Soft Tissue Sarcoma with Lower Limb Impairment: Development of a Specific Rehabilitation Protocol Based on Demolitive and Reconstructive Surgery Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Rehabilitation Protocol
2.4. Clinical Assessment
- The mBI is a 10-item measure of physical disability with a score range of 0 to 100, often used to assess abilities and functioning in activities of daily living [23].
- The NRS, an 11-point (0–10) numerical rating scale, measures the patient’s perceived level of discomfort [24].
- The APECS employs an 8-point rating scale (0–7) to assess walking ability and independence [25].
2.5. Statistical Analysis
3. Results
- 15 patients: demolitive surgery without secondary reconstruction (primary closure).
- 3 patients: demolitive and reconstructive surgery with local perforator flaps.
- 5 patients: demolitive and reconstructive surgery with free flaps (harvested from the latissimus dorsi muscle in four cases and the contralateral gracile muscle in one).
- 8 patients: demolitive and reconstructive surgery with FFMT (harvested from the contralateral thigh in four cases, from the latissimus dorsi muscle in three cases, and from the upper limb in one).
4. Discussion
5. Limits
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gamboa, A.C.; Gronchi, A.; Cardona, K. Soft-tissue Sarcoma in Adults: An Update on the Current State of Histiotype-specific Management in an Era of Personalized Medicine. CA Cancer J. Clin. 2020, 70, 200–229. [Google Scholar] [CrossRef] [PubMed]
- PDQ Adult Treatment Editorial Board. Soft Tissue Sarcoma Treatment (PDQ®): Health Professional Version; National Cancer Institute: Bethesda, MD, USA, 2002. [Google Scholar]
- Miwa, S.; Yamamoto, N.; Tsuchiya, H. Sarcoma: Molecular Pathology, Diagnostics, and Therapeutics. Int. J. Mol. Sci. 2023, 24, 5833. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, I.-M.; Cote, G.M.; Hornick, J.L. Contemporary Sarcoma Diagnosis, Genetics, and Genomics. J. Clin. Oncol. 2018, 36, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, R.; Mori, T.; Okita, Y.; Shiraishi, Y.; Endo, M. A Multidisciplinary Approach to Soft-Tissue Sarcoma of the Extremities. Expert. Rev. Anticancer. Ther. 2020, 20, 893–900. [Google Scholar] [CrossRef]
- Casali, P.G.; Blay, J.-Y. Soft Tissue Sarcomas: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2010, 21, v198–v203. [Google Scholar] [CrossRef] [PubMed]
- Dei Tos, A.P.; Bonvalot, S.; Haas, R. The Key Role of Pathology, Surgery and Radiotherapy in the Initial Management of Soft Tissue Sarcoma. Future Oncol. 2018, 14, 15–23. [Google Scholar] [CrossRef]
- Brunetti, B.; Morelli Coppola, M.; Tenna, S.; Salzillo, R.; Petrucci, V.; Pazzaglia, M.; Valeri, S.; Alloni, R.; Vincenzi, B.; Tonini, G.; et al. Thigh Reconstruction between Form and Function: An Algorithm for Flap Selection Based on a Series of 70 Oncological Patients. Microsurgery 2024, 44. [Google Scholar] [CrossRef]
- Gerrand, C.; Furtado, S. Issues of Survivorship and Rehabilitation in Soft Tissue Sarcoma. Clin. Oncol. 2017, 29, 538–545. [Google Scholar] [CrossRef]
- Parsons, J.A.; Davis, A.M. Rehabilitation and Quality-of-Life Issues in Patients with Extremity Soft Tissue Sarcoma. Curr. Treat. Options Oncol. 2004, 5, 477–488. [Google Scholar] [CrossRef]
- Stout, N.L.; Santa Mina, D.; Lyons, K.D.; Robb, K.; Silver, J.K. A Systematic Review of Rehabilitation and Exercise Recommendations in Oncology Guidelines. CA Cancer J. Clin. 2021, 71, 149–175. [Google Scholar] [CrossRef]
- Strobel, E.S.; Fritschka, E. Current Aspects of Oncological Rehabilitation. MMW Fortschr. Med. 2006, 148, 49–51. [Google Scholar]
- Wu, J.; Chi, H.; Kok, S.; Chua, J.M.W.; Huang, X.-X.; Zhang, S.; Mah, S.; Foo, L.-X.; Peh, H.-Y.; Lee, H.-B.; et al. Multimodal Prerehabilitation for Elderly Patients with Sarcopenia in Colorectal Surgery. Ann. Coloproctol. 2024, 40, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Tobias, K.; Gillis, T. Rehabilitation of the Sarcoma Patient-Enhancing the Recovery and Functioning of Patients Undergoing Management for Extremity Soft Tissue Sarcomas. J. Surg. Oncol. 2015, 111, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, H.; Imura, Y.; Wakamatsu, T.; Takenaka, S. Comorbidity, Body Mass Index, and Performance Status as Prognostic Factors in Older Patients with Soft-Tissue Sarcoma. J. Geriatr. Oncol. 2022, 13, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Andrews, C.C.; Siegel, G.; Smith, S. Rehabilitation to Improve the Function and Quality of Life of Soft Tissue and Bony Sarcoma Patients. Patient Relat. Outcome Meas. 2020, 10, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R. Rehabilitation Strategies and Outcomes of the Sarcoma Patient. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 171–180. [Google Scholar] [CrossRef]
- Riou, J.-P.A.; Cohen, J.R.; Johnson, H. Factors Influencing Wound Dehiscence. Am. J. Surg. 1992, 163, 324–330. [Google Scholar] [CrossRef]
- Hohenberger, P.; Schwarzbach, M.H.M. Management of Locally Recurrent Soft Tissue Sarcoma after Prior Surgery and Radiation Therapy. In Treatment of Bone and Soft Tissue Sarcomas; Springer: Berlin/Heidelberg, Germany, 2009; pp. 271–283. [Google Scholar]
- Abouarab, M.H.; Salem, I.L.; Degheidy, M.M.; Henn, D.; Hirche, C.; Eweida, A.; Uhl, M.; Kneser, U.; Kremer, T. Therapeutic Options and Postoperative Wound Complications after Extremity Soft Tissue Sarcoma Resection and Postoperative External Beam Radiotherapy. Int. Wound J. 2018, 15, 148–158. [Google Scholar] [CrossRef]
- Springfield, D.S. Surgical Wound Healing. Cancer Treat. Res. 1993, 67, 81–98. [Google Scholar]
- Chi, D.; Raman, S.; Tawaklna, K.; Zhu, W.Y.; Keane, A.M.; Bruce, J.G.; Parikh, R.; Tung, T.H. Free Functional Muscle Transfer for Lower Extremity Reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2023, 86, 288–299. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Downie, W.W.; Leatham, P.A.; Rhind, V.M.; Wright, V.; Branco, J.A.; Anderson, J.A. Studies with Pain Rating Scales. Ann. Rheum. Dis. 1978, 37, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.H.; Chun, M.H.; Kim, B.R.; Han, E.Y.; Park, J.Y. Bowel Function in Acute Stroke Patients. Ann. Rehabil. Med. 2011, 35, 337. [Google Scholar] [CrossRef] [PubMed]
- Busk, H.; Holm, P.; Skou, S.T.; Seitner, S.; Siemsen, T.; Wienecke, T. Inter-Rater Reliability and Agreement of 6 Minute Walk Test and 10 Meter Walk Test at Comfortable Walk Speed in Patients with Acute Stroke. Physiother. Theory Pract. 2023, 39, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Okamoto, M.; Kito, M.; Yoshimura, Y.; Aoki, K.; Suzuki, S.; Takazawa, A.; Komatsu, Y.; Ideta, H.; Ishida, T.; et al. Muscle Strength and Functional Recovery for Soft-Tissue Sarcoma of the Thigh: A Prospective Study. Int. J. Clin. Oncol. 2023, 28, 922–927. [Google Scholar] [CrossRef]
- Watanabe, T.; Momosaki, R.; Suzuki, S.; Abo, M. Preoperative Rehabilitation for Patients Undergoing Colorectal Cancer Surgery: A Retrospective Cohort Study. Support. Care Cancer 2020, 28, 2293–2297. [Google Scholar] [CrossRef]
- Geng, C.; Yang, G.; Zhou, H.; Wang, H.; Li, Y.; Leng, Y.; Zhang, Z.; Jian, Y.; Chen, W. Low Barthel Index Score Is a Poor Prognostic Factor for Newly Diagnosed Multiple Myeloma Patients. Clin. Exp. Med. 2023, 23, 2593–2600. [Google Scholar] [CrossRef]
- Shibahashi, H.; Murakawa, M.; Matsuda, K.; Takakubo, Y.; Takagi, M. Barthel Index and Age as Predictors of Discharge Destination in Patients with Glioblastoma. Cancer Investig. 2024, 42, 619–626. [Google Scholar] [CrossRef]
- Dos Santos Barros, V.; Bassi-Dibai, D.; Guedes, C.L.R.; Morais, D.N.; Coutinho, S.M.; de Oliveira Simões, G.; Mendes, L.P.; da Cunha Leal, P.; Dibai-Filho, A.V. Barthel Index Is a Valid and Reliable Tool to Measure the Functional Independence of Cancer Patients in Palliative Care. BMC Palliat. Care 2022, 21, 124. [Google Scholar] [CrossRef]
- Morishima, T.; Sato, A.; Nakata, K.; Matsumoto, Y.; Koeda, N.; Shimada, H.; Maruhama, T.; Matsuki, D.; Miyashiro, I. Barthel Index-Based Functional Status as a Prognostic Factor in Young and Middle-Aged Adults with Newly Diagnosed Gastric, Colorectal and Lung Cancer: A Multicentre Retrospective Cohort Study. BMJ Open 2021, 11, e046681. [Google Scholar] [CrossRef]
- Cheville, A.L.; Basford, J.R. Role of Rehabilitation Medicine and Physical Agents in the Treatment of Cancer-Associated Pain. J. Clin. Oncol. 2014, 32, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M. Functional Outcome in Extremity Soft Tissue Sarcoma. Semin. Radiat. Oncol. 1999, 9, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Spierer, M.M.; Alektiar, K.M.; Zelefsky, M.J.; Brennan, M.F.; Cordiero, P.G. Tolerance of Tissue Transfers to Adjuvant Radiation Therapy in Primary Soft Tissue Sarcoma of the Extremity. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus Postoperative Radiotherapy in Soft-Tissue Sarcoma of the Limbs: A Randomised Trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef]
- Hoang, M.N.; Nyqvist, E.; Hesla, A.C.; Tsagkozis, P.; Löfgren, J. Wound Healing after Surgery for Soft Tissue Sarcomas, and the Effect of Primary Plastic Reconstruction—A Retrospective Cohort Study. Eur. J. Surg. Oncol. 2024, 50, 108348. [Google Scholar] [CrossRef]
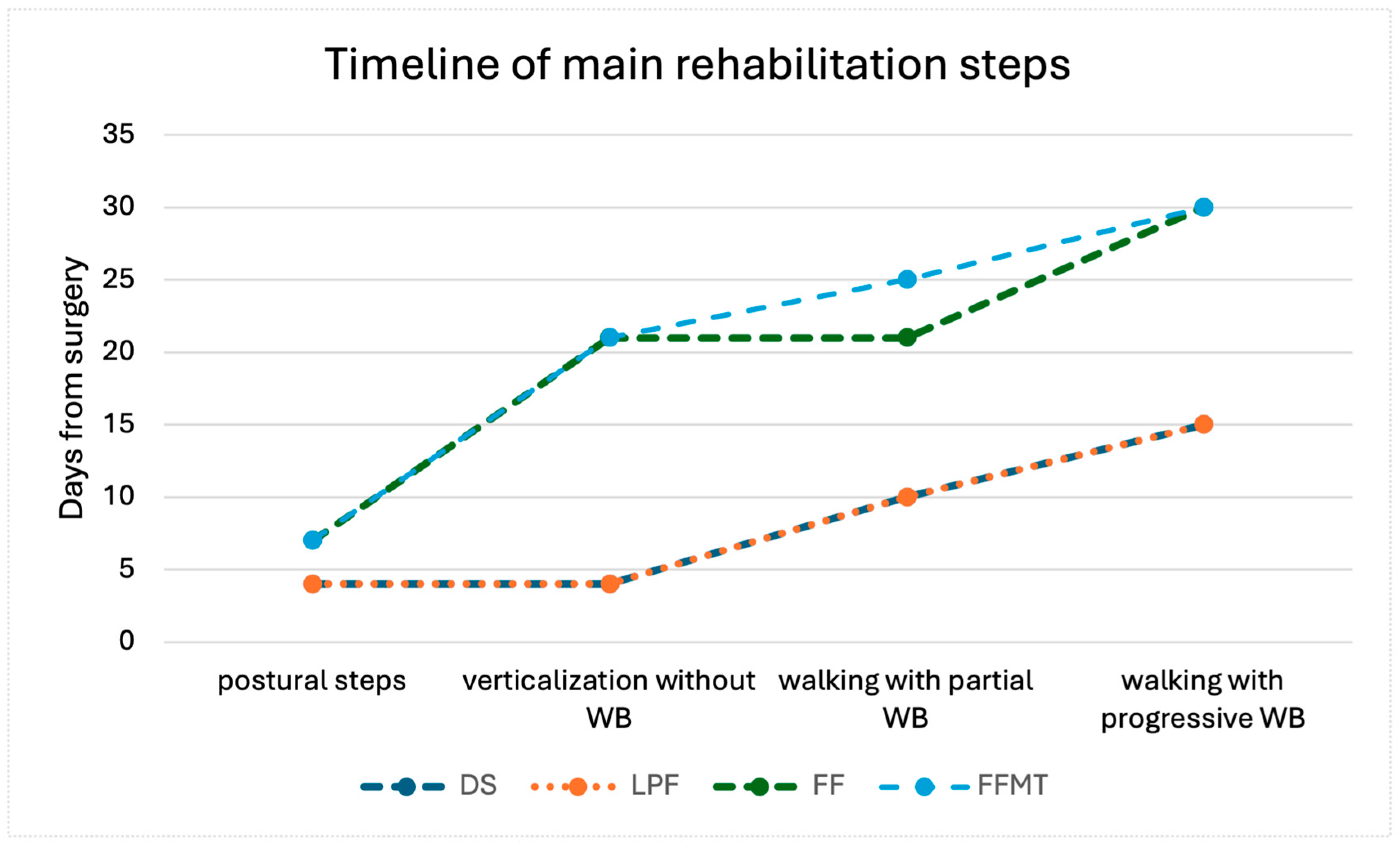


| Rehabilitation Program | Demolition Surgery Without Reconstruction | Local Perforator Flaps | Free Flap * | Free Function Muscle Transfer * |
|---|---|---|---|---|
| 0–7 days | 0–7 days | 0–15 days | 0–15 days |
| 0–7 days | 0–7 days | >21 days | >21 days |
| NA | NA | 15–21 days | 15–30 days |
| NA | NA | >15 days | >15 days |
| 7–15 days | 7–15 days | >21 days | 20–30 days |
| 7–15 days | 7–15 days | >15 days | >30 days |
| 7–15 days | 7–15 days | >15 days | 45–60 days |
| >15 days | >15 days | >30 days | >30 days (with brace) |
| >30 days | >30 days | >30 days | >60 days |
| Subjects n = 31 | ||
|---|---|---|
| Time from the surgery to the admission to the rehabilitation ward (days, Mean ± SD) | Patient without surgical complication (n = 25) | 7.76 ± 5.0 |
| Patient with surgical complication (n = 6) | 82.5 ± 38.3 | |
| Length of stay (days, Mean ± SD) | 68.5 ± 32.6 | |
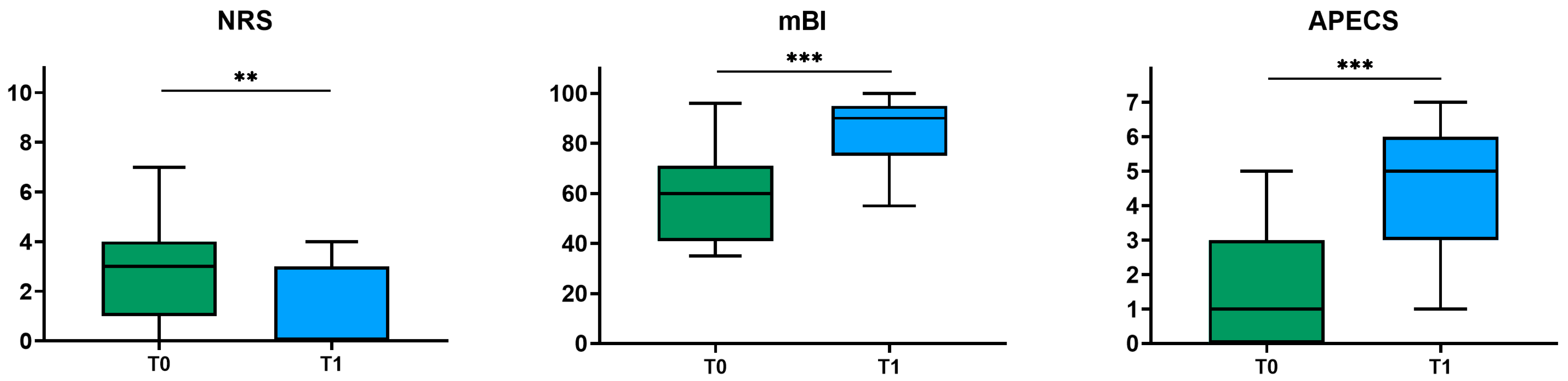
| modified Barthel Index (Mean ± SD) | 59.6 ± 17.3 | |
| Adapted Patient Evaluation System (Mean ± SD) | 1.5 ± 1.5 | |
| Numeric Rating Scale (Mean ± SD) | 2.7 ± 2.1 | |
| ID | Sex | Age | Tumor | Side | Type of Surgery | Resection | Reconstruction | Medical Therapy | Modified Barthel Index (T0) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 73 | Pleomorphic sarcoma | L | FFMT | SM, ST, gracilis | Latissimus dorsi | RT + CT + Hyperthermia | 93 |
| 2 | F | 80 | Liposarcoma | L | DS | LHBF, adductor brevis | No | No | 77 |
| 3 | M | 48 | Liposarcoma | R | FF | TFL, sartorius, VM | Latissimus dorsi | CT | 63 |
| 4 | F | 73 | Atypical lipomatous tumor | R | DS | VM | No | No | 56 |
| 5 | F | 53 | Leiomyosarcoma | R | FF | VM, VI, RF | Gracilis (controlateral) | CT | 85 |
| 6 | M | 77 | Liposarcoma | R | DS | Gracilis, adductor longus and brevis | No | RT + Hyperthermia | 73 |
| 7 | M | 46 | Myxofibrosarcoma | L | FFMT | VM, VI, RF | RF (controlateral) | RT + Hyperthermia | 40 |
| 8 | F | 46 | Pleomorphic sarcoma | L | DS | VM | No | No | 81 |
| 9 | F | 59 | Atypical lipomatous tumor | L | DS | VM | No | No | 66 |
| 10 | M | 77 | Myxofibrosarcoma | L | FFMT | VM, VI, RF | RF (controlateral) | RT + CT + Hyperthermia | 36 |
| 11 | M | 59 | Pleomorphic sarcoma | L | FFMT | LHBF, VL | Latissimus dorsi | RT + Hyperthermia | 38 |
| 12 | F | 79 | Malignant peripheral nerve sheath tumors | R | LPF | VM, VI, sartorius, adductor magnus | SCIP | No | 43 |
| 13 | F | 50 | Leiomyosarcoma | R | LPF | VM, VI, RF | PAP | No | 63 |
| 14 | M | 56 | Liposarcoma | L | DS | Iliopsoas | No | CT | 55 |
| 15 | M | 76 | Atypical lipomatous tumor | R | DS | Biceps femoris | No | No | 60 |
| 16 | F | 76 | Atypical lipomatous tumor | L | DS | VM, VI, VL, RF | No | No | 40 |
| 17 | F | 70 | Synovial sarcoma | R | FFMT | Tibialis posterior, extensor digitorum longus | Radial flexor of the carpus, palmaris longus | RT+ Hyperthermia | 82 |
| 18 | F | 53 | Mesenchymal tissue neoplasms | L | LPF | RF, VI | Gracilis | No | 96 |
| 19 | M | 32 | Synovial sarcoma | R | FFMT | VM, VI, VL | Latissimus dorsi | CT | 63 |
| 20 | M | 72 | Pleomorphic sarcoma | R | FFMT | VM, VI, VL, RF | RF, VM (controlateral) | RT + CT | 71 |
| 21 | F | 53 | Synovial sarcoma | L | FF | Plantar muscles | Latissimus dorsi | RT + CT | 52 |
| 22 | M | 70 | Liposarcoma | R | FF | Sartorius, gracilis, adductor magnus, SM, ST | Latissimus dorsi | RT + CT | 40 |
| 23 | F | 72 | Dedifferentiated sarcoma | L | DS | Iliopsoas, sartorius, RF, VL, VI | No | No | 41 |
| 24 | M | 72 | Dedifferentiated sarcoma | L | DS | LHBF, SM | No | No | 47 |
| 25 | F | 86 | Liposarcoma | L | FF | Extensor hallucis longus, tibialis anterior, soleus | Latissimus dorsi | RT | 57 |
| 26 | F | 60 | Pleomorphic sarcoma | L | FFMT | RF | VL (controlateral) | RT + Hyperthermia | 35 |
| 27 | F | 46 | Liposarcoma | L | DS | Iliopsoas | No | No | 52 |
| 28 | M | 69 | Myxofibrosarcoma | L | DS | VL, TFL | No | No | 64 |
| 29 | M | 64 | Leiomyosarcoma | R | DS | Iliopsoas | No | No | 70 |
| 30 | F | 45 | Spindle cell sarcoma | L | DS | SM, ST, LHBF | No | No | 70 |
| 31 | M | 72 | Atypical lipomatous tumor | L | DS | VM, RF | No | No | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galluccio, C.; Germanotta, M.; Valeri, S.; Brunetti, B.; Vincenzi, B.; Tenna, S.; Pagnoni, C.; Alloni, R.; Angelucci, M.; Salzillo, R.; et al. Soft Tissue Sarcoma with Lower Limb Impairment: Development of a Specific Rehabilitation Protocol Based on Demolitive and Reconstructive Surgery Types. J. Clin. Med. 2024, 13, 7023. https://doi.org/10.3390/jcm13237023
Galluccio C, Germanotta M, Valeri S, Brunetti B, Vincenzi B, Tenna S, Pagnoni C, Alloni R, Angelucci M, Salzillo R, et al. Soft Tissue Sarcoma with Lower Limb Impairment: Development of a Specific Rehabilitation Protocol Based on Demolitive and Reconstructive Surgery Types. Journal of Clinical Medicine. 2024; 13(23):7023. https://doi.org/10.3390/jcm13237023
Chicago/Turabian StyleGalluccio, Caterina, Marco Germanotta, Sergio Valeri, Beniamino Brunetti, Bruno Vincenzi, Stefania Tenna, Chiara Pagnoni, Rossana Alloni, Michela Angelucci, Rosa Salzillo, and et al. 2024. "Soft Tissue Sarcoma with Lower Limb Impairment: Development of a Specific Rehabilitation Protocol Based on Demolitive and Reconstructive Surgery Types" Journal of Clinical Medicine 13, no. 23: 7023. https://doi.org/10.3390/jcm13237023
APA StyleGalluccio, C., Germanotta, M., Valeri, S., Brunetti, B., Vincenzi, B., Tenna, S., Pagnoni, C., Alloni, R., Angelucci, M., Salzillo, R., Morelli Coppola, M., Valeri, A., Passa, R., Falchini, F., Pavan, A., Cortellini, L., Lattanzi, S., & Aprile, I. G. (2024). Soft Tissue Sarcoma with Lower Limb Impairment: Development of a Specific Rehabilitation Protocol Based on Demolitive and Reconstructive Surgery Types. Journal of Clinical Medicine, 13(23), 7023. https://doi.org/10.3390/jcm13237023










