Abstract
Aim: To examine the oral health of children and adolescents with and without diabetes mellitus. Background: Diabetes mellitus is the most common metabolic disease in childhood and demonstrates an increasing incidence. Many children live with gingivitis as a precursor to periodontitis. If left untreated, it can cause the development of periodontitis. The links between periodontitis and diabetes mellitus are known but have been little studied in the age group of children and adolescents. Materials and Methods: Clinical examination and collection of sulcus fluid from participants aged 5 to 21 years was performed. The following data were collected: demographic variables, caries prevalence, DMF-T, VPI, PUFA, salivary flow rate, HbA1c, PSI, and the concentration of IL-1β, IL-6, MMP-8, and TNF-α. Results: Patients with diabetes mellitus showed a significantly lower salivary flow rate with higher concentrations of MMP-8 and IL-1β. The data indicate that at this age, regular visits to the dentist are of great importance for the promotion of oral health in children and adolescents regardless of diabetes and that patients with diabetes mellitus in particular benefit from prevention, as they belong to the periodontitis risk group. Conclusions: Patients with low salivary flow rates and increased inflammatory mediators are high-risk patients for whom dental preventive measures play a major role.
1. Introduction
Diabetes mellitus is the most common chronic metabolic disease in children and adolescents, with increasing prevalence (1.52 million persons under 20) and incidence (149,500 new cases per year) worldwide [1]. The common feature of all types of diabetes is chronic hyperglycemia [2]. Type 1 diabetes mellitus is based on the destruction of the beta cells of the pancreas, resulting in an absolute insulin deficiency. Type 2 involves a reduced insulin effect, which is caused by peripheral insulin resistance [3]. The insulin receptor density decreases, which means that body cells absorb less glucose. The resulting high blood glucose concentration leads to insulin hypersecretion, which is why the resistance is intensified when insulin secretion decreases. A progressive reduction in the function of the beta cells or a disorder of glucose-dependent insulin secretion develops [4]. With around 175 compared to 3100 new cases of type 1 diabetes in Germany each year, the incidence of type 2 diabetes in children and adolescents is therefore significantly lower [5]. Specific types of diabetes due to other causes or type 3 diabetes is a group of diseases that have hyperglycemia as the main symptom but cannot be caused by other types of diabetes [6]. Gestational diabetes is present when glucose tolerance disorder is first diagnosed during pregnancy [7].
Hypoglycemia is the most common acute complication of diabetes mellitus [8]. Mild hypoglycemia can be treated by the patient by ingesting rapidly usable carbohydrates. In contrast, patients with severe hypoglycemia require outside help, as neurological symptoms can occur [9]. Hyperglycemia can be developed in unrecognized or inadequately treated diabetes mellitus. The development of microvascular and macrovascular diseases is considered a long-term risk. These include retino-, neuro-, and nephropathy as well as arteriosclerosis. If diseases such as nephropathy or retinopathy already occur in childhood and adolescence, this indicates a more severe course in the development of microvascular and macrovascular complications [8,10,11,12]. In addition, the occurrence of these diseases reduces life expectancy [13].
Reviews show that people with diabetes mellitus often have limited knowledge of oral health and inadequate oral hygiene behavior with a lower utilization of dental services [14]. In particular, patients with uncontrolled diabetes mellitus appear to be at increased risk of developing oral health problems [15,16,17,18,19,20]. Diabetes mellitus, periodontitis, and tooth decay are common diseases and share behavioral risk factors such as an unhealthy diet and lack of exercise [21,22].
Caries is caused by biofilm-related demineralization of the teeth and affects 43.6% of 6- to 7-year-olds in Germany [23]. This disease is preventable by reducing the frequency of sugar intake and maintaining adequate oral hygiene. According to the WHO, there is a causal link between high sugar consumption, diabetes mellitus, obesity, and dental caries [24]. However, the data on caries prevalence and caries experience in children with and without diabetes are limited and inconsistent [25,26,27,28,29,30,31,32]. More frequent meal intake, higher sugar concentration in saliva, and lower saliva flow rate are cited as causes for the differences in oral health [14,33,34,35,36]. Furthermore, poor oral hygiene and poorly controlled diabetes mellitus lead to a drop in the pH value and a reduction in the cleansing function of saliva [32]. Children and adolescents with diabetes mellitus appear to have an increased prevalence of xerostomia and a reduced salivary flow rate compared to control subjects [37]. Individuals with a low unstimulated salivary flow rate are more at risk of developing carious lesions due to the loss of the protective properties of saliva [38] and have poorer oral health with an increased number of decayed and filled teeth compared to patients with a physiological salivary flow rate [39]. At the same time, salivary flow rate correlates with diabetes control: the worse the metabolic control, the more frequently xerostomia and hyposalivation occur [39].
Periodontitis is a multifactorial disease that causes progressive destruction of the periodontium [40]. The bidirectional relationship between diabetes mellitus and periodontitis is considered certain, although the underlying mechanisms have not yet been conclusively clarified [15]. People with diabetes mellitus develop periodontitis at a younger age than healthy people [41]. The disease manifests itself as early as childhood [42] or adolescence [43]. The pathomechanisms have primarily been investigated in adults, but a similar pathogenesis is suspected in children [44]. As a systemic infection, periodontitis triggers increased tissue resistance to insulin [45]. At the same time, the onset and progression of periodontitis are influenced by microangiopathy, impaired immune system, reduced resistance to infection, altered oral microbiome, and dysfunction of collagen metabolism [46].
The null hypotheses were:
- Children and adolescents with diabetes mellitus do not have poorer dental health than their healthy peers.
- Diabetics have a higher salivary flow rate than healthy people.
- Oral health-related quality of life does not differ between the study groups.
- There are no differences in the concentrations of inflammatory parameters in the sulcus fluid between the study groups.
2. Materials and Methods
In this case-control study, the oral health of children and adolescents with and without diabetes mellitus was compared. People were recruited for the study during consultation hours at the Section for Preventive Dentistry and Pediatric Dentistry of the Department for Orthodontics and the Section for Diabetology of the Clinic for Pediatric and Adolescent Medicine at Jena University Hospital. Children and adolescents with and without diabetes mellitus were included.
2.1. Inclusion and Exclusion Criteria
Children and adolescents from the age of 5 to the age of 21 with diabetes mellitus and healthy children and adolescents were included. Children and adolescents in the diabetes group were included in the study regardless of their diabetes type. Since the influence of chronic hyperglycemia and long-term metabolic control on oral health is to be investigated, the cause or type of diabetes can be disregarded in this study. The following were excluded: healthy children and adolescents with other general medical conditions such as metabolic, cardiovascular, or tumor diseases; healthy children and adolescents who regularly take medication; children and adolescents who have taken antibiotics in the three months before the start of the study; adolescents who smoke (nicotine consumption); children and adolescents with fixed orthodontic appliances; missing or unsigned consent form. If the participants were minors, the informed consents were signed by the parents or guardians of the children.
2.2. Clinical Examination
The children and adolescents were examined according to WHO standards without taking X-ray images, using a WHO probe, dental forceps, and mouth mirror under dental lighting or an inspection lamp [47]. The teeth were dried using gauze swabs. The findings were recorded anonymously and coded on a documentation sheet. The caries prevalence and experience was recorded according to the WHO standard using the dmf-t/DMF-T index at dentin caries level [47]. In addition, initial carious lesions at enamel caries level were included in the findings and the index was extended to the tooth surface-related evaluation (“surface”) (dmf-s-/DMF-S-; dmfi-s/DMFI-S-Index). According to the Basic methods for oral health surveys (WHO), the dmf-t/DMF-T index was used with addition of initial caries lesions.
The pufa/PUFA index [48] was used to assess odontogenic infections as a result of untreated caries and the Visible Plaque Index [49] was used to classify the oral hygiene status.
The amount of saliva was determined using the saliva flow rate. Samples were collected in the morning or afternoon at least 2 h after eating, drinking, smoking, or tooth brushing [50]. The patient allowed saliva to drip into a scaled glass cup over a period of 5 min. After the time had elapsed, all the saliva in the mouth was spat into the funnel and the flow rate was read off the scaled tube. From this, the saliva flow rate per minute was calculated.
Sulcus fluid was collected by the examiners inserting paper strips into the sulcus of teeth 11, 12, 21, and 22.
A standardized evidence-based questionnaire (Oral Health Impact Profile OHIP-14) was used to determine oral health-related quality of life. If the study participants were too young, their parents or guardians helped to fill out the forms.
The diabetes parameters (HbA1c, CPR, albumin content in urine, BMI) were collected as part of the routine examinations in the diabetes consultation. The children and adolescents were examined by two calibrated dentists. WHO-compliant calibration training [47] was carried out in advance by an epidemiologically experienced study and test dentist.
2.3. Sulcus Fluid Sample Preparation and ELISA
Frozen paper strips with sulcus fluid samples were incubated with 100 µL 1X Phosphate Buffered Saline (1X PBS) in Eppendorf tubes for 1 h at 4 °C to resolve proteins. Strips were clipped into the lid of the tube and centrifuged for 5 min at 1000× g at 4 °C [51]. For each sample, protein amount was measured with a BCA Protein-Assay-Kit (Thermo Fisher Scientific, Carlsbad, CA, USA) according to the manufacture’s guidelines. Sulcus fluid samples were analyzed for IL-1β, IL-6, MMP-8, and TNF-α using appropriate enzyme-linked immunosorbent assays (ELISA) according to the manufacturer’s guidelines. All samples were measured as technical duplicates. For each sample, measured cytokine concentration was correlated to the protein amount.
2.4. Statistics
The case number planning for this study was carried out by the Institute of Medical Statistics, Informatics and Data Science, Friedrich Schiller University Jena. The basis for the case number calculation were the periodontological aspects with the comparison of the inflammation parameters. In a pilot study, the amount of the inflammatory parameter interleukin 1-β was measured in sulcus fluid (mean value in patients without diabetes mellitus 0.658 ± 0.218 pg/mL vs. 1.365 ± 0.319 pg/mL in patients with diabetes mellitus). Based on this, the number of cases was planned at a power of 80% and a significance level of 5%, resulting in a number of 49 cases per group.
Statistical analyses were performed with Graph Pad Prism 9 (https://www.graphpad.com; version number: 10.1.2, accessed on 26 November 2021). All experiments were performed in technical duplicates. Student’s t-test, Fisher’s exact test, and chi-square test were used as statistical tests. Significance levels: * p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001.
3. Results
3.1. Population
Patient recruitment and examination took place from 11 December 2019 to 25 August 2020 at the Children’s Hospital of the University Hospital Jena and was made more difficult due to the corona pandemic, as children and adolescents with diabetes mellitus represent a risk group. The total population comprised 92 children and adolescents, 54 of whom had diabetes. The average age was 11.8 ± 4.1 years and differed significantly between the groups. Overall, 48.9% of participants were female, compared to 52.6% in the control group and 46.3% in the diabetes group.
3.2. Diabetes Mellitus Data
Diabetes control was assessed based on the HbA1c value (Table 1).

Table 1.
Diabetes control based on HbA1c value [52].
There was no significant difference between the genders. There was no significance for the relationship between BMI and diabetes control (Table 2).

Table 2.
BMI distribution [53] after diabetes control.
Urine albumin levels correlated positively with diabetes control according to HbA1c levels. The annual diabetes program could not be carried out routinely during the corona pandemic. As a result, only isolated CRP values and 24-h blood pressure profiles were collected, which could not be statistically evaluated.
3.3. Caries Data
The caries prevalence of healthy individuals was 57.9% (CI 41.5–74.3%) and that of diabetics 42.6% (CI 29.0–56.2%) and did not differ significantly.
Deciduous teeth of children with diabetes mellitus showed a significantly lower caries experience than those without metabolic disease (Table 3). The diabetes setting correlated significantly and moderately negatively with the dmf-t/dmfi-t/dmf-s/dmfi-s.

Table 3.
Caries experience in first dentition.
There was no difference in caries experience in permanent dentition (Table 4). There was no significance for the weak positive correlation between caries experience and diabetes control according to the HbA1c value.

Table 4.
Caries experience in second dentition.
Among all study participants, only one participant with diabetes mellitus showed caries-related ulceration.
At 90.5% (CI 82.7–98.2%), patients without diabetes mellitus did not show a significantly higher degree of restoration than patients with diabetes mellitus (81.4% (CI 71.7–91.2%)). The same applies to the restoration index (control group 90.2% (CI 82.2–98.1%) vs. diabetes group 81.4% (CI 71.7–91.2%)).
Healthy subjects had an average Visible Plaque Index of 48.2% (CI 37.3–59.1%). At 63.0% (CI 53.9–72.1%), patients with diabetes mellitus achieved a significantly higher value. Diabetes control, according to the HbA1c value, had no influence on the VPI.
Metabolically healthy patients had a significantly higher salivary flow rate of 0.29 ± 0.22 mL/min compared to children and adolescents with diabetes mellitus with 0.19 ± 0.15 mL/min (Figure 1). Diabetes control, according to the HbA1c value, showed no detectable influence on the salivary flow rate.
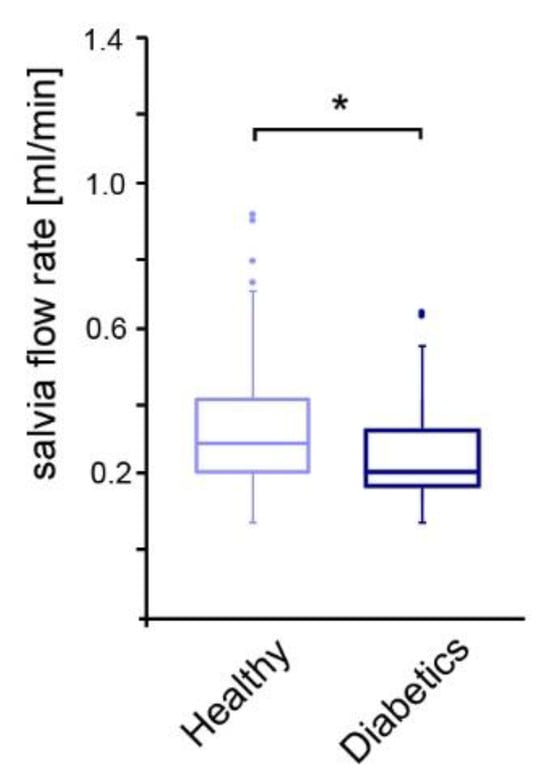
Figure 1.
Comparison of salivary flow rate among the study groups. * p-value < 0.05, dots: outliers.
3.4. Oral Health-Related Quality of Life
According to the total values of the Oral Health Impact Profile (OHIP-G 14), the study groups had a significant difference in oral health-related quality of life. Participants without diabetes mellitus had a higher mean sum score (4.19 ± 5.13) than participants with diabetes mellitus (2.04 ± 2.94).
3.5. Diabetic Patients Showed Increased IL-1β and MMP-8 Levels in Sulcus Fluids
Persons with diabetes mellitus develop a chronic low-grade inflammation, which should be detected in the sulcus fluid. Concentrations of pro-inflammatory cytokines IL-1β, IL-6, TNF-α, and MMP-8 were measured in sulcus fluids of diabetic patients. While IL-6 and TNF-α levels were comparable to the healthy controls, significantly increased concentrations of IL-1β and MMP-8 were detected in the group of diabetic participants (Figure 2A–D).
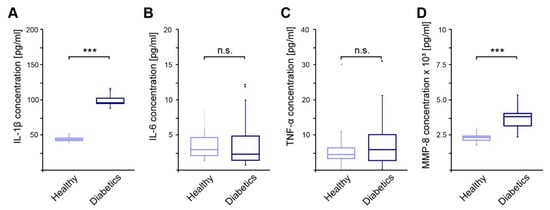
Figure 2.
Concentrations of pro-inflammatory cytokines measured in sulcus fluids of diabetic patients in relation to healthy controls. (A)—Concentration of IL-1β; (B)—Concentration of IL-6; (C)—Concentration of TNF-α; (D)—Concentration of MMP-8; ***—p-value < 0.001; n.s.—not significant.
4. Discussion
This study analyzed whether children and adolescents with diabetes mellitus have poorer dental health, with an increased caries prevalence and greater caries experience, compared to a healthy control group. Children and adolescents with diabetes mellitus were diagnosed with a lower caries prevalence compared to those without. In the literature, the prevalence of caries in minors with diabetes mellitus varies between 30% and 100%, depending on the study [54,55,56,57,58,59,60,61,62]. Contrary to the international state of knowledge, no correlation between metabolic control and caries prevalence could be established. In a systematic review and meta-analysis, Wang et al. investigated the prevalence of caries in children and adolescents with type 1 diabetes and found that patients with diabetes mellitus who were well controlled had a significantly lower prevalence of caries compared to those with poor control [63].
There were differences between the study groups in the caries experience of the primary dentition. Healthy people had significantly more caries than people with metabolic disease (1.3 vs. 0.5 dmf-t). The international literature is inconsistent. The majority of studies show that people without diabetes mellitus have less caries experience than those with diabetes mellitus [28,64,65,66,67], although the results are usually not significant. Some authors document a higher caries experience of the healthy study participants, also without significance [33]. The caries experience of permanent dentition showed no significant differences between patients with and without diabetes mellitus. Other studies also came to this conclusion [28,43,66,68].
Neither the degree of restoration nor the restoration index showed significant differences between the study groups.
Due to the small number, no significance could be determined for the PUFA index. International comparative values differ greatly because of the geographical location, caries experience, and level of education [69].
In the survey presented, patients in the diabetes group had a significantly higher VPI than those in the comparison group. VPI can be used to estimate the caries risk, as it represents a risk factor for caries development [70]. The documented discrepancy between the amount of plaque and caries experience can be explained by the examination modality. Healthy individuals came to the Center for Dental, Oral and Maxillofacial Medicine for a scheduled dental appointment, while participants with diabetes mellitus were only informed about the possibility of participating in the study during their pediatric examination. It can therefore be assumed that the children in the healthy group brushed their teeth beforehand with the knowledge of an upcoming visit to the dentist, whereas this was not performed in the group of children with diabetes.
Furthermore, the null hypothesis that diabetics have a higher salivary flow rate was disproved. People without diabetes mellitus showed significantly higher unstimulated salivary flow rates compared to those with diabetes. Numerous studies have also come to this conclusion [67,71,72]. Due to the different studies (age group, time of investigation, circadian rhythm, etc.) and the limited data available, the specific values are only comparable to a limited extent.
Consistent with oral health, participants without diabetes mellitus had a significantly higher OHIP-G sum score compared to those with diabetes mellitus. Although there were no significant differences between the groups for individual questions, which corresponds to the hypothesis, almost significant differences were found for questions on oral functionality. The control group was significantly younger, and the healthy participants were more likely to be in the second mixed dentition phase. It is possible that the change of teeth also impaired oral health-related quality of life.
The outbreak of the COVID-19 pandemic and the patient group of the Section of Preventive Dentistry and Pediatric Dentistry, which does not correspond to the epidemiological mean, had a limiting effect on the study.
It is well established that diabetes is a risk factor for the development of periodontitis. However, the exact pathomechanism is still the subject of intensive research. Priority has been given to adults, although a similar mechanism is suspected in children and adolescents.
As pro-inflammatory markers in the sulcus fluid, the cytokines IL-1b and IL-6 can be used to assess the inflammatory state of the periodontium [73].
The increased IL-1b levels in diabetics indicate an increased inflammatory state compared to the healthy control group. This corresponds to the current state of knowledge, and it has also been shown that there is a correlation between glycemic control and IL-1b concentration. Furthermore, younger diabetics appear to be increasingly affected by high IL-1b levels [74], which could be related to the cytokine storm associated with the first stage of type 1 diabetes [75].
IL-6 is causally involved in the development of insulin resistance in type 2 diabetes mellitus [76,77]. Since the patients in this study are primarily suffering from type 1 diabetes, it is therefore unlikely that any differences between healthy individuals and diabetics could be detected.
TNF-α stimulates IL-1b and IL-6 [78] and increases insulin resistance by phosphorylating insulin receptors. In this study, no difference in TNF-α concentrations was found between the study groups. Dos Santos Haber et al. report a change in the cytokine profile; over the course of the diabetes diagnosis, the TNF-α level increases [79]. It is possible that the duration of the disease in the population studied was still too short for the TNF-α concentration to differ from that of healthy individuals.
MMP8 has been established as a classic marker for periodontal inflammation. Diabetics exhibited elevated concentrations, but only the combination with clinical parameters and periodontal pathogens makes it possible to differentiate between stable and acute courses [80,81,82]. Due to the function of MMP8 in tissue degradation, an increased concentration represents a risk factor for the development of periodontitis.
Limitations
The regionality and the clientele of the Section of Preventive Dentistry and Pediatric Dentistry had a limiting effect on this study. Due to its function as a maximum care provider, patients with a low social status and limited financial resources are treated. Despite a general decline in caries, groups from a low social milieu are increasingly at risk of developing caries [83]. To obtain more representative results, patients in private practices or in kindergartens and schools should be examined. In addition, the start of the study occurring shortly before the start of the global coronavirus pandemic had a limiting effect on the study. Due to the increased risk for diabetics, fewer medical check-ups took place, resulting in a small number of cases. The documented discrepancy between the amount of plaque and caries experience can be explained by the examination modality. Healthy individuals came to the Center for Dental, Oral and Maxillofacial Medicine for a scheduled dental appointment, while participants with diabetes mellitus were only informed about the possibility of participating in the study during their pediatric examination. It can therefore be assumed that the children in the healthy group brushed their teeth beforehand with the knowledge of an upcoming visit to the dentist, whereas this was not performed in the group of children with diabetes.
5. Conclusions
Finally, it can be stated that patients with low salivary flow rates and increased inflammatory mediators are high-risk patients for whom dental preventive measures play a major role.
Author Contributions
Conceptualization, Y.W. and P.S.; methodology, Y.W.; validation, Y.W., J.S. and P.S.; formal analysis, P.S.; investigation, P.S., J.S.; resources, C.J., A.D., J.S. and P.S.; data curation, P.S.; writing—original draft preparation, P.S. and J.S.; writing—review and editing, Y.W.; visualization, P.S. and J.S.; supervision, Y.W.; project administration, Y.W.; funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Deutsche Gesellschaft für Zahn-, Mund- und Kieferheilkunde, science fund 11 November 2019. We acknowledge support by the German Research Foundation Projekt-Nr. 512648189 and the Open Access Publication Fund of the Thueringer Universitaets- und Landesbibliothek Jena.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of University Hospital Jena (2019-1387_1-BO; Deutsches Register Klinischer Studien DRKS00015088 and date of approval 15 May 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- International Diabetes Federation. About Diabetes. 2024. Available online: https://idf.org/ (accessed on 24 October 2024).
- Mauri-Obradors, E.; Estrugo-Devesa, A.; Jané-Salas, E.; Viñas, M.; López-López, J. Oral manifestations of Diabetes Mellitus. A systematic review. Med. Oral. Patol. Oral. Cir. Bucal 2017, 22, e586–e594. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Singhal, M.; Jialal, I. Type 2 Diabetes; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Harreiter, J.; Roden, M. Diabetes mellitus—Definition, Klassifikation, Diagnose, Screening und Prävention (Update 2019). Wien. Klin. Wochenschr. 2019, 131, 6–15. [Google Scholar] [CrossRef]
- Rosenbauer, J.; Neu, A.; Rothe, U.; Seufert, J.; Holl, R.W. Diabetestypen sind nicht auf Altersgruppen beschränkt: Typ-1-Diabetes bei Erwachsenen und Typ-2-Diabetes bei Kindern und Jugendlichen. J. Health Monit. 2019, 35, 80–86. [Google Scholar]
- Deutsche Diabetes Gesellschaft. S3-Leitlinie Therapie des Typ-1-Diabetes 2. Auflage; Deutsche Diabetes Gesellschaft: Berlin, Germany, 2018. [Google Scholar]
- Hartling, L.; Dryden, D.M.; Guthrie, A.; Muise, M.; Vandermeer, B.; Donovan, L. Benefits and harms of treating gestational diabetes mellitus: A systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann. Intern. Med. 2013, 159, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J. Pediatr. 1994, 125, 177–188. [Google Scholar] [CrossRef]
- Jacobson, A.M.; Musen, G.; Ryan, C.M.; Silvers, N.; Cleary, P.; Waberski, B.; Burwood, A.; Weinger, K.; Bayless, M.; Dahms, W.; et al. Long-term effect of diabetes and its treatment on cognitive function. N. Engl. J. Med. 2007, 356, 1842–1852. [Google Scholar] [CrossRef]
- Moore, W.V.; Donaldson, D.L.; Chonko, A.M.; Ideus, P.; Wiegmann, T.B. Ambulatory blood pressure in type I diabetes mellitus. Comparison to presence of incipient nephropathy in adolescents and young adults. Diabetes 1992, 41, 1035–1041. [Google Scholar] [CrossRef]
- Holl, R.W.; Lang, G.E.; Grabert, M.; Heinze, E.; Lang, G.K.; Debatin, K.M. Diabetic retinopathy in pediatric patients with type-1 diabetes: Effect of diabetes duration, prepubertal and pubertal onset of diabetes, and metabolic control. J. Pediatr. 1998, 132, 790–794. [Google Scholar] [CrossRef]
- Nathan, D.M.; Cleary, P.A.; Backlund, J.Y.; Genuth, S.M.; Lachin, J.M.; Orchard, T.J.; Raskin, P.; Zinman, B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med. 2005, 353, 2643–2653. [Google Scholar] [CrossRef]
- Fuhr, J.C.; Ramos, M.E.K.; Piovesan, F.; Renner, L.O.; Siqueira, L.O. Relationship of advanced glycation end-products in hypertension in diabetic patients: A systematic review. J. Bras. Nefrol. 2022, 44, 557–572. [Google Scholar] [CrossRef]
- Ismail, A.F.; McGrath, C.P.; Yiu, C.K. Oral health of children with type 1 diabetes mellitus: A systematic review. Diabetes Res. Clin. Pract. 2015, 108, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Genco, R. Diabetes and periodontal diseases: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84 (Suppl. S4), S106–S112. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontology 2000 2013, 62, 59–94. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.J.; Preshaw, P.M.; Lalla, E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J. Periodontol. 2013, 84 (Suppl. S4), S113–S134. [Google Scholar] [CrossRef]
- Engebretson, S.; Kocher, T. Evidence that periodontal treatment improves diabetes outcomes: A systematic review and meta-analysis. J. Clin. Periodontol. 2013, 40 (Suppl. S14), S153–S163. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Guo, X.; Luo, X.; Wang, D. The effect of periodontal treatment on hemoglobin a1c levels of diabetic patients: A systematic review and meta-analysis. PLoS ONE 2014, 9, e108412. [Google Scholar] [CrossRef]
- Simpson, T.C.; Weldon, J.C.; Worthington, H.V.; Needleman, I.; Wild, S.H.; Moles, D.R.; Stevenson, B.; Furness, S.; Iheozor-Ejiofor, Z. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst. Rev. 2015, 2015, Cd004714. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, X.F.; Chen, J.; Xia, L.; Cao, A.; Zhang, Y.; Wang, J.; Li, H.; Yang, K.; Guo, K.; et al. Combined lifestyle factors and risk of incident type 2 diabetes and prognosis among individuals with type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. Diabetologia 2020, 63, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.M.; Lee, S.; Hwang, W.; Son, E.; Kim, T.W.; Kim, K.; Kim, Y.H. Obesity and periodontitis: A systematic review and updated meta-analysis. Front. Endocrinol. 2022, 13, 999455. [Google Scholar] [CrossRef]
- Basner, R.; Santamaria, R.M.; Schmoeckel, J.; Schüler, E.; Splieth, C.H. Epidemiologische Begleituntersuchungen zur Gruppenprophylaxe 2016. In Deutsche Arbeitsgemeinschaft für Jugendzahnpflege; DAJ: Bonn, Germany, 2017. [Google Scholar]
- World Health Organisation. Oral Health. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 24 October 2024).
- Orbak, R.; Simsek, S.; Orbak, Z.; Kavrut, F.; Colak, M. The influence of type-1 diabetes mellitus on dentition and oral health in children and adolescents. Yonsei Med. J. 2008, 49, 357–365. [Google Scholar] [CrossRef]
- Novotna, M.; Podzimek, S.; Broukal, Z.; Lencova, E.; Duskova, J. Periodontal Diseases and Dental Caries in Children with Type 1 Diabetes Mellitus. Mediat. Inflamm. 2015, 2015, 379626. [Google Scholar] [CrossRef] [PubMed]
- El-Tekeya, M.; El Tantawi, M.; Fetouh, H.; Mowafy, E.; Abo Khedr, N. Caries risk indicators in children with type 1 diabetes mellitus in relation to metabolic control. Pediatr. Dent. 2012, 34, 510–516. [Google Scholar] [PubMed]
- Rafatjou, R.; Razavi, Z.; Tayebi, S.; Khalili, M.; Farhadian, M. Dental Health Status and Hygiene in Children and Adolescents with Type 1 Diabetes Mellitus. J. Res. Health Sci. 2016, 16, 122–126. [Google Scholar] [PubMed]
- Ismail, A.F.; McGrath, C.P.; Yiu, C.K.Y. Oral health status of children with type 1 diabetes: A comparative study. J. Pediatr. Endocrinol. Metab. 2017, 30, 1155–1159. [Google Scholar] [CrossRef]
- Akpata, E.S.; Alomari, Q.; Mojiminiyi, O.A.; Al-Sanae, H. Caries experience among children with type 1 diabetes in Kuwait. Pediatr. Dent. 2012, 34, 468–472. [Google Scholar]
- Arheiam, A.; Omar, S. Dental caries experience and periodontal treatment needs of 10- to 15-year old children with type 1 diabetes mellitus. Int. Dent. J. 2014, 64, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Miko, S.; Ambrus, S.J.; Sahafian, S.; Dinya, E.; Tamas, G.; Albrecht, M.G. Dental caries and adolescents with type 1 diabetes. Br. Dent. J. 2010, 208, E12. [Google Scholar] [CrossRef]
- Gupta, V.K.; Malhotra, S.; Sharma, V.; Hiremath, S.S. The Influence of Insulin Dependent Diabetes Mellitus on Dental Caries and Salivary Flow. Int. J. Chronic Dis. 2014, 2014, 790898. [Google Scholar] [CrossRef]
- Díaz Rosas, C.Y.; Cárdenas Vargas, E.; Castañeda-Delgado, J.E.; Aguilera-Galaviz, L.A.; Aceves Medina, M.C. Dental, periodontal and salivary conditions in diabetic children associated with metabolic control variables and nutritional plan adherence. Eur. J. Paediatr. Dent. 2018, 19, 119–126. [Google Scholar] [CrossRef]
- Hoseini, A.; Mirzapour, A.; Bijani, A.; Shirzad, A. Salivary flow rate and xerostomia in patients with type I and II diabetes mellitus. Electron. Physician 2017, 9, 5244–5249. [Google Scholar] [CrossRef]
- Ferizi, L.; Dragidella, F.; Spahiu, L.; Begzati, A.; Kotori, V. The Influence of Type 1 Diabetes Mellitus on Dental Caries and Salivary Composition. Int. J. Dent. 2018, 2018, 5780916. [Google Scholar] [CrossRef] [PubMed]
- López-Pintor, R.M.; Casañas, E.; González-Serrano, J.; Serrano, J.; Ramírez, L.; de Arriba, L.; Hernández, G. Xerostomia, Hyposalivation, and Salivary Flow in Diabetes Patients. J. Diabetes Res. 2016, 2016, 4372852. [Google Scholar] [CrossRef] [PubMed]
- Ekström, J.; Khosravani, N.; Castagnola, M.; Messana, I. Saliva and the Control of Its Secretion. In Dysphagia: Diagnosis and Treatment, Ekberg, O., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 21–57. [Google Scholar]
- Moore, P.A.; Guggenheimer, J.; Etzel, K.R.; Weyant, R.J.; Orchard, T. Type 1 diabetes mellitus, xerostomia, and salivary flow rates. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endodontology 2001, 92, 281–291. [Google Scholar] [CrossRef]
- Sanz, M.; Tonetti, M. New Classification of Periodontal and Peri-Implant Diseases; European Federation of Periodontology: Madrid, Spain, 2019. [Google Scholar]
- Thorstensson, H. Periodontal disease in adult insulin-dependent diabetics. Swed. Dent. J. Suppl. 1995, 107, 1–68. [Google Scholar]
- Lalla, E.; Cheng, B.; Lal, S.; Kaplan, S.; Softness, B.; Greenberg, E.; Goland, R.S.; Lamster, I.B. Diabetes mellitus promotes periodontal destruction in children. J. Clin. Periodontol. 2007, 34, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Lalla, E.; Cheng, B.; Lal, S.; Tucker, S.; Greenberg, E.; Goland, R.; Lamster, I.B. Periodontal changes in children and adolescents with diabetes: A case-control study. Diabetes Care 2006, 29, 295–299. [Google Scholar] [CrossRef]
- Zainal Abidin, Z.; Zainuren, Z.A.; Noor, E.; Mohd Nor, N.S.; Mohd Saffian, S.; Abdul Halim, R. Periodontal health status of children and adolescents with diabetes mellitus: A systematic review and meta-analysis. Aust. Dent. J. 2021, 66 (Suppl. S1), S15–S26. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef]
- Iughetti, L.; Marino, R.; Bertolani, M.F.; Bernasconi, S. Oral health in children and adolescents with IDDM—A review. J. Pediatr. Endocrinol. Metab. 1999, 12 (Suppl. S2), 603–610. [Google Scholar] [CrossRef]
- World Health Organisation. Oral Health Surveys, Basic Methods, 5th ed.; World Health Organisation: Geneva, Switzerland, 2013. [Google Scholar]
- Monse, B.; Heinrich-Weltzien, R.; Benzian, H.; Holmgren, C.; van Palenstein Helderman, W. PUFA—An index of clinical consequences of untreated dental caries. Community Dent. Oral. Epidemiol. 2010, 38, 77–82. [Google Scholar] [CrossRef]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar] [PubMed]
- Coelho, A.; Paula, A.; Mota, M.; Laranjo, M.; Abrantes, M.; Carrilho, F.; Ferreira, M.; Silva, M.; Botelho, F.; Carrilho, E. Dental caries and bacterial load in saliva and dental biofilm of type 1 diabetics on continuous subcutaneous insulin infusion. J. Appl. Oral. Sci. 2018, 26, e20170500. [Google Scholar] [CrossRef] [PubMed]
- Guentsch, A.; Kramesberger, M.; Sroka, A.; Pfister, W.; Potempa, J.; Eick, S. Comparison of Gingival Crevicular Fluid Sampling Methods in Patients with Severe Chronic Periodontitis. J. Periodontol. 2011, 82, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Rewers, M.; Pihoker, C.; Donaghue, K.; Hanas, R.; Swift, P.; Klingensmith, G.J. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr. Diabetes 2007, 8, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Neuhauser, P.D.H.; Schienkiewitz, D.A.; Rosario, A.S.; Dortschy, R.; Kurth, D.B.-M. Referenzperzentile für Anthropometrische Maßzahlen und Blutdruck aus der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland (KiGGS); Robert Koch Institut: Berlin, Germany, 2013. [Google Scholar]
- Abeuova, B.; Tuleutaeyva, S.; Ashirbekova, Z. Dental health in children with diabetes mellitus. Eur. J. Pediatr. 2017, 176, 1479. [Google Scholar]
- Ofilada, E.J. Oral health status of children attending a summer camp for diabetes children. J. ASEAN Fed. Endocr. Soc. 2015, 30, 138. [Google Scholar] [CrossRef][Green Version]
- Ofilada, E.J.L.; Jimeno, C.A. A survey on the barriers to dental care among individuals with type 1 diabetes. Endocrinology 2013, 51, 1–6. [Google Scholar]
- Carneiro, V.L.; Fraiz, F.C.; Ferreira, F.d.M.; Pintarelli, T.P.; Oliveira, A.C.B.; Boguszewski, M.C.S. The influence of glycemic control on the oral health of children and adolescents with diabetes mellitus type 1. Arch. Endocrinol. Metab. 2015, 59, 535–540. [Google Scholar] [CrossRef]
- Miranda, O.; Troncoso, P.; Rodriguez, S.; Aravena, T.; Jimenez Del, R.P. Dental caries and hygiene oral index in children with diabetes mellitus type 1. Rev. Chil. Pediatr. Chile 2013, 84, 527–531. [Google Scholar] [CrossRef]
- Gómez-Díaz, R.A.; Ramírez-Soriano, E.; Tanus Hajj, J.; Bautista Cruz, E.; Jiménez Galicia, C.; Villasis-Keever, M.A.; Aguilar-Salinas, C.A.; Wacher, N.H. Association between carotid intima-media thickness, buccodental status, and glycemic control in pediatric type 1 diabetes. Pediatr. Diabetes 2012, 13, 552–558. [Google Scholar] [CrossRef]
- Alavi, A.A.; Amirhakimi, E.; Karami, B. The prevalence of dental caries in 5–18-year-old insulin-dependent diabetics of Fars Province, southern Iran. Arch. Iran. Med. 2006, 9, 254–260. [Google Scholar] [PubMed]
- Twetman, S.; Johansson, I.; Birkhed, D.; Nederfors, T. Caries incidence in young type 1 diabetes mellitus patients in relation to metabolic control and caries-associated risk factors. Caries Res. 2002, 36, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, K.M.; Knuuttila, M.L.; Käär, M.L. Relationship between caries and level of metabolic balance in children and adolescents with insulin-dependent diabetes mellitus. Caries Res. 1997, 31, 13–18. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, L.; Yu, H.; Zhao, L. Prevalence of dental caries in children and adolescents with type 1 diabetes: A systematic review and meta-analysis. BMC Oral Health 2019, 19, 213. [Google Scholar] [CrossRef]
- López del Valle, L.M.; Ocasio-López, C. Comparing the oral health status of diabetic and non-diabetic children from Puerto Rico: A case-control pilot study. P. R. Health Sci. J. 2011, 30, 123–127. [Google Scholar]
- Alves, C.; Menezes, R.; Brandão, M. Salivary flow and dental caries in Brazilian youth with type 1 diabetes mellitus. Indian. J. Dent. Res. 2012, 23, 758–762. [Google Scholar] [CrossRef]
- Tagelsir, A.; Cauwels, R.; van Aken, S.; Vanobbergen, J.; Martens, L.C. Dental caries and dental care level (restorative index) in children with diabetes mellitus type 1. Int. J. Paediatr. Dent. 2011, 21, 13–22. [Google Scholar] [CrossRef] [PubMed]
- López, M.E.; Colloca, M.E.; Páez, R.G.; Schallmach, J.N.; Koss, M.A.; Chervonagura, A. Salivary characteristics of diabetic children. Braz. Dent. J. 2003, 14, 26–31. [Google Scholar] [CrossRef]
- Lai, S.; Cagetti, M.G.; Cocco, F.; Cossellu, D.; Meloni, G.; Campus, G.; Lingström, P. Evaluation of the difference in caries experience in diabetic and non-diabetic children—A case control study. PLoS ONE 2017, 12, e0188451. [Google Scholar] [CrossRef]
- Ozsin Ozler, C.; Uzamis Tekcicek, M.; Ozdemir, P.; Guciz Dogan, B. Pufa Index and Related Factors Among 36- to 71-month-old Children in Turkey: A Cross-Sectional Study. Oral. Health Prev. Dent. 2018, 16, 467–472. [Google Scholar] [CrossRef]
- Fejerskov, O.; Nyvad, B.; Kidd, E. Dental Caries: The Disease and Its Clinical Management; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Javed, F.; Sundin, U.; Altamash, M.; Klinge, B.; Engström, P.E. Self-perceived oral health and salivary proteins in children with type 1 diabetes. J. Oral. Rehabil. 2009, 36, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.R.; Passos, I.A.; Sampaio, F.C.; Soares, M.S.M.; Oliveira, R.J. Flow rate, pH and calcium concentration of saliva of children and adolescents with type 1 diabetes mellitus. Braz. J. Med. Biol. Res. 2009, 42, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Pintos, T.; Regueira-Iglesias, A.; Seijo-Porto, I.; Balsa-Castro, C.; Castelo-Baz, P.; Nibali, L.; Tomás, I. Accuracy of periodontitis diagnosis obtained using multiple molecular biomarkers in oral fluids: A systematic review and meta-analysis. J. Clin. Periodontol. 2023, 50, 1420–1443. [Google Scholar] [CrossRef] [PubMed]
- Cano-Cano, F.; Gómez-Jaramillo, L.; Ramos-García, P.; Arroba, A.I.; Aguilar-Diosdado, M. IL-1β Implications in Type 1 Diabetes Mellitus Progression: Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1303. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Piperi, C.; Ziakas, P.D.; Apostolopoulos, N.V.; Makrilakis, K.; Syriou, V.; Diamanti-Kandarakis, E.; Kaltsas, G.; Kalofoutis, A. Cytokine secretion in long-standing diabetes mellitus type 1 and 2: Associations with low-grade systemic inflammation. J. Clin. Immunol. 2008, 28, 314–321. [Google Scholar] [CrossRef]
- Schultz, O.; Oberhauser, F.; Saech, J.; Rubbert-Roth, A.; Krone, W.; Laudes, M. Die Inhibition von Interleukin-6 verbessert die Insulinsensitivität und senkt die Lipoprotein (a) Serumkonzentration beim Menschen. Diabetol. Stoffwechs. 2010, 5, P93. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H.; Liaqat, A.; Kamal, S.; Qadir, M.I.; Rasul, A. Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 229–236. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Dos Santos Haber, J.F.; Barbalho, S.M.; Sgarbi, J.A.; de Argollo Haber, R.S.; de Labio, R.W.; Laurindo, L.F.; Chagas, E.F.B.; Payão, S.L.M. The Relationship between Type 1 Diabetes Mellitus, TNF-α, and IL-10 Gene Expression. Biomedicines 2023, 11, 1120. [Google Scholar] [CrossRef]
- Kraft-Neumärker, M.; Lorenz, K.; Koch, R.; Hoffmann, T.; Mäntylä, P.; Sorsa, T.; Netuschil, L. Full-mouth profile of active MMP-8 in periodontitis patients. J. Periodontal Res. 2012, 47, 121–128. [Google Scholar] [CrossRef]
- Hernández, M.; Gamonal, J.; Tervahartiala, T.; Mäntylä, P.; Rivera, O.; Dezerega, A.; Dutzan, N.; Sorsa, T. Associations between matrix metalloproteinase-8 and -14 and myeloperoxidase in gingival crevicular fluid from subjects with progressive chronic periodontitis: A longitudinal study. J. Periodontol. 2010, 81, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.S.; Griffiths, G.S.; Stafford, G.P.; Al-Zubidi, M.I.; Rawlinson, A.; Douglas, C.W.I. Investigation of a Novel Predictive Biomarker Profile for the Outcome of Periodontal Treatment. J. Periodontol. 2017, 88, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Splieth, C.H.; Christiansen, J.; Foster Page, L.A. Caries Epidemiology and Community Dentistry: Chances for Future Improvements in Caries Risk Groups. Outcomes of the ORCA Saturday Afternoon Symposium, Greifswald, 2014. Part 1. Caries Res. 2016, 50, 9–16. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).