Sutureless Bioprostheses for Aortic Valve Replacement: An Updated Systematic Review with Long-Term Results
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.2.1. Search Strategy
2.2.2. Data Collection Process
2.2.3. Data Abstraction/Synthesis
2.3. Outcomes
2.4. Ethical Approval
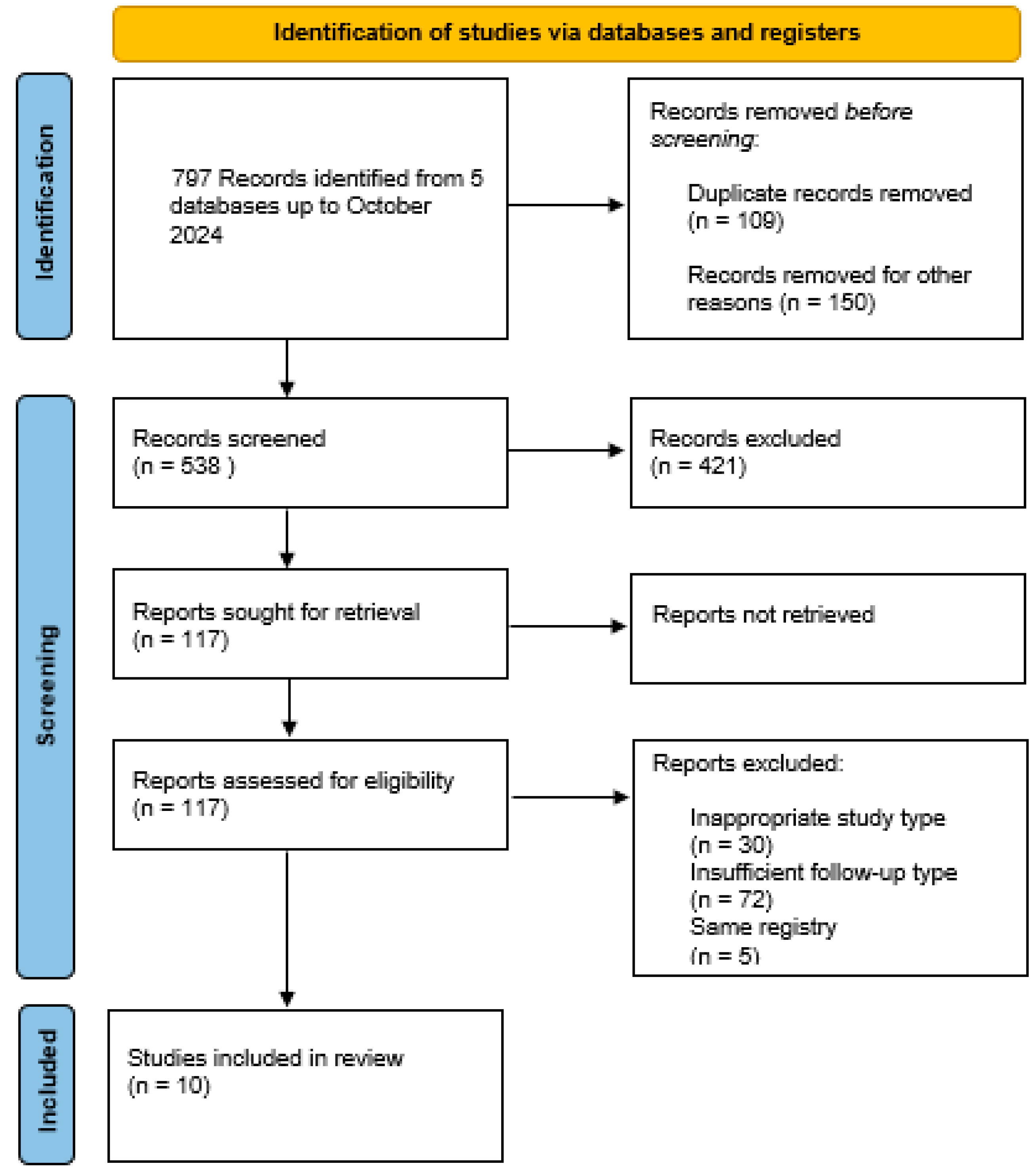
3. Results
3.1. Patients
3.2. Primary Outcomes
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.J.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. PARTNER 2 Investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Debb, G.M.; Maini, B.; Gada, H.; et al. SURTAVI Investigators. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; O’Brien, S.M.; Wu, C.; Sikora, J.A.; Griffith, B.P.; Gammie, J.S. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: Changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J. Thorac. Cardiovasc. Surg. 2009, 137, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Makkar, R.R.; Krishnaswami, A.; Hermiller, J.; Greenbaum, A.; Babaliaros, V.; Shah, P.B.; Bailey, S.H.; Bapat, V.; Kapadia, S.; et al. Valve-in-Surgical-Valve With SAPIEN 3 for transcatheter aortic valve replacement based on Society of Thoracic Surgeons predicted risk of mortality. Circ. Cardiovasc. Interv. 2021, 14, e010288. [Google Scholar] [CrossRef]
- Meuris, B.; Borger, M.A.; Bourguignon, T.; Siepe, M.; Grabenwöger, M.; Laufer, G.; Binder, K.; Polvani, G.; Stefano, P.; Coscioni, E.; et al. Durability of bioprosthetic aortic valves in patients under the age of 60years—Rationale and design of the international INDURE registry. J. Cardiothorac. Surg. 2020, 15, 119. [Google Scholar] [CrossRef]
- Iung, B.; Baron, G.; Tornos, P.; Gohlke-Bärwolf, C.; Butchart, E.G.; Vahanian, A. Valvular heart disease in the comunity: An european experience. Curr. Probl. Cardiol. 2007, 32, 609–661. [Google Scholar] [CrossRef]
- Mohr, F.W.; Holzhey, D.; Möllmann, H.; Beckmann, A.; Veit, C.; Figulla, H.R.; Cremer, J.; Kuck, K.-H.; Lange, R.; Zahn, R.; et al. The German Aortic Valve Registry: 1-year results from 13,680 patients with aortic valve disease. Eur. J. Cardiothorac. Surg. 2014, 46, 808–816. [Google Scholar] [CrossRef]
- Berretta, P.; Andreas, M.; Carrel, T.P.; Solinas, M.; Teoh, K.; Fishlein, T.; Santarpino, G.; Folliguet, T.; Villa, E.; Meuris, B.; et al. Minimally invasive aortic valve replacement with sutureless and rapid deployment valves: A report from an international registry (Sutureless and Rapid Deployment International Registry). Eur. J. Cardiothorac. Surg. 2019, 56, 793–799. [Google Scholar] [CrossRef]
- D’Onofrio, A.; Salizzoni, S.; Rubino, A.S.; Besola, L.; Filippini, C.; Alfieri, O.; Colombo, A.; Agrifoglio, M.; Fishlein, T.; Rapetto, F.; et al. The rise of new technologies for aortic valve stenosis: A comparison of sutureless and transcatheter aortic valve implantation. J. Thorac. Cardiovasc. Surg. 2016, 152, 99–109. [Google Scholar] [CrossRef]
- Di Eusanio, M.; Phan, K.; Berretta, P.; Carrel, T.P.; Andreas, M.; Santarpino, G.; Di Bartolomeoo, R.; Folliguet, T.; Meuris, B.; Mignosa, C.; et al. Sutureless and Rapid Deployment Aortic Valve Replacement International Registry (SURD-IR): Early results from 3343 patients. Eur. J. Cardiothorac. Surg. 2018, 54, 768–773. [Google Scholar] [CrossRef]
- Meco, M.; Montisci, A.; Miceli, A.; Panisi, P.; Donatelli, F.; Cirri, S.; Ferrarini, M.; Lio, A.; Glauber, M. Sutureless Perceval aortic valve versus conventional stented bioprostheses: Meta-analysis of postoperative and midterm results in isolated aortic valve replacement. J. Am. Heart Assoc. 2018, 7, e006091. [Google Scholar] [CrossRef] [PubMed]
- Flameng, W.; Herregods, M.C.; Hermans, H.; Van der Mieren, G.; Vercalsteren, M.; Poortmans, G.; Hemelrijck, J.V.; Meuris, B. Effect of sutureless implantation of the Perceval S aortic valve bioprosthesis on intraoperative and early postoperative outcomes. J. Thorac. Cardiovasc. Surg. 2011, 142, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Woldendorp, K.; Doyle, M.P.; Bannon, P.G.; Misfeld, M.; Yan, T.D.; Santarpino, G.; Berretta, P.; Di Eusanio, M.; Meuris, B.; Cerillo, A.G.; et al. Aortic valve replacement using stented or sutureless/rapid deployment prosthesis via either full-sternotomy or a minimally invasive approach: A network meta-analysis. Ann. Cardiothorac. Surg. 2020, 9, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Glauber, M.; Di Bacco, L.; Cuenca, J.; Di Bartolomeo, R.; Baghai, M.; Zakova, D.; Fishlein, T.; Troise, G.; Viganò, G.; Solinas, M. Minimally invasive aortic valve replacement with sutureless valves: Results from an international prospective registry. Innovations 2020, 15, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.L.; Flynn, C.D.; Mamo, A.A.; Tian, D.H.; Kappert, U.; Wilbring, M.; Folliguet, T.; Fiore, A.; Miceli, A.; D’Onofrio, A.; et al. Long-term outcomes of sutureless and rapid-deployment aortic valve replacement: A systematic review and meta-analysis. Ann. Cardiothorac. Surg. 2020, 9, 265–279. [Google Scholar] [CrossRef]
- Jolliffe, J.; Moten, S.; Tripathy, A.; Skillington, P.; Tatoulis, J.; Muneretto, C.; Di Bacco, L.; Figueiredo Galvao, H.B.; Goldblatt, J. Perceval valve intermediate outcomes: A systematic review and meta-analysis at 5-year follow-up. J. Cardiothorac. Surg. 2023, 18, 129. [Google Scholar] [CrossRef]
- Moher, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264. [Google Scholar] [CrossRef]
- Yayan, J.; Schiffner, R. Weaning failure in elderly patients: A systematic review and meta-analysis. J. Clin. Med. 2024, 13, 6429. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernàn, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Meuris, B.; Flameng, W.J.; Laborde, F.; Folliguet, T.A.; Haverich, A.; Shrestha, M. Five-year results of the pilot trial of a sutureless valve. J. Thorac. Cardiovasc. Surg. 2015, 150, 84–88. [Google Scholar] [CrossRef]
- Fischlein, T.; Meuris, B.; Folliguet, T.; Hakim-Meibodi, K.; Misfeld, M.; Carrel, T.; Zembala, M.; Cerutti, E.; Asch, F.M.; Haverich, A. Midterm outcomes with a sutureless aortic bioprosthesis in a prospective multicenter cohort study. J. Thorac. Cardiovasc. Surg. 2022, 164, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Szecel, D.; Eurlings, R.; Rega, F.; Verbrugghe, P.; Meuris, B. Perceval sutureless aortic valve implantation: Midterm outcomes. Ann. Thorac. Surg. 2021, 111, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Muneretto, C.; Solinas, M.; Folliguet, T.; Di Bartolomeo, R.; Repossini, A.; Laborde, F.; Rambaldini, M.; Santarpino, G.; Di Bacco, L.; Fishlein, T. Sutureless versus transcatheter aortic valves in elderly patients with aortic stenosis at intermediate risk: A multi-institutional study. J. Thorac. Cardiovasc. Surg. 2022, 163, 925–935. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Bozso, S.J.; Lakey, O.; Hong, Y.; Wang, S.; Nagendran, J. Rapid deployment valves versus conventional tissue valves for aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2022, 163, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Pollari, F.; Mamdooh, H.; Hitzl, W.; Grossman, I.; Vogt, F.; Fishlein, T. Ten years’ experience with the sutureless aortic valve replacement: Incidence and predictors for survival and valve durability at follow-up. Eur. J. Cardiothorac. Surg. 2023, 63, ezac572. [Google Scholar] [CrossRef] [PubMed]
- Concistré, G.; Baghai, M.; Santarpino, G.; Royse, A.; Sherner, M.; Troise, G.; Glauber, M.; Solinas, M. Clinical and hemodynamic outcomes of the Perceval sutureless aortic valve from a real-world registry. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 36, ivad103. [Google Scholar] [CrossRef]
- Dokollari, A.; Margaryan, R.; Torregrossa, G.; Sicouri, S.; Cameli, M.; Mandoli, G.E.; Prifti, E.; Veshti, A.; Bonacchi, M.; Gelsomino, S. Risk predictors that impact long-term prognosis in patients undergoing aortic valve replacement with the Perceval sutureless bioprosthesis. Cardiovasc. Revasc. Med. 2023, 55, 10–19. [Google Scholar] [CrossRef]
- Lamberigts, M.; Szecel, D.; Rega, F.; Verbrugghe, P.; Dubois, C.; Meuris, B. Sutureless aortic valves in isolated and combined procedures: Thirteen years of experience in 784 patients. J. Thorac. Cardiovasc. Surg. 2024, 167, 1724–1732. [Google Scholar] [CrossRef]
- Schizas, N.; Samiotis, I.; Nazou, G.; Iliopoulos, D.C.; Anagnostopoulos, I.; Kousta, M.; Papaioannou, N.; Argiriou, M.; Dedeilias, P. Perceval-S over time. Clinical outcomes after ten years of usage. J. Cardiothorac. Surg. 2024, 19, 192. [Google Scholar] [CrossRef]
- Shrestha, M.; Folliguet, T.; Meuris, B.; Dibie, A.; Bara, C.; Herregods, M.C.; Khaladj, N.; Hagl, C.; Flameng, W.; Laborde, F.; et al. Sutureless Perceval S aortic valve replacement: A multicenter prospective pilot trial. J. Heart Valve Dis. 2009, 18, 698–702. [Google Scholar]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tsai, Y.C.; Niles, N.; Tchantchaleishvili, V.; Di Eusanio, M.; Yan, T.D.; Phan, K. Transcatheter aortic valve implantation (TAVI) versus sutureless aortic valve replacement (SUAVR) for aortic stenosis: A systematic review and meta-analysis of matched studies. J. Thorac. Dis. 2016, 8, 3283–3293. [Google Scholar] [CrossRef] [PubMed]
- Chiariello, G.A.; Villa, E.; Messina, A.; Tomba, M.D.; Cirillo, M.; Brunelli, F.; Zean, M.; Troise, G. Perceval valve-in-valve implant for full root xenograft failure. J. Card. Surg. 2017, 32, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Chiariello, G.A.; Villa, E.; Dalla Tomba, M.; Cirillo, M.; Brunelli, F.; Mhagna, Z.; Troise, G. Sutureless prosthesis for failed small Mitroflow valves: The Perceval-after-Mitroflow procedure. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 865–868. [Google Scholar] [CrossRef]
- Shalabi, A.; Spiegelstein, D.; Sternik, L.; Feinberg, M.S.; Kogan, A.; Levin, S.; Orlov, B.; Nachum, E.; Lipey, A.; Raanani, E.; et al. Sutureless versus stented valve in aortic valve replacement in patients with small annulus. Ann. Thorac. Surg. 2016, 102, 118–122. [Google Scholar] [CrossRef]
- Chiariello, G.A.; Bruno, P.; Villa, E.; Pasquini, A.; Pavone, N.; Cammeroni, F.; Andrea, M.; Colizzi, C.; Nesta, M.; Iafrancesco, M.; et al. Aortic valve replacement in elderly patients with small aortic annulus: Results with three different bioprostheses. Innovations 2019, 14, 27–36. [Google Scholar] [CrossRef]
- Nguyen, A.; Fortin, W.; Mazine, A.; Bouchard, D.; Carrier, M.; El Hamamsy, I.; Lamarche, Y.; Demers, P. Sutureless aortic valve replacement in patients who have bicuspid aortic valve. J. Thorac. Cardiovasc. Surg. 2015, 150, 851–857. [Google Scholar] [CrossRef][Green Version]
- Chiariello, G.A.; Villa, E.; Messina, A.; Troise, G. Dislocation of a sutureless prosthesis after type I bicuspid aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2018, 156, e87–e89. [Google Scholar] [CrossRef]
- Miceli, A.; Berretta, P.; Fiore, A.; Andreas, M.; Solinas, M.; Santarpino, G.; Kappert, U.; Misfeld, M.; Savini, C.; Albertini, A.; et al. Sutureless and rapid deployment implantation in bicuspid aortic valve: Results from the sutureless and rapid-deployment aortic valve replacement international registry. Ann. Cardiothorac. Surg. 2020, 9, 298–304. [Google Scholar] [CrossRef]
- Gersak, B.; Fischlein, T.; Folliguet, T.A.; Meuris, B.; Teoh, K.H.; Moten, S.C.; Solinas, M.; Miceli, A.; Oberwalder, P.J.; Rambaldini, M.; et al. Sutureless, rapid deployment valves and stented bioprosthesis in aortic valve replacement: Recommendations of an International Expert Consensus Panel. Eur. J. Cardio-Thorac. Surg. 2016, 49, 709–718. [Google Scholar] [CrossRef]
- Anselmi, A.; Ruggieri, V.G.; Belhaj Soulami, R.; Flécher, E.; Langanay, T.; Corbineau, H.; Leguerrier, A.; Verhoye, J.-P. Hemodynamic results and mid-term follow-up of 850 19 to 23 mm Perimount Magna Ease valves. Thorac. Cardiovasc. Surg. 2019, 67, 274–281. [Google Scholar]
- Riess, F.-C.; Cramer, E.; Hansen, L.; Schifelers, S.; Wahl, G.; Wallrath, J.; Winkel, S.; Kremer, P. Clinical results of the Medtronic Mosaic porcine bioprosthesis up to 13 years. Eur. J. Cardiothorac. Surg. 2010, 37, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Bruno, P.; Cammertoni, F.; Rosenhek, R.; Mazza, A.; Pavone, N.; Iafrancesco, M.; Nesta, M.; Chiariello, G.A.; Spalletta, C.; Graziano, G.; et al. Improved patient recovery with minimally invasive aortic valve surgery: A propensity-matched study. Innovations 2019, 14, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Landes, U.; Dvir, D.; Schoels, W.; Tron, C.; Ensminger, S.; Simonato, M.; Säfer, U.; Bunc, M.; Aldea, G.S.; Cerillo, A.; et al. Transcatheter aortic valve-in-valve implantation in degenerative rapid deployment bioprostheses. EuroIntervention 2019, 15, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fabre, O.; Radutoiu, M.; Carjaliu, I.; Rebet, O.; Gautier, L.; Hysi, I. Recent improvement in operative techniques lead to lower pacemaker rate after Perceval implant. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac182. [Google Scholar] [CrossRef] [PubMed]
- Meuris, B.; Lamberigts, M.; Szecel, D. The importance of sizing in sutureless valves. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac206. [Google Scholar] [CrossRef]
| Author | Study Design | Year | Trial/Registries | Study Period | Patient Number | Median Follow-Up (Years) | Maximum Follow-Up (Years) | Risk of Bias (ROBINS-1) |
|---|---|---|---|---|---|---|---|---|
| Meuris et al. [20] | PC | 2015 | Pilot (5 years) | 2007–2008 | 30 | 4.2 | 5 | Serious |
| Fishlein et al. [21] | PC | 2021 | CAVALIER | 2010–2013 | 658 | 3.8 | 5 | Serious |
| Szecel et al. [22] | RC | 2021 | Institutional data | 2007–2017 | 468 | 3.1 | 11.2 | Serious |
| Muneretto et al. [23] | RC | 2022 | Institutional data | 2008–2015 | 481 | 5 | - | Moderate |
| White et al. [24] | RC | 2022 | Institutional data | 2013–2019 | 295 | 2.4 | 5 | Serious |
| Pollari et al. [25] | RC | 2023 | Institutional data | 2010–2020 | 547 | 3.8 | 10.6 | Serious |
| Concistre et al. [26] | PC | 2023 | SURE-AVR | 2011–2021 | 1652 | 1.2 | 8 | Serious |
| Dokollari et al. [27] | RC | 2023 | Institutional data | 2013–2020 | 101 | 1.5 | 7 | Serious |
| Lamberigts et al. [28] | RC | 2024 | Institutional data | 2007–2019 | 784 | 7 | 13.6 | Serious |
| Shizas N et al. [29] | RC | 2024 | Institutional data | 2013–2020 | 205 | 6.7 | 10 | Moderate |
| Author | Age Mean (SD) Years | Gender (Male) n (%) | BSA Mean (SD) m2 | Diabetes n (%) | COPD n (%) | Dyslipidemia n (%) | Renal Failure n (%) | Previous NV Events n (%) | PVD n (%) | NYHA > III n (%) | Euroscore Mean (SD)% | STS Score Mean (SD)% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meuris et al. [20] | 80.4 (3.8) | 8 (27) | 1.8 (0.2) | N/A | N/A | N/A | N/A | N/A | N/A | 30 (100) | 13.1 (7.2) | N/A |
| Fishlein et al. [21] | 78.3 (5.6) | 234 (35.6) | 1.8 (0.2) | 191 (29) | 103 (15.7) | N/A | 97 (14.8) | 75 (11.4) | 112 (17) | 418 (63.5) | 10.2 (7.8) | 7.2 (7.4) |
| Szecel et al. [22] | 79 (5) | 206 (44) | 1.8 (0.2) | 116 (25) | 75 (16) | N/A | N/A | N/A | 122 (26) | 278 (59.4) | N/A | 5.8 (5.5) |
| Muneretto et al. [23] | 79 (5) | 174 (36.2) | N/A | 154 (32) | 89 (18.5) | N/A | 59 (12.3) | 31 (6.4) | 81 (16.8) | 285 (59.3) | 13.6 (18.4) | 5.7 (6.4) |
| White et al. [24] | 72.4 (9.9) | 188 (63.7) | N/A | 75 (25.4) | 40 (13.6) | 166 (56.3) | 12 (4.1) | 14 (4.7) | 12 (4.1) | N/A | N/A | N/A |
| Pollari et al. [25] | 76.4 (5.2) | 268 (49) | 1.88 (0.2) | 170 (31) | 73 (13) | 409 (75) | 20 (3.6) | 15 (3) | 161 (29.4) | N/A | 13 (11) | N/A |
| Concistre et al. [26] | 75.3 (7) | 761 (46) | N/A | 514 (31.1) | 228 (13.8) | 937 (56.7) | 184 (11.1) | 107 (6.5) | 103 (8.5) | N/A | N/A | N/A |
| Dokollari et al. [27] | 71.2 (7.6) | 55 (54.4) | 1.9 (0.25) | 41 (40.6) | N/A | 76 (75.2) | N/A | 14 (14) | N/A | 61 (61) | 3.5 (4.4) | N/A |
| Lamberigts et al. [28] | 78.5 (5.8) | 279 (48.3) | 1.8 (0.2) | 30 (3.8) | 119 (15.2) | N/A | N/A | N/A | 196 (25) | 406 (51.7) | N/A | N/A |
| Shizas N et al. [29] | 76.4 (34.1) | 70 (34.1) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Author | FS n (%) | Minimally Invasive n (%) | Redo Operation n (%) | Combined Operation n (%) | Combined CABG n (%) | CPB Time Mean (SD) Min | ACC Time Mean (SD) Min | AV Sten n (%) | AV Reg n (%) | AV Sten and Reg n (%) | BAV n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meuris et al. [20] | 30 (100) | N/A | 3 (10) | 14 (46.6) | 14 (46.6) | 46.4 (6.7) | 29.3 (8) | 23 (76.7) | 0 (0) | 7 (23.3) | N/A |
| Fishlein et al. [21] | 439 (66.7) | 219 (33.3) | 446 (68) * | 207 (31.5) | 154 (23.4) | 58.7 (20.2) | 35.5 (12.4) | 430 (65.3) | 2 (0.3) | 226 (34.3) | 12 (1.8) |
| Szecel et al. [22] | 328 (70) | 140 (29.9) | N/A | 267 (57) | 184 (39) | 66 (22) | 39 (13) | N/A | 8 (1.7) | N/A | 11 (2.3) |
| Muneretto et al. [23] | 256 (53.2) | 225 (46.7) | 35 (7.3) | N/A | N/A | 56 (25) | 35 (16) | 481 (100) | 0 (0) | 0 (0) | 31 (6.4) |
| White et al. [24] | N/A | N/A | N/A | 94 (31.8) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Pollari et al. [25] | 162 (29.6) | 385 (70.3) | 21 (4) | 173 (31.6) | 141 (26) | 59.4 (20) | 36.1 (11) | 544 (99.1) | 3 (0.5) | 0 (0) | 69 (13) |
| Concistre et al. [26] | 899 (54.4) | 744 (45) | 270 (16.3) | 593 (35.9) | 426 (25.8) | 77.4 (30.8) | 51 (20.5) | 1233 (74.6) | 89 (5.4) | 300 (18.2) | 132 (8) |
| Dokollari et al. [27] | 101 (100) | 0 (0) | 24 (24) | 0 (0) | 0 (0) | 65 (29.6) | 47.3 (21.3) | 88 (88) | 0 (0) | 13 (13) | 25 (25) |
| Lamberigts et al. [28] | 541 (69) | 243 (31) | N/A | 435 (55.4) | 239 (30.5) | N/A | N/A | N/A | N/A | N/A | N/A |
| Shizas N et al. [29] | 96 (47) | 109 (53) | N/A | 68 (33) | 63 (30.7) | 59.1 (15.3) | 49.1 (13.4) | 185 (90.2) | 4 (1.9) | N/A | N/A |
| Author | N | Deaths n (%) | cv Deaths n (%) | SVD ** n (%) | PVL ** n (%) | PVE n (%) | Overall Reintervention n (%) | Redo for SVD *** n (%) | Surgica Redo (Explant) for SVD n (%) | V-in-V for SVD n (%) | Redo (Explant) for PVL n (%) | Redo (Explant) for PVE n (%) | NV Events n (%) | PMK n (%) | Valve Thrombosis n (%) | Hemolysis n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meuris et al. [20] | 29 | 6 (20.6) | 1 (3.4) | 0 (0) | 1 (3.4) | 2 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.4) | 0 (0) | 0 (0) |
| Fishlein et al. [21] | 599 | 131 (21.9) | 59 (9.8) | 13 (2.1) | 6 (1) | 17 (2.8) | 24 (4) | 13 (2.1) | 7 (1.2) | 6 (1) | 0 (0) | 8 (1.3) | 18 (3) | 15 (2.5) | 0 (0) | 1 (0.2) |
| Szecel et al. [22] | 453 | 97 (21.4) | 27 (5.9) | 10 (2.2) | 14 (3) | 5 (1.1) | 5 (1.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (1.1) | N/A | 11 (2.4) | 0 (0) | N/A |
| Muneretto et al. [23] | 287 * | 46 (16.1) | 5 (1.7) | 2 (0.7) | 1 (0.3) | 2 (0.7) | 3 (1) | 1 (0.3) | 1 (0.3) | 0 (0) | 1 (0.3) | 2 (0.7) | 4 (1.4) | 5 (1.8) | 0 (0) | N/A |
| White et al. [24] | 288 | 19 (6.5) | N/A | N/A | N/A | N/A | 0 (0) | N/A | N/A | N/A | N/A | N/A | 12 (4.1) | 20 (7) | 0 (0) | N/A |
| Pollari et al. [25] | 529 | 110 (20.7) | 13 (2.4) | 23 (4.8) | 1 (0.2) | 10 (1.9) | 23 (4.3) | 19 (4) | 4 (0.8) | 15 (3.1) | 0 (0) | 4 (0.7) | 5 (0.9) | 9 (1.7) | 0 (0) | N/A |
| Concistre et al. [26] | 1639 | 127 (7.7) | 55 (3.3) | 10 (0.6) | 0 (0) | 14 (0.8) | 23 (1.4) | 10 (0.6) | 1 (0.06) | 9 (0.5) | 6 (0.3) | 7 (0.4) | 12 (0.7) | 48 (3) | 0 (0) | N/A |
| Dokollari et al. [27] | 99 | 12 (12) | 5 (5) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 10 (10) | 5 (5) | N/A | N/A |
| Lamberigts et al. [28] | 758 | 208 (27.4) | N/A | 15 (1.9) | 9 (1.2) | 13 (1.7) | 14 (1.8) | 3 (0.4) | 0 (0) | 3 (0.4) | 0 (0) | 11 (1.4) | 11 (1.4) | N/A | N/A | N/A |
| Shizas N et al. [29] | 196 | 47 (24) | 35 (18) | 1 (0.5) | 1 (0.5) | 0 (0) | 5 (2.5) | 1 (0.5) | 0 (0) | 1 (0.5) | 1 (0.5) | 0 (0) | N/A | 14 (7.1) | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiariello, G.A.; Di Mauro, M.; Villa, E.; Koulouroudias, M.; Bruno, P.; Mazza, A.; Pasquini, A.; D’Avino, S.; De Angelis, G.; Corigliano, K.; et al. Sutureless Bioprostheses for Aortic Valve Replacement: An Updated Systematic Review with Long-Term Results. J. Clin. Med. 2024, 13, 6829. https://doi.org/10.3390/jcm13226829
Chiariello GA, Di Mauro M, Villa E, Koulouroudias M, Bruno P, Mazza A, Pasquini A, D’Avino S, De Angelis G, Corigliano K, et al. Sutureless Bioprostheses for Aortic Valve Replacement: An Updated Systematic Review with Long-Term Results. Journal of Clinical Medicine. 2024; 13(22):6829. https://doi.org/10.3390/jcm13226829
Chicago/Turabian StyleChiariello, Giovanni Alfonso, Michele Di Mauro, Emmanuel Villa, Marinos Koulouroudias, Piergiorgio Bruno, Andrea Mazza, Annalisa Pasquini, Serena D’Avino, Gaia De Angelis, Kiara Corigliano, and et al. 2024. "Sutureless Bioprostheses for Aortic Valve Replacement: An Updated Systematic Review with Long-Term Results" Journal of Clinical Medicine 13, no. 22: 6829. https://doi.org/10.3390/jcm13226829
APA StyleChiariello, G. A., Di Mauro, M., Villa, E., Koulouroudias, M., Bruno, P., Mazza, A., Pasquini, A., D’Avino, S., De Angelis, G., Corigliano, K., Marcolini, A., Zancanaro, E., Saitto, G., Meani, P., Massetti, M., & Lorusso, R. (2024). Sutureless Bioprostheses for Aortic Valve Replacement: An Updated Systematic Review with Long-Term Results. Journal of Clinical Medicine, 13(22), 6829. https://doi.org/10.3390/jcm13226829









