Fibrinogen and Prothrombin Complex Concentrate: The Importance of the Temporal Sequence—A Post-Hoc Analysis of Two Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Population
2.3. Data Collection
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Despotis, G.; Avidan, M.; Eby, C. Prediction and management of bleeding in cardiac surgery. J. Thromb. Haemost. 2009, 7 (Suppl. S1), 111–117. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M.; Ashikhmina, E.; Brinkman, N.J.; Barbara, D.W. Perioperative Use of Coagulation Factor Concentrates in Patients Undergoing Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Natorska, J.; Mazur, P.; Undas, A. Increased bleeding risk in patients with aortic valvular stenosis: From new mechanisms to new therapies. Thromb. Res. 2016, 139, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S. State of the art—How I manage coagulopathy in cardiac surgery patients. Br. J. Haematol. 2014, 164, 779–789. [Google Scholar] [CrossRef]
- Inada, E. Blood coagulation and autologous blood transfusion in cardiac surgery. J. Clin. Anesth. 1990, 2, 393–406. [Google Scholar] [CrossRef]
- Agarwal, S.; Abdelmotieleb, M. Viscoelastic testing in cardiac surgery. Transfusion 2020, 60 (Suppl. S6), S52–S60. [Google Scholar] [CrossRef]
- Sodha, N.R.; Boodhwani, M.; Bianchi, C.; Ramlawi, B.; Sellke, F.W. Aprotinin in cardiac surgery. Expert Rev. Cardiovasc. Ther. 2006, 4, 151–160. [Google Scholar] [CrossRef]
- Casselman, F.P.A.; Lance, M.D.; Ahmed, A.; Ascari, A.; Blanco-Morillo, J.; Bolliger, D.; Eid, M.; Erdoes, G.; Gerhardus Haumann, R.; Jeppsson, A.; et al. 2024 EACTS/EACTAIC Guidelines on patient blood management in adult cardiac surgery in collaboration with EBCP. Eur. J. Cardiothorac. Surg. 2024, ezae352. [Google Scholar] [CrossRef]
- Ranucci, M.; Baryshnikova, E.; Crapelli, G.B.; Rahe-Meyer, N.; Menicanti, L.; Frigiola, A.; Surgical Clinical Outcome REsearch (SCORE) Group. Randomized, double-blinded, placebo-controlled trial of fibrinogen concentrate supplementation after complex cardiac surgery. J. Am. Heart Assoc. 2015, 4, e002066. [Google Scholar] [CrossRef]
- Weber, C.F.; Görlinger, K.; Meininger, D.; Herrmann, E.; Bingold, T.; Moritz, A.; Cohn, L.H.; Zacharowski, K. Point-of-care testing: A prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology 2012, 117, 531–547. [Google Scholar] [CrossRef]
- Monaco, F.; Nardelli, P.; Denaro, G.; De Luca, M.; Franco, A.; Bertoglio, L.; Castiglioni, A.; Zangrillo, A. First experience with a ROTEM-enhanced transfusion algorithm in patients undergoing aortic arch replacement with frozen elephant trunk technique. A theranostic approach to patient blood management. J. Clin. Anesth. 2020, 66, 109910. [Google Scholar] [CrossRef] [PubMed]
- Karkouti, K.; Callum, J.; Wijeysundera, D.N.; Rao, V.; Crowther, M.; Grocott, H.P.; Pinto, R.; Scales, D.C.; TACS Investigators. Point-of-Care Hemostatic Testing in Cardiac Surgery: A Stepped-Wedge Clustered Randomized Controlled Trial. Circulation 2016, 134, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.J.A.J.M.; van Egmond, L.T.; Henskens, Y.M.C.; Roekaerts, P.M.; Maessen, J.G.; Ten Cate, H.; Buhre, W.F.; Lancé, M.D. Shifts of Transfusion Demand in Cardiac Surgery After Implementation of Rotational Thromboelastometry-Guided Transfusion Protocols: Analysis of the HEROES-CS (HEmostasis Registry of patiEntS in Cardiac Surgery) Observational, Prospective Open Cohort Database. J. Cardiothorac. Vasc. Anesth. 2019, 33, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Scolletta, S.; Simioni, P.; Campagnolo, V.; Celiento, M.; Fontanari, P.; Guadagnucci, A.; Guarracino, F.; Haxhiademi, D.; Paniccia, R.; Simeone, F.; et al. Patient blood management in cardiac surgery: The “Granducato algorithm”. Int. J. Cardiol. 2019, 289, 37–42. [Google Scholar] [CrossRef]
- Auci, E.; Vetrugno, L.; Riccardi, I.; Brussa, A.; Orso, D.; Baroselli, A.; Gigante, A.; Cecotti, R.; Bassi, F.; Livi, U.; et al. Multiple Electrode Aggregometry After Cardiopulmonary Bypass to Assess Platelet (Dys)-Function and Transfusion Threshold: A Concordance Study. J. Cardiothorac. Vasc. Anesth. 2020, 34, 3306–3313. [Google Scholar] [CrossRef]
- Karkouti, K.; McCluskey, S.A.; Callum, J.; Freedman, J.; Selby, R.; Timoumi, T.; Roy, D.; Rao, V. Evaluation of a novel transfusion algorithm employing point-of-care coagulation assays in cardiac surgery: A retrospective cohort study with interrupted time-series analysis. Anesthesiology 2015, 122, 560–570. [Google Scholar] [CrossRef]
- Raphael, J.; Mazer, C.D.; Subramani, S.; Schroeder, A.; Abdalla, M.; Ferreira, R.; Roman, P.E.; Patel, N.; Welsby, I.; Greilich, P.E.; et al. Society of Cardiovascular Anesthesiologists Clinical Practice Improvement Advisory for Management of Perioperative Bleeding and Hemostasis in Cardiac Surgery Patients. Anesth. Analg. 2019, 129, 1209–1221. [Google Scholar] [CrossRef]
- Baryshnikova, E.; Aloisio, T.; Di Dedda, U.; Anguissola, M.; Barbaria, A.; Caravella, G.; Ranucci, M. A Randomized Controlled Trial Comparing Effectiveness of Different Fibrinogen Preparations in Restoring Clot Firmness. Anesth. Analg. 2024. [Google Scholar] [CrossRef]
- Kim, S.M.; Sohn, C.H.; Kwon, H.; Ryoo, S.M.; Ahn, S.; Seo, D.W.; Kim, W.Y. Thromboelastography as an early prediction method for hypofibrinogenemia in emergency department patients with primary postpartum hemorrhage. Scand. J. Trauma Resusc. Emerg. Med. 2024, 32, 85. [Google Scholar] [CrossRef]
- Grottke, O.; Mallaiah, S.; Karkouti, K.; Saner, F.; Haas, T. Fibrinogen Supplementation and Its Indications. Semin. Thromb. Hemost. 2020, 46, 38–49. [Google Scholar] [CrossRef]
- Mace, H.; Lightfoot, N.; McCluskey, S.; Selby, R.; Roy, D.; Timoumi, T.; Karkouti, K. Validity of Thromboelastometry for Rapid Assessment of Fibrinogen Levels in Heparinized Samples During Cardiac Surgery: A Retrospective, Single-center, Observational Study. J. Cardiothorac. Vasc. Anesth. 2016, 3, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Gertler, R.; Wiesner, G.; Tassani-Prell, P.; Braun, S.L.; Martin, K. Are the point-of-care diagnostics MULTIPLATE and ROTEM valid in the setting of high concentrations of heparin and its reversal with protamine? J. Cardiothorac. Vasc. Anesth. 2011, 25, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, F.; Perret, A.; Ferrari, E.; Marcucci, C.M.; Flèche, J.; Crosset, M.; Schoettker, P.; Marcucci, C. Validation of rotational thromboelastometry during cardiopulmonary bypass: A prospective, observational in-vivo study. Eur. J. Anaesthesiol. 2014, 31, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.; Spahn, D.R.; Innerhofer, P.; Spannagl, M.; Rossaint, R. Clinical review: Prothrombin complex concentrates–evaluation of safety and thrombogenicity. Crit. Care 2011, 15, 201. [Google Scholar] [CrossRef] [PubMed]
- Theusinger, O.M.; Stein, P.; Levy, J.H. Point of care and factor concentrate-based coagulation algorithms. Transfus. Med. Hemother. 2015, 42, 115–121. [Google Scholar] [CrossRef]
- Erdoes, G.; Koster, A.; Ortmann, E.; Meesters, M.I.; Bolliger, D.; Baryshnikova, E.; Martinez Lopez De Arroyabe, B.; Ahmed, A.; Lance, M.D.; Ranucci, M.; et al. A European consensus statement on the use of four-factor prothrombin complex concentrate for cardiac and non-cardiac surgical patients. Anaesthesia 2021, 76, 381–392. [Google Scholar] [CrossRef]
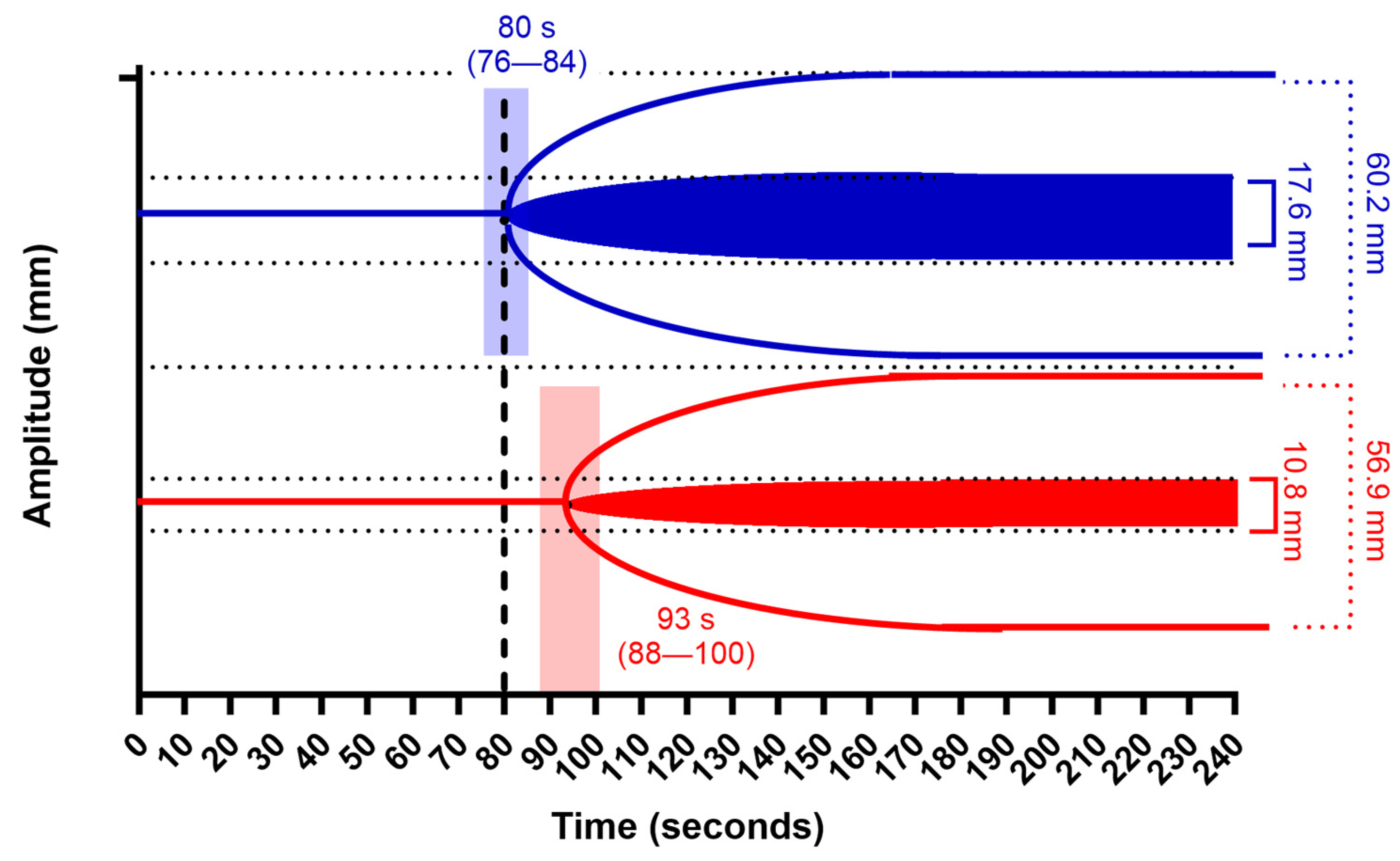
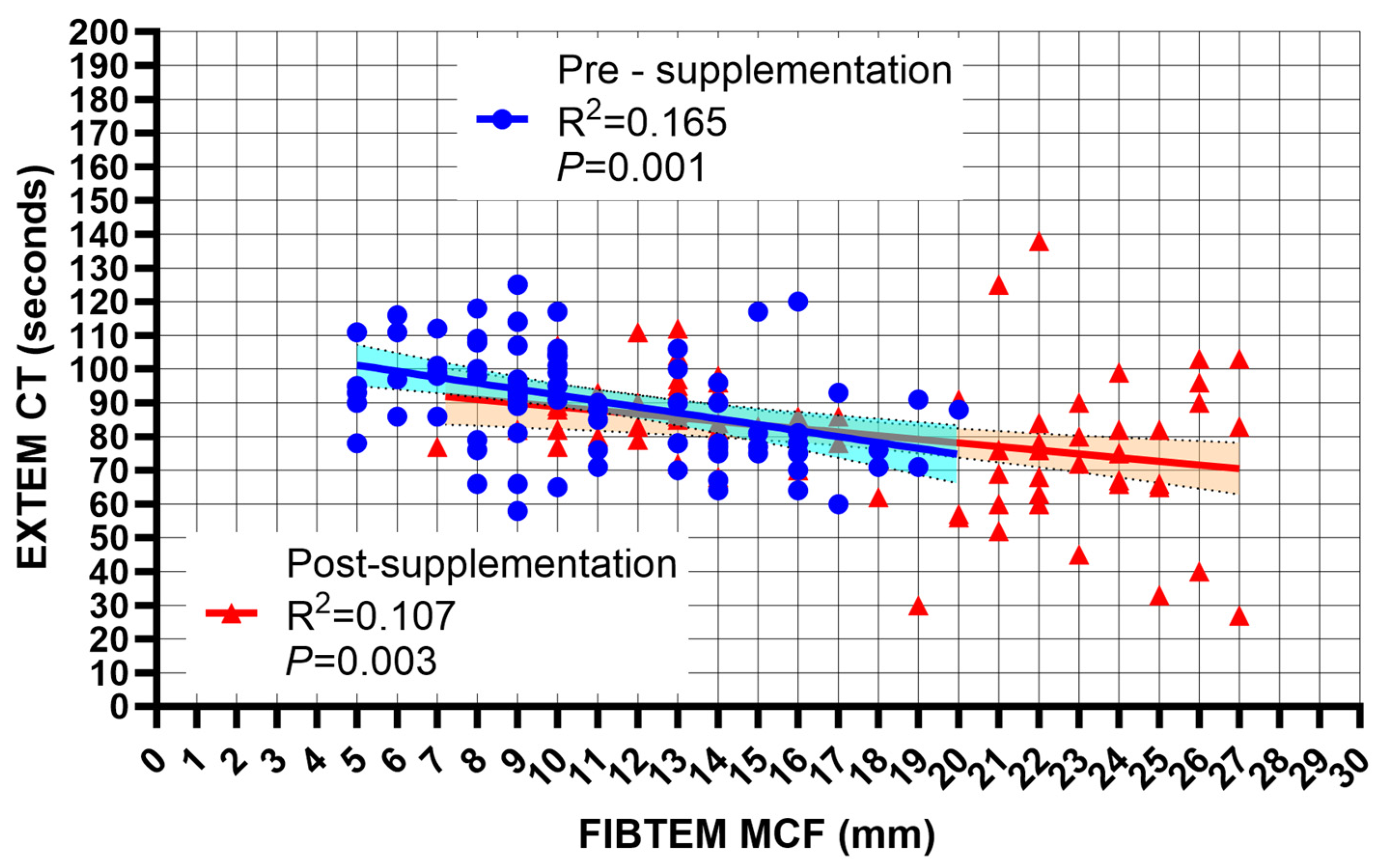

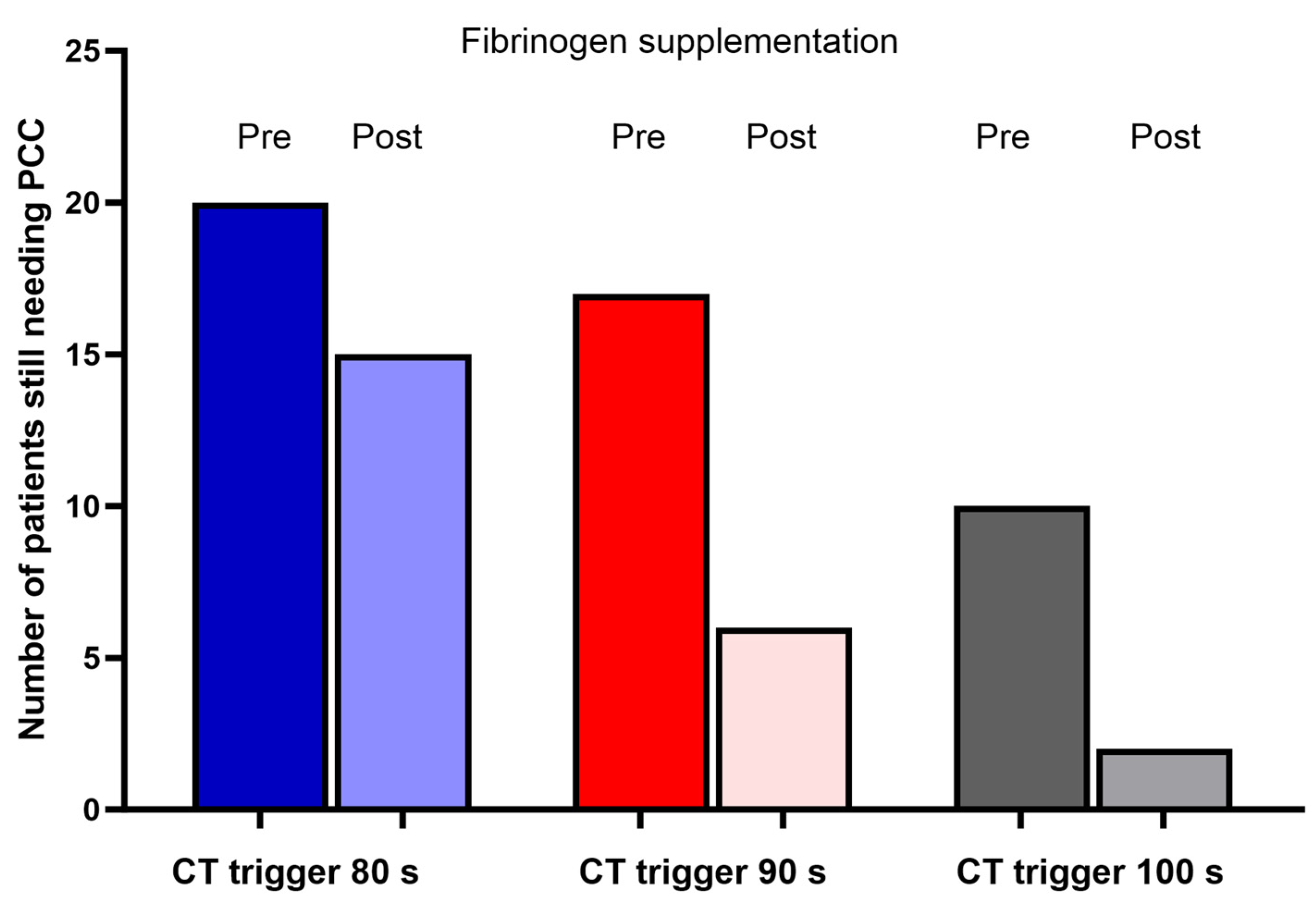
| Pre-Supplementation | Post-Supplementation | Mean Difference | p | ||
|---|---|---|---|---|---|
| Weight (kg) | 74.9 (15.1) | ||||
| Fibrinogen dose (mg) | 3541 (1893) | ||||
| Fibrinogen dose/kg (mg) | 47.4 (23.0) | ||||
| FIBTEM MCF (mm) | 10.8 (3.8) | 17.6 (5.8) | 6.9 (6.0 to 7.8) | 0.001 | |
| EXTEM MCF (mm) | 56.9 (9.9) | 60.2 (5.7) | 3.3 (1.6 to 4.8) | 0.001 | |
| CT EXTEM (s) | 93.1 (27.2) | 80.1 (18.9) | −13.1 (−17.8 to −8.4) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranucci, M.; Aloisio, T.; Di Dedda, U.; Anguissola, M.; Barbaria, A.; Baryshnikova, E. Fibrinogen and Prothrombin Complex Concentrate: The Importance of the Temporal Sequence—A Post-Hoc Analysis of Two Randomized Controlled Trials. J. Clin. Med. 2024, 13, 7137. https://doi.org/10.3390/jcm13237137
Ranucci M, Aloisio T, Di Dedda U, Anguissola M, Barbaria A, Baryshnikova E. Fibrinogen and Prothrombin Complex Concentrate: The Importance of the Temporal Sequence—A Post-Hoc Analysis of Two Randomized Controlled Trials. Journal of Clinical Medicine. 2024; 13(23):7137. https://doi.org/10.3390/jcm13237137
Chicago/Turabian StyleRanucci, Marco, Tommaso Aloisio, Umberto Di Dedda, Martina Anguissola, Alessandro Barbaria, and Ekaterina Baryshnikova. 2024. "Fibrinogen and Prothrombin Complex Concentrate: The Importance of the Temporal Sequence—A Post-Hoc Analysis of Two Randomized Controlled Trials" Journal of Clinical Medicine 13, no. 23: 7137. https://doi.org/10.3390/jcm13237137
APA StyleRanucci, M., Aloisio, T., Di Dedda, U., Anguissola, M., Barbaria, A., & Baryshnikova, E. (2024). Fibrinogen and Prothrombin Complex Concentrate: The Importance of the Temporal Sequence—A Post-Hoc Analysis of Two Randomized Controlled Trials. Journal of Clinical Medicine, 13(23), 7137. https://doi.org/10.3390/jcm13237137







