K.; et al. The Mosaic of the Cardiac Amyloidosis Diagnosis: Role of Imaging in Subtypes and Stages of the Disease. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1307–1315. [Google Scholar] [CrossRef]
- Flodrova, P.; Flodr, P.; Pika, T.; Vymetal, J.; Holub, D.; Dzubak, P.; Hajduch, M.; Scudla, V. Cardiac Amyloidosis: From Clinical Suspicion to Morphological Diagnosis. Pathology 2018, 50, 261–268. [Google Scholar] [CrossRef]
- Vergaro, G.; Aimo, A.; Barison, A.; Genovesi, D.; Buda, G.; Passino, C.; Emdin, M. Keys to Early Diagnosis of Cardiac Amyloidosis: Red Flags from Clinical, Laboratory and Imaging Findings. Eur. J. Prev. Cardiol. 2020, 27, 1806–1815. [Google Scholar] [CrossRef]
- Chompoopong, P.; Mauermann, M.L.; Siddiqi, H.; Peltier, A. Amyloid Neuropathy: From Pathophysiology to Treatment in Light-Chain Amyloidosis and Hereditary Transthyretin Amyloidosis. Ann. Neurol. 2024, 96, 423–440. [Google Scholar] [CrossRef]
- Mazzeo, A.; Russo, M.; Di Bella, G.; Minutoli, F.; Stancanelli, C.; Gentile, L.; Baldari, S.; Carerj, S.; Toscano, A.; Vita, G. Transthyretin-Related Familial Amyloid Polyneuropathy (TTR-FAP): A Single-Center Experience in Sicily, an Italian Endemic Area. J. Neuromuscul. Dis. 2015, 2, S39–S48. [Google Scholar] [CrossRef] [PubMed]
- Monda, E.; Cirillo, C.; Verrillo, F.; Palmiero, G.; Falco, L.; Aimo, A.; Emdin, M.; Merlo, M.; Limongelli, G. Genotype-Phenotype Correlations in ATTR Amyloidosis: A Clinical Update. Heart Fail. Clin. 2024, 20, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Di Nunzio, D.; Recupero, A.; de Gregorio, C.; Zito, C.; Carerj, S.; Di Bella, G. Echocardiographic Findings in Cardiac Amyloidosis: Inside Two-Dimensional, Doppler, and Strain Imaging. Curr. Cardiol. Rep. 2019, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Alwan, L.; Benz, D.C.; Cuddy, S.A.M.; Dobner, S.; Shiri, I.; Caobelli, F.; Bernhard, B.; Stämpfli, S.F.; Eberli, F.; Reyes, M.; et al. Current and Evolving Multimodality Cardiac Imaging in Managing Transthyretin Amyloid Cardiomyopathy. JACC Cardiovasc. Imaging 2024, 17, 195–211. [Google Scholar] [CrossRef]
- Di Bella, G.; Pizzino, F. Myocardial Deformation Analysis and Late-Gadolinium Enhancement: Important Markers of Cardiac Amyloidosis Involvement That Can Masquerade as a False-Negative Diagnosis. Circ. J. 2018, 82, 2688. [Google Scholar] [CrossRef]
- Di Bella, G.; Pizzino, F.; Minutoli, F. Letter by Di Bella et al Regarding Article, “Effect of Combined Systolic and Diastolic Functional Parameter Assessment for Differentiation of Cardiac Amyloidosis from Other Causes of Concentric Left Ventricular Hypertrophy”. Circ. Cardiovasc. Imaging 2014, 7, 215. [Google Scholar] [CrossRef]
- Merlo, M.; Porcari, A.; Pagura, L.; Cameli, M.; Vergaro, G.; Musumeci, B.; Biagini, E.; Canepa, M.; Crotti, L.; Imazio, M.; et al. A National Survey on Prevalence of Possible Echocardiographic Red Flags of Amyloid Cardiomyopathy in Consecutive Patients Undergoing Routine Echocardiography: Study Design and Patients Characterization—The First Insight from the AC-TIVE Study. Eur. J. Prev. Cardiol. 2022, 29, e173–e177. [Google Scholar] [CrossRef]
- Trimarchi, G.; Carerj, S.; Di Bella, G.; Manganaro, R.; Pizzino, F.; Restelli, D.; Pelaggi, G.; Lofrumento, F.; Licordari, R.; Taverna, G.; et al. Clinical Applications of Myocardial Work in Echocardiography: A Comprehensive Review. J. Cardiovasc. Echogr. 2024, 34, 99–113. [Google Scholar] [CrossRef]
- de Gregorio, C.; Trimarchi, G.; Faro, D.C.; De Gaetano, F.; Campisi, M.; Losi, V.; Zito, C.; Tamburino, C.; Di Bella, G.; Monte, I.P. Myocardial Work Appraisal in Transthyretin Cardiac Amyloidosis and Nonobstructive Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2023, 208, 173–179. [Google Scholar] [CrossRef]
- de Gregorio, C.; Trimarchi, G.; Faro, D.C.; Poleggi, C.; Teresi, L.; De Gaetano, F.; Zito, C.; Lofrumento, F.; Koniari, I.; Licordari, R.; et al. Systemic Vascular Resistance and Myocardial Work Analysis in Hypertrophic Cardiomyopathy and Transthyretin Cardiac Amyloidosis with Preserved Left Ventricular Ejection Fraction. J. Clin. Med. 2024, 13, 1671. [Google Scholar] [CrossRef]
- Laptseva, N.; Rossi, V.A.; Sudano, I.; Schwotzer, R.; Ruschitzka, F.; Flammer, A.J.; Duru, F. Arrhythmic Manifestations of Cardiac Amyloidosis: Challenges in Risk Stratification and Clinical Management. J. Clin. Med. 2023, 12, 2581. [Google Scholar] [CrossRef] [PubMed]
- Mistrulli, R.; Ferrera, A.; Muthukkattil, M.L.; Battistoni, A.; Gallo, G.; Barbato, E.; Spera, F.R.; Magrì, D. Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy and Cardiac Amyloidosis: From Clinical Management to Catheter Ablation Indication. J. Clin. Med. 2024, 13, 501. [Google Scholar] [CrossRef]
- Di Bella, G.; Minutoli, F.; Piaggi, P.; Casale, M.; Mazzeo, A.; Zito, C.; Oreto, G.; Baldari, S.; Vita, G.; Pingitore, A.; et al. Usefulness of Combining Electrocardiographic and Echocardiographic Findings and Brain Natriuretic Peptide in Early Detection of Cardiac Amyloidosis in Subjects with Transthyretin Gene Mutation. Am. J. Cardiol. 2015, 116, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Licordari, R.; Trimarchi, G.; Teresi, L.; Restelli, D.; Lofrumento, F.; Perna, A.; Campisi, M.; de Gregorio, C.; Grimaldi, P.; Calabrò, D.; et al. Cardiac Magnetic Resonance in HCM Phenocopies: From Diagnosis to Risk Stratification and Therapeutic Management. J. Clin. Med. 2023, 12, 3481. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, V.; Aimo, A.; Todiere, G.; Barison, A.; Fabiani, I.; Panichella, G.; Genovesi, D.; Bonino, L.; Clemente, A.; Cademartiri, F.; et al. Role of Imaging in Cardiomyopathies. Card. Fail. Rev. 2023, 9, e08. [Google Scholar] [CrossRef]
- Aquaro, G.D.; De Gori, C.; Faggioni, L.; Parisella, M.L.; Cioni, D.; Lencioni, R.; Neri, E. Diagnostic and Prognostic Role of Late Gadolinium Enhancement in Cardiomyopathies. Eur. Heart J. Suppl. 2023, 25, C130–C136. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Piechnik, S.K.; Banypersad, S.M.; Fontana, M.; Ntusi, N.B.; Ferreira, V.M.; Whelan, C.J.; Myerson, S.G.; Robson, M.D.; Hawkins, P.N.; et al. Noncontrast T1 Mapping for the Diagnosis of Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2013, 6, 488–497. [Google Scholar] [CrossRef]
- Di Bella, G.; Pizzino, F.; Aquaro, G.D. A Negative LGE Is Inconclusive to Exclude an Early Cardiac Amyloidosis: It’s the Time for a T1 Mapping in Clinical Practice. Int. J. Cardiol. 2017, 247, 45. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhu, J.; Chen, M.; Zhu, M.; Dai, Y.; Hu, C. Prognostic Value of Native T1 and Extracellular Volume in Patients with Immunoglubin Light-Chain Amyloidosis. BMC Cardiovasc. Disord. 2024, 24, 112. [Google Scholar] [CrossRef]
- Wechalekar, A.D.; Fontana, M.; Quarta, C.C.; Liedtke, M. AL Amyloidosis for Cardiologists: Awareness, Diagnosis, and Future Prospects: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2022, 4, 427–441. [Google Scholar] [CrossRef]
- Morfino, P.; Aimo, A.; Giorgetti, A.; Genovesi, D.; Merlo, M.; Limongelli, G.; Castiglione, V.; Vergaro, G.; Emdin, M. The Role of Scintigraphy with Bone Radiotracers in Cardiac Amyloidosis. Heart Fail. Clin. 2024, 20, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Perugini, E.; Guidalotti, P.L.; Salvi, F.; Cooke, R.M.T.; Pettinato, C.; Riva, L.; Leone, O.; Farsad, M.; Ciliberti, P.; Bacchi-Reggiani, L.; et al. Noninvasive Etiologic Diagnosis of Cardiac Amyloidosis Using 99mTc-3,3-Diphosphono-1,2-Propanodicarboxylic Acid Scintigraphy. J. Am. Coll. Cardiol. 2005, 46, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Grigoratos, C.; Aimo, A.; Rapezzi, C.; Genovesi, D.; Barison, A.; Aquaro, G.D.; Vergaro, G.; Pucci, A.; Passino, C.; Marzullo, P.; et al. Diphosphonate Single-Photon Emission Computed Tomography in Cardiac Transthyretin Amyloidosis. Int. J. Cardiol. 2020, 307, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Musetti, V.; Greco, F.; Castiglione, V.; Aimo, A.; Palmieri, C.; Genovesi, D.; Giorgetti, A.; Emdin, M.; Vergaro, G.; McDonnell, L.A.; et al. Tissue Characterization in Cardiac Amyloidosis. Biomedicines 2022, 10, 3054. [Google Scholar] [CrossRef]
- Rognoni, P.; Mazzini, G.; Caminito, S.; Palladini, G.; Lavatelli, F. Dissecting the Molecular Features of Systemic Light Chain (AL) Amyloidosis: Contributions from Proteomics. Medicina 2021, 57, 916. [Google Scholar] [CrossRef]
- Palstrøm, N.B.; Rojek, A.M.; Møller, H.E.H.; Hansen, C.T.; Matthiesen, R.; Rasmussen, L.M.; Abildgaard, N.; Beck, H.C. Classification of Amyloidosis by Model-Assisted Mass Spectrometry-Based Proteomics. Int. J. Mol. Sci. 2022, 23, 319. [Google Scholar] [CrossRef]
- Lalario, A.; Saro, R.; Sinagra, G.; Merlo, M.; Porcari, A. Clinical Use of Biomarkers in Cardiac Amyloidosis. Heart Fail. Clin. 2024, 20, 283–294. [Google Scholar] [CrossRef]
- De Michieli, L.; Cipriani, A.; Iliceto, S.; Dispenzieri, A.; Jaffe, A.S. Cardiac Troponin in Patients with Light Chain and Transthyretin Cardiac Amyloidosis: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol 2024, 6, 1–15. [Google Scholar] [CrossRef]
- Kumar, S.K.; Gertz, M.A.; Lacy, M.Q.; Dingli, D.; Hayman, S.R.; Buadi, F.K.; Short-Detweiler, K.; Zeldenrust, S.R.; Leung, N.; Greipp, P.R.; et al. Recent Improvements in Survival in Primary Systemic Amyloidosis and the Importance of an Early Mortality Risk Score. Mayo Clin. Proc. 2011, 86, 12–18. [Google Scholar] [CrossRef]
- Lee Chuy, K.; Drill, E.; Yang, J.C.; Landau, H.; Hassoun, H.; Nahhas, O.; Chen, C.L.; Yu, A.F.; Steingart, R.M.; Liu, J.E. Incremental Value of Global Longitudinal Strain for Predicting Survival in Patients with Advanced AL Amyloidosis. JACC CardioOncol 2020, 2, 223–231. [Google Scholar] [CrossRef]
- Usuku, H.; Yamamoto, E.; Sueta, D.; Shinriki, R.; Oike, F.; Tabata, N.; Ishii, M.; Hanatani, S.; Hoshiyama, T.; Kanazawa, H.; et al. A New Staging System Using Right Atrial Strain in Patients with Immunoglobulin Light-Chain Cardiac Amyloidosis. ESC Heart Fail. 2024, 11, 1612–1624. [Google Scholar] [CrossRef] [PubMed]
- Monte, I.P.; Faro, D.C.; Trimarchi, G.; de Gaetano, F.; Campisi, M.; Losi, V.; Teresi, L.; Di Bella, G.; Tamburino, C.; de Gregorio, C. Left Atrial Strain Imaging by Speckle Tracking Echocardiography: The Supportive Diagnostic Value in Cardiac Amyloidosis and Hypertrophic Cardiomyopathy. J. Cardiovasc. Dev. Dis. 2023, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Wang, Z.; Huang, J.; Fan, F.; Yang, F.; Qiu, L.; Zhao, K.; Qiu, J.; Yang, Y.; Ma, W.; et al. Early Diagnostic and Prognostic Value of Myocardial Strain Derived from Cardiovascular Magnetic Resonance in Patients with Cardiac Amyloidosis. Cardiovasc. Diagn. Ther. 2023, 13, 979–993. [Google Scholar] [CrossRef]
- Boretto, P.; Patel, N.H.; Patel, K.; Rana, M.; Saglietto, A.; Soni, M.; Ahmad, M.; Sin Ying Ho, J.; De Filippo, O.; Providencia, R.A.; et al. Prognosis Prediction in Cardiac Amyloidosis by Cardiac Magnetic Resonance Imaging: A Systematic Review with Meta-Analysis. Eur. Heart J. Open 2023, 3, oead092. [Google Scholar] [CrossRef]
- Fuentes-Abolafio, I.J.; Stubbs, B.; Pérez-Belmonte, L.M.; Bernal-López, M.R.; Gómez-Huelgas, R.; Cuesta-Vargas, A.I. Physical Functional Performance and Prognosis in Patients with Heart Failure: A Systematic Review and Meta-Analysis. BMC Cardiovasc. Disord. 2020, 20, 512. [Google Scholar] [CrossRef]
- Mapelli, M.; Salvioni, E.; Mattavelli, I.; Vignati, C.; Galotta, A.; Magrì, D.; Apostolo, A.; Sciomer, S.; Campodonico, J.; Agostoni, P. Cardiopulmonary Exercise Testing and Heart Failure: A Tale Born from Oxygen Uptake. Eur. Heart J. Suppl. 2023, 25, C319–C325. [Google Scholar] [CrossRef]
- Malhotra, R.; Bakken, K.; D’Elia, E.; Lewis, G.D. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016, 4, 607–616. [Google Scholar] [CrossRef]
- Schwinger, R.H.G. Pathophysiology of Heart Failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef]
- Nadruz, W.; West, E.; Sengeløv, M.; Santos, M.; Groarke, J.D.; Forman, D.E.; Claggett, B.; Skali, H.; Shah, A.M. Prognostic Value of Cardiopulmonary Exercise Testing in Heart Failure with Reduced, Midrange, and Preserved Ejection Fraction. J. Am. Heart Assoc. 2017, 6, e006000. [Google Scholar] [CrossRef]
- O’Neill, J.O.; Young, J.B.; Pothier, C.E.; Lauer, M.S. Peak Oxygen Consumption as a Predictor of Death in Patients with Heart Failure Receiving Beta-Blockers. Circulation 2005, 111, 2313–2318. [Google Scholar] [CrossRef]
- Verwerft, J.; Soens, L.; Wynants, J.; Meysman, M.; Jogani, S.; Plein, D.; Stroobants, S.; Herbots, L.; Verbrugge, F.H. Heart Failure with Preserved Ejection Fraction: Relevance of a Dedicated Dyspnoea Clinic. Eur. Heart J. 2023, 44, 1544–1556. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, A.; Burns, P.; Correia, J.; Jamieson, P.; Moxon, P.; Purvis, J.; Thomas, M.; Tighe, H.; Sylvester, K.P. ARTP Statement on Cardiopulmonary Exercise Testing 2021. BMJ Open Respir Res 2021, 8, e001121. [Google Scholar] [CrossRef] [PubMed]
- Balady, G.J.; Arena, R.; Sietsema, K.; Myers, J.; Coke, L.; Fletcher, G.F.; Forman, D.; Franklin, B.; Guazzi, M.; Gulati, M.; et al. Clinician’s Guide to Cardiopulmonary Exercise Testing in Adults: A Scientific Statement from the American Heart Association. Circulation 2010, 122, 191–225. [Google Scholar] [CrossRef] [PubMed]
- Keteyian, S.J.; Patel, M.; Kraus, W.E.; Brawner, C.A.; McConnell, T.R.; Piña, I.L.; Leifer, E.S.; Fleg, J.L.; Blackburn, G.; Fonarow, G.C.; et al. Variables Measured During Cardiopulmonary Exercise Testing as Predictors of Mortality in Chronic Systolic Heart Failure. J. Am. Coll. Cardiol. 2016, 67, 780–789. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Olson, T.P.; Obokata, M.; Melenovsky, V.; Borlaug, B.A. Hemodynamic Correlates and Diagnostic Role of Cardiopulmonary Exercise Testing in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2018, 6, 665–675. [Google Scholar] [CrossRef]
- Nayor, M.; Houstis, N.E.; Namasivayam, M.; Rouvina, J.; Hardin, C.; Shah, R.V.; Ho, J.E.; Malhotra, R.; Lewis, G.D. Impaired Exercise Tolerance in Heart Failure with Preserved Ejection Fraction: Quantification of Multiorgan System Reserve Capacity. JACC Heart Fail. 2020, 8, 605–617. [Google Scholar] [CrossRef]
- Mueller, S.; Haller, B.; Feuerstein, A.; Winzer, E.B.; Beckers, P.; Haykowsky, M.J.; Gevaert, A.B.; Hommel, J.; Azevedo, L.F.; Duvinage, A.; et al. Peak O2-Pulse Predicts Exercise Training-Induced Changes in Peak VO2 in Heart Failure with Preserved Ejection Fraction. ESC Heart Fail. 2022, 9, 3393–3406. [Google Scholar] [CrossRef]
- Radtke, T.; Crook, S.; Kaltsakas, G.; Louvaris, Z.; Berton, D.; Urquhart, D.S.; Kampouras, A.; Rabinovich, R.A.; Verges, S.; Kontopidis, D.; et al. ERS Statement on Standardisation of Cardiopulmonary Exercise Testing in Chronic Lung Diseases. Eur. Respir. Rev. 2019, 28, 180101. [Google Scholar] [CrossRef]
- Battaglini, D.; Al-Husinat, L.; Normando, A.G.; Leme, A.P.; Franchini, K.; Morales, M.; Pelosi, P.; Rocco, P.R. Personalized Medicine Using Omics Approaches in Acute Respiratory Distress Syndrome to Identify Biological Phenotypes. Respir. Res. 2022, 23, 318. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Glaab, T.; Taube, C. Practical Guide to Cardiopulmonary Exercise Testing in Adults. Respir. Res. 2022, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society. American College of Chest Physicians ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Del Punta, L.; De Biase, N.; Armenia, S.; Di Fiore, V.; Maremmani, D.; Gargani, L.; Mazzola, M.; De Carlo, M.; Mengozzi, A.; Lomonaco, T.; et al. Combining Cardiopulmonary Exercise Testing with Echocardiography: A Multiparametric Approach to the Cardiovascular and Cardiopulmonary Systems. Eur. Heart J.-Imaging Methods Pract. 2023, 1, qyad021. [Google Scholar] [CrossRef] [PubMed]
- Fujii, B.; Matsuda, Y.; Ohno, H.; Hamada, Y.; Takashiba, K.; Ebihara, H.; Hyakuna, E.; Tani, S. A Case of Cardiac Amyloidosis Presenting with Symptoms of Exertional Syncope. Clin. Cardiol. 1991, 14, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Hein, S.; Aus Dem Siepen, F.; Bauer, R.; Katus, H.A.; Kristen, A.V. Peak V’O2 Is an Independent Predictor of Survival in Patients with Cardiac Amyloidosis. Amyloid 2018, 25, 167–173. [Google Scholar] [CrossRef]
- Clemmensen, T.S.; Soerensen, J.; Hansson, N.H.; Tolbod, L.P.; Harms, H.J.; Eiskjær, H.; Mikkelsen, F.; Wiggers, H.; Andersen, N.F.; Poulsen, S.H. Myocardial Oxygen Consumption and Efficiency in Patients with Cardiac Amyloidosis. J. Am. Heart Assoc. 2018, 7, e009974. [Google Scholar] [CrossRef]
- Trikas, A.; Rallidis, L.; Hawkins, P.; Oakley, C.M.; Nihoyannopoulos, P. Comparison of Usefulness between Exercise Capacity and Echocardiographic Indexes of Left Ventricular Function in Cardiac Amyloidosis. Am. J. Cardiol. 1999, 84, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, D.; Pan, S.; Latif, F.; Goldsmith, R.L.; Saith, S.E.; Mapara, M.Y.; Chakraborty, R.; Lentzsch, S.; Maurer, M.S. Cardiopulmonary Exercise Testing in Patients with Cardiac Amyloidosis. Clin. Lymphoma Myeloma Leuk. 2021, 21, 545–548. [Google Scholar] [CrossRef]
- Nativi-Nicolau, J.; Stehlik, J.; Al-Dulaimi, R.; Rodriguez, C.; Jaramillo, J.; Conte, J.; Kovacsovics, T.; Cowley, J.; Abraham, J.; Barrel, K.; et al. Chronotropic Incompetence and Autonomic Dysfunction as Mechanisms of Dyspnoea in Patients with Late Stage Cardiac Amyloidosis. Amyloid 2019, 26, 134–135. [Google Scholar] [CrossRef]
- Nicol, M.; Deney, A.; Lairez, O.; Vergaro, G.; Emdin, M.; Carecci, A.; Inamo, J.; Montfort, A.; Neviere, R.; Damy, T.; et al. Prognostic Value of Cardiopulmonary Exercise Testing in Cardiac Amyloidosis. Eur. J. Heart Fail. 2021, 23, 231–239. [Google Scholar] [CrossRef]
- Banydeen, R.; Monfort, A.; Inamo, J.; Neviere, R. Diagnostic and Prognostic Values of Cardiopulmonary Exercise Testing in Cardiac Amyloidosis. Front. Cardiovasc. Med. 2022, 9, 898033. [Google Scholar] [CrossRef] [PubMed]
- Monfort, A.; Thevenet, E.; Lacavalerie, M.R.; Banydeen, R.; Inamo, J.; Neviere, R. Determinants of Ventilatory Inefficiency in Transthyretin Cardiac Amyloidosis: The Role of Excessive Ventilatory Drive. Front. Physiol. 2022, 13, 1002238. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, A.; Fumagalli, C.; Razvi, Y.; Porcari, A.; Rauf, M.U.; Martinez-Naharro, A.; Venneri, L.; Moody, W.; Steeds, R.P.; Petrie, A.; et al. Prognostic Value of a 6-Minute Walk Test in Patients with Transthyretin Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2024, 84, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Vita, G.L.; Stancanelli, C.; Gentile, L.; Barcellona, C.; Russo, M.; Di Bella, G.; Vita, G.; Mazzeo, A. 6MWT Performance Correlates with Peripheral Neuropathy but Not with Cardiac Involvement in Patients with Hereditary Transthyretin Amyloidosis (hATTR). Neuromuscul. Disord. 2019, 29, 213–220. [Google Scholar] [CrossRef]
- Luks, A.M.; Glenny, R.W.; Robertson, H.T. Interpreting the Results of Cardiopulmonary Exercise Tests. In Introduction to Cardiopulmonary Exercise Testing; Luks, A.M., Glenny, R.W., Robertson, H.T., Eds.; Springer: New York, NY, USA, 2013; pp. 53–75. ISBN 978-1-4614-6283-5. [Google Scholar]
- Monfort, A.; Banydeen, R.; Demoniere, F.; Courty, B.; Codiat, R.; Neviere, R.; Inamo, J. Restrictive Cardiac Phenotype as Primary Cause of Impaired Aerobic Capacity in Afro-Caribbean Patients with Val122ile Variant Transthyretin Amyloid Cardiomyopathy. Amyloid 2020, 27, 145–152. [Google Scholar] [CrossRef]
- Clemmensen, T.S.; Mølgaard, H.; Sörensen, J.; Eiskjaer, H.; Andersen, N.F.; Mellemkjaer, S.; Andersen, M.J.; Tolbod, L.P.; Harms, H.J.; Poulsen, S.H. Inotropic Myocardial Reserve Deficiency Is the Predominant Feature of Exercise Haemodynamics in Cardiac Amyloidosis. Eur. J. Heart Fail. 2017, 19, 1457–1465. [Google Scholar] [CrossRef]
- Bhuiyan, T.; Helmke, S.; Patel, A.R.; Ruberg, F.L.; Packman, J.; Cheung, K.; Grogan, D.; Maurer, M.S. Pressure-Volume Relationships in Patients with Transthyretin (ATTR) Cardiac Amyloidosis Secondary to V122I Mutations and Wild-Type Transthyretin: Transthyretin Cardiac Amyloid Study (TRACS). Circ. Heart Fail. 2011, 4, 121–128. [Google Scholar] [CrossRef]
- Argirò, A.; Silverii, M.V.; Burgisser, C.; Fattirolli, F.; Baldasseroni, S.; di Mario, C.; Zampieri, M.; Biagioni, G.; Mazzoni, C.; Chiti, C.; et al. Serial Changes in Cardiopulmonary Exercise Testing Parameters in Untreated Patients with Transthyretin Cardiac Amyloidosis. Can. J. Cardiol. 2024, 40, 364–369. [Google Scholar] [CrossRef]
- Badr Eslam, R.; Öztürk, B.; Rettl, R.; Capelle, C.D.J.; Qin, H.; Binder, C.; Dachs, T.-M.; Camuz Ligios, L.; Duca, F.; Dalos, D.; et al. Impact of Tafamidis and Optimal Background Treatment on Physical Performance in Patients with Transthyretin Amyloid Cardiomyopathy. Circ. Heart Fail. 2022, 15, e008381. [Google Scholar] [CrossRef]
- Maurer, M.S.; Kale, P.; Fontana, M.; Berk, J.L.; Grogan, M.; Gustafsson, F.; Hung, R.R.; Gottlieb, R.L.; Damy, T.; González-Duarte, A.; et al. Patisiran Treatment in Patients with Transthyretin Cardiac Amyloidosis. N. Engl. J. Med. 2023, 389, 1553–1565. [Google Scholar] [CrossRef]
- Wernhart, S.; Michel, L.; Carpinteiro, A.; Luedike, P.; Rassaf, T. (Non)-Exertional Variables of Cardiopulmonary Exercise Testing in Heart Failure with and Without Cardiac Amyloidosis. Curr. Heart Fail. Rep. 2024, 21, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Briasoulis, A.; Theodorakakou, F.; Rempakos, A.; Petropoulos, I.; Gavriatopoulou, M.; Androulakis, E.; Stamatelopoulos, K.; Kallianos, A.; Trakada, G.; Dimopoulos, M.A.; et al. Cardiopulmonary Exercise Physiology in AL Amyloidosis Patients with Cardiac Involvement and Its Association with Cardiac Imaging Parameters. JCM 2022, 11, 5437. [Google Scholar] [CrossRef] [PubMed]
- Cantone, A.; Serenelli, M.; Sanguettoli, F.; Maio, D.; Fabbri, G.; Dal Passo, B.; Agostoni, P.; Grazzi, G.; Campo, G.; Rapezzi, C. Cardiopulmonary Exercise Testing Predicts Prognosis in Amyloid Cardiomyopathy: A Systematic Review and Meta-analysis. ESC Heart Fail. 2023, 10, 2740–2744. [Google Scholar] [CrossRef] [PubMed]
- Poggio, R.; Arazi, H.C.; Giorgi, M.; Miriuka, S.G. Prediction of Severe Cardiovascular Events by VE/Vco2 Slope versus Peak Vo2 in Systolic Heart Failure: A Meta-Analysis of the Published Literature. Am. Heart J. 2010, 160, 1004–1014. [Google Scholar] [CrossRef]
- Weatherald, J.; Sattler, C.; Garcia, G.; Laveneziana, P. Ventilatory Response to Exercise in Cardiopulmonary Disease: The Role of Chemosensitivity and Dead Space. Eur. Respir. J. 2018, 51, 1700860. [Google Scholar] [CrossRef]
- Yunis, A.; Doros, G.; Luptak, I.; Connors, L.H.; Sam, F. Use of Ventilatory Efficiency Slope as a Marker for Increased Mortality in Wild-Type Transthyretin Cardiac Amyloidosis. Am. J. Cardiol. 2019, 124, 122–130. [Google Scholar] [CrossRef]
- Olson, T.P.; Beck, K.C.; Johnson, B.D. Pulmonary Function Changes Associated with Cardiomegaly in Chronic Heart Failure. J. Card. Fail. 2007, 13, 100–107. [Google Scholar] [CrossRef]
- Kawakami, R.; Nakada, Y.; Hashimoto, Y.; Ueda, T.; Nakagawa, H.; Nishida, T.; Onoue, K.; Soeda, T.; Watanabe, M.; Saito, Y. Prevalence and Prognostic Significance of Pulmonary Function Test Abnormalities in Hospitalized Patients with Acute Decompensated Heart Failure with Preserved and Reduced Ejection Fraction. Circ. J. 2021, 85, 1426–1434. [Google Scholar] [CrossRef]
- Van Iterson, E.H.; Cho, L.; Tonelli, A.; Finet, J.E.; Laffin, L.J. All-cause Mortality Predicted by Peak Oxygen Uptake Differs Depending on Spirometry Pattern in Patients with Heart Failure and Reduced Ejection Fraction. ESC Heart Fail. 2021, 8, 2731–2740. [Google Scholar] [CrossRef]
- Banydeen, R.; Eggleston, R.; Deney, A.; Monfort, A.; Ryu, J.H.; Vergaro, G.; Castiglione, V.; Lairez, O.; Emdin, M.; Inamo, J.; et al. Risk Stratification in Transthyretin Cardiac Amyloidosis: The Added Value of Lung Spirometry. J. Clin. Med. 2023, 12, 3684. [Google Scholar] [CrossRef]
- Ando, Y.; Adams, D.; Benson, M.D.; Berk, J.L.; Planté-Bordeneuve, V.; Coelho, T.; Conceição, I.; Ericzon, B.-G.; Obici, L.; Rapezzi, C.; et al. Guidelines and New Directions in the Therapy and Monitoring of ATTRv Amyloidosis. Amyloid 2022, 29, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.; Carpinteiro, A.; Luedike, P.; Buehning, F.; Wernhart, S.; Rassaf, T.; Michel, L. Current Therapies and Future Horizons in Cardiac Amyloidosis Treatment. Curr. Heart Fail. Rep. 2024, 21, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.M.; Ruberg, F.L.; Ambardekar, A.V.; Brannagan, T.H.; Cheng, R.K.; Clarke, J.O.; Dember, L.M.; Frantz, J.G.; Hershberger, R.E.; Maurer, M.S.; et al. 2023 ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient with Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2023, 81, 1076–1126. [Google Scholar] [CrossRef]
- Tomasoni, D.; Bonfioli, G.B.; Aimo, A.; Adamo, M.; Canepa, M.; Inciardi, R.M.; Lombardi, C.M.; Nardi, M.; Pagnesi, M.; Riccardi, M.; et al. Treating Amyloid Transthyretin Cardiomyopathy: Lessons Learned from Clinical Trials. Front. Cardiovasc. Med. 2023, 10, 1154594. [Google Scholar] [CrossRef] [PubMed]
- Stelmach-Gołdyś, A.; Zaborek-Łyczba, M.; Łyczba, J.; Garus, B.; Pasiarski, M.; Mertowska, P.; Małkowska, P.; Hrynkiewicz, R.; Niedźwiedzka-Rystwej, P.; Grywalska, E. Physiology, Diagnosis and Treatment of Cardiac Light Chain Amyloidosis. JCM 2022, 11, 911. [Google Scholar] [CrossRef] [PubMed]
- Vaxman, J.; Dispenzieri, A. The Role of Autologous Stem Cell Transplantation in Amyloidosis. Oncology 2021, 35, 471–478. [Google Scholar] [CrossRef]
- Kastritis, E.; Palladini, G.; Minnema, M.C.; Wechalekar, A.D.; Jaccard, A.; Lee, H.C.; Sanchorawala, V.; Gibbs, S.; Mollee, P.; Venner, C.P.; et al. Daratumumab-Based Treatment for Immunoglobulin Light-Chain Amyloidosis. N. Engl. J. Med. 2021, 385, 46–58. [Google Scholar] [CrossRef]
- Dima, D.; Mazzoni, S.; Anwer, F.; Khouri, J.; Samaras, C.; Valent, J.; Williams, L. Diagnostic and Treatment Strategies for AL Amyloidosis in an Era of Therapeutic Innovation. JCO Oncol. Pract. 2023, 19, 265–275. [Google Scholar] [CrossRef]
- Sidana, S.; Milani, P.; Binder, M.; Basset, M.; Tandon, N.; Foli, A.; Dispenzieri, A.; Gertz, M.A.; Hayman, S.R.; Buadi, F.K.; et al. A Validated Composite Organ and Hematologic Response Model for Early Assessment of Treatment Outcomes in Light Chain Amyloidosis. Blood Cancer J. 2020, 10, 41. [Google Scholar] [CrossRef]
- Rettl, R.; Mann, C.; Duca, F.; Dachs, T.-M.; Binder, C.; Ligios, L.C.; Schrutka, L.; Dalos, D.; Koschutnik, M.; Donà, C.; et al. Tafamidis Treatment Delays Structural and Functional Changes of the Left Ventricle in Patients with Transthyretin Amyloid Cardiomyopathy. Eur. Heart J.-Cardiovasc. Imaging 2022, 23, 767–780. [Google Scholar] [CrossRef]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.D.; Waddington-Cruz, M.; Berk, J.L.; Polydefkis, M.; Dyck, P.J.; Wang, A.K.; Planté-Bordeneuve, V.; Barroso, F.A.; Merlini, G.; Obici, L.; et al. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Adams, D.; Kristen, A.; Grogan, M.; González-Duarte, A.; Maurer, M.S.; Merlini, G.; Damy, T.; Slama, M.S.; Brannagan, T.H.; et al. Effects of Patisiran, an RNA Interference Therapeutic, on Cardiac Parameters in Patients with Hereditary Transthyretin-Mediated Amyloidosis: Analysis of the APOLLO Study. Circulation 2019, 139, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Coelho, T.; Marques, W.; Dasgupta, N.R.; Chao, C.-C.; Parman, Y.; França, M.C.; Guo, Y.-C.; Wixner, J.; Ro, L.-S.; Calandra, C.R.; et al. Eplontersen for Hereditary Transthyretin Amyloidosis with Polyneuropathy. JAMA 2023, 330, 1448. [Google Scholar] [CrossRef]
- Dalia, T.; Acharya, P.; Chan, W.-C.; Sauer, A.J.; Weidling, R.; Fritzlen, J.; Goyal, A.; Miller, D.; Knipper, E.; Porter, C.B.; et al. Prognostic Role of Cardiopulmonary Exercise Testing in Wild-Type Transthyretin Amyloid Cardiomyopathy Patients Treated with Tafamidis. J. Card. Fail. 2021, 27, 1285–1289. [Google Scholar] [CrossRef]
- Nakaya, Y.; Ogimoto, A.; Kitaoka, H. Changes in Exercise Tolerance over Time in Patients with Transthyretin Amyloidosis Cardiomyopathy Treated with Tafamidis: A Preliminary Study. Int. Heart J. 2023, 64, 647–653. [Google Scholar] [CrossRef]
- Porcari, A.; Cappelli, F.; Nitsche, C.; Tomasoni, D.; Sinigiani, G.; Longhi, S.; Bordignon, L.; Masri, A.; Serenelli, M.; Urey, M.; et al. SGLT2 Inhibitor Therapy in Patients with Transthyretin Amyloid Cardiomyopathy. J. Am. Coll. Cardiol. 2024, 83, 2411–2422. [Google Scholar] [CrossRef]
- Patel, R.K.; Bandera, F.; Venneri, L.; Porcari, A.; Razvi, Y.; Ioannou, A.; Chacko, L.; Martinez-Naharro, A.; Rauf, M.U.; Knight, D.; et al. Cardiopulmonary Exercise Testing in Evaluating Transthyretin Amyloidosis. JAMA Cardiol. 2024, 9, 367–376. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugliatti, P.; Trimarchi, G.; Barocelli, F.; Pizzino, F.; Di Spigno, F.; Tedeschi, A.; Piccione, M.C.; Irrera, P.; Aschieri, D.; Niccoli, G.; et al. Advancing Cardiac Amyloidosis Care Through Insights from Cardiopulmonary Exercise Testing. J. Clin. Med. 2024, 13, 7285. https://doi.org/10.3390/jcm13237285
Pugliatti P, Trimarchi G, Barocelli F, Pizzino F, Di Spigno F, Tedeschi A, Piccione MC, Irrera P, Aschieri D, Niccoli G, et al. Advancing Cardiac Amyloidosis Care Through Insights from Cardiopulmonary Exercise Testing. Journal of Clinical Medicine. 2024; 13(23):7285. https://doi.org/10.3390/jcm13237285
Chicago/Turabian StylePugliatti, Pietro, Giancarlo Trimarchi, Federico Barocelli, Fausto Pizzino, Francesco Di Spigno, Andrea Tedeschi, Maurizio Cusmà Piccione, Pierangela Irrera, Daniela Aschieri, Giampaolo Niccoli, and et al. 2024. "Advancing Cardiac Amyloidosis Care Through Insights from Cardiopulmonary Exercise Testing" Journal of Clinical Medicine 13, no. 23: 7285. https://doi.org/10.3390/jcm13237285
APA StylePugliatti, P., Trimarchi, G., Barocelli, F., Pizzino, F., Di Spigno, F., Tedeschi, A., Piccione, M. C., Irrera, P., Aschieri, D., Niccoli, G., Paradossi, U., & Di Bella, G. (2024). Advancing Cardiac Amyloidosis Care Through Insights from Cardiopulmonary Exercise Testing. Journal of Clinical Medicine, 13(23), 7285. https://doi.org/10.3390/jcm13237285









